Abstract
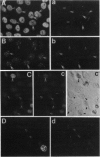
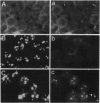
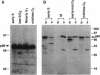
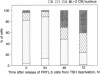
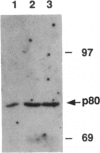
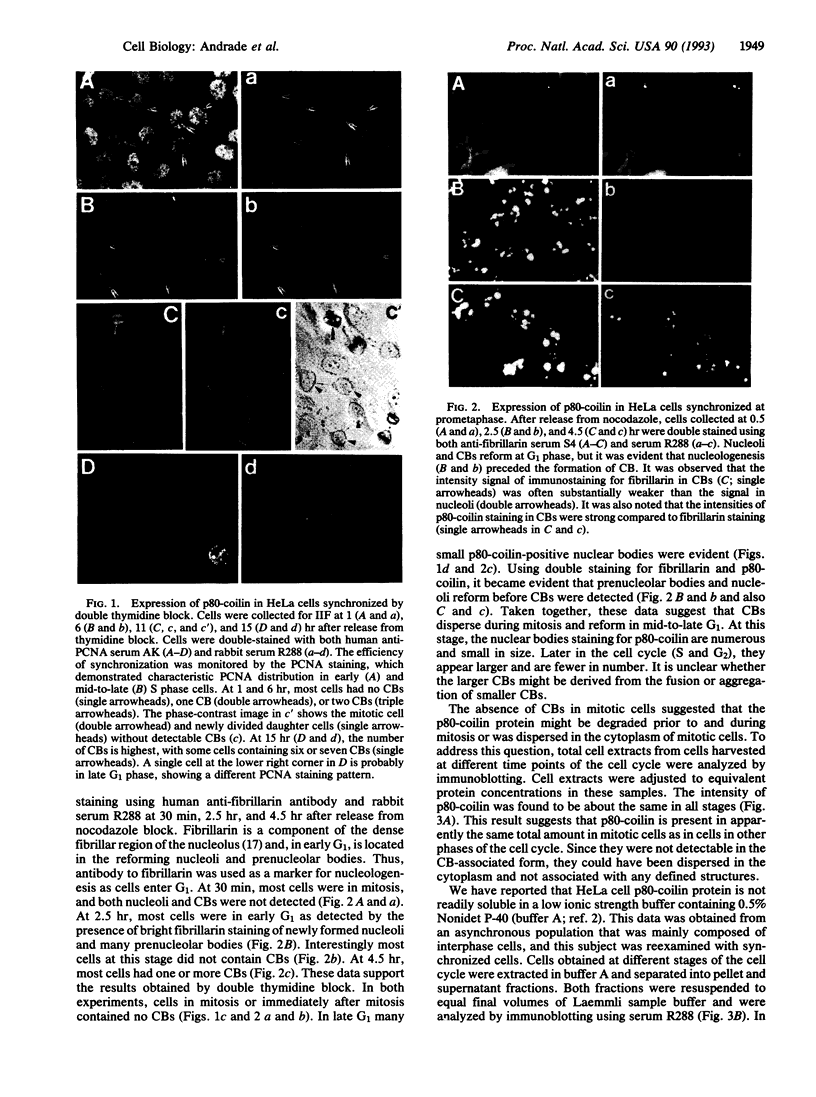
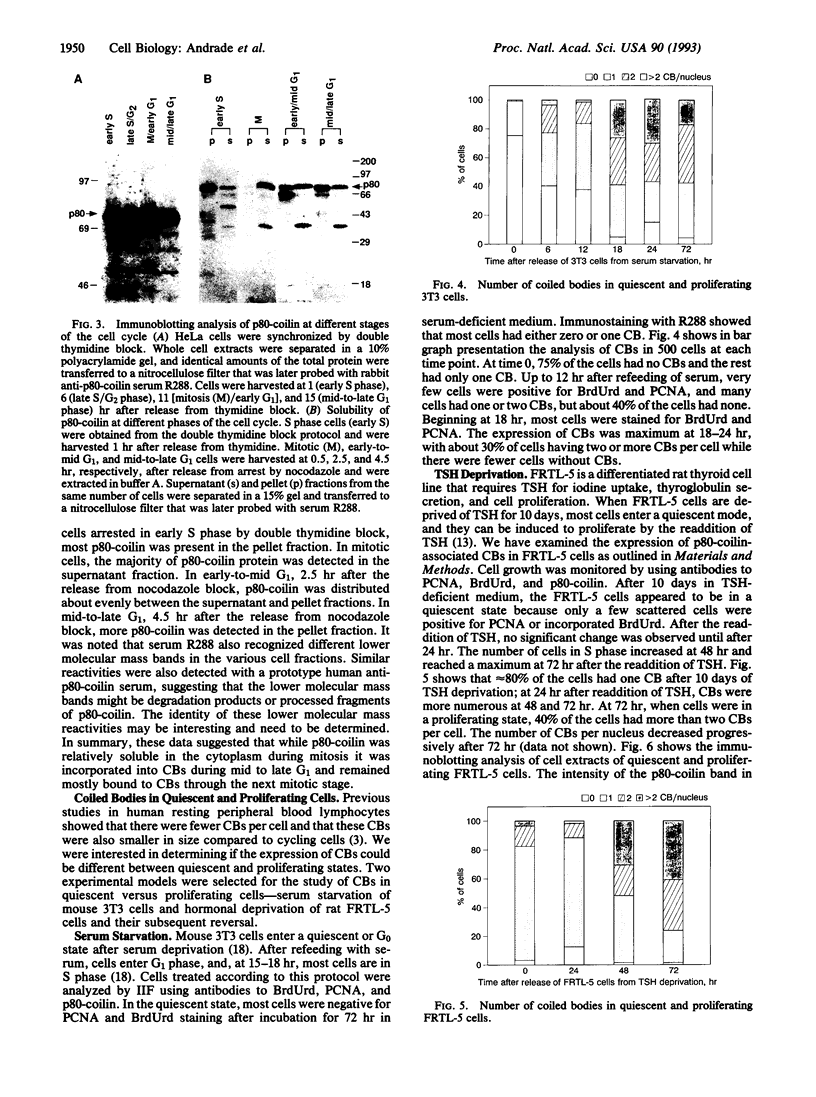
Coiled bodies (CBs) are small, round structures found in the nucleoplasm of most eukaryotic cells. Human autoantibodies to a 80-kDa protein, p80-coilin, are immunohistologic markers for CBs. A polyclonal rabbit antiserum (R288) raised against recombinant p80-coilin was shown to have similar immunochemical properties as human autoantibodies and was used to analyze the expression of p80-coilin-associated CBs in cell cultures synchronized by double thymidine block, nocodazole arrest, serum starvation, or hormonal deprivation. By employing thymidine block and nocodazole arrest of HeLa cells, CBs were observed in immunofluorescent studies to be largest in size in the S and G2 phases of the cell cycle. These large CBs might have coalesced into one or two such structures per cell from smaller and more numerous CBs of three to eight per cell during the mid G1 phase of the cell cycle. No CB-like structures were observed in mitosis and early G1. However, immunoblotting analyses showed that the total amount of p80-coilin remained approximately the same throughout the cell cycle. When HeLa cells were separated into soluble and particulate fractions, p80-coilin was detected predominantly in the soluble fraction in mitosis and early G1, whereas it was present predominantly in the particulate fraction in late G1, S, and G2 when structurally distinct CBs were observed. In the analysis of CBs in two experimental models of cell proliferation (reversal of 3T3 serum starvation and FRTL-5 thyrotropin deprivation), proliferating cells contained larger, brighter, and more numerous CBs as well as a > 2-fold increase in the total amount of p80-coilin compared to that in quiescent cells. The expression of p80-coilin in quiescent cells induced to proliferate and the cyclic formation and breakdown of CBs might be consistent with the notion that CBs may be specialized centers related to the maturation of mRNA, but this evidence is indirect and needs further definitive study.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTSMA D., BUDKE L., VOS O. STUDIES ON SYNCHRONOUS DIVISION OF TISSUE CULTURE CELLS INITIATED BY EXCESS THYMIDINE. Exp Cell Res. 1964 Jan;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987 Oct;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. F. Regulation of fibroblast cell cycle by serum. Nature. 1976 Mar 18;260(5548):248–250. doi: 10.1038/260248a0. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri G. L., Ryerse J. S. Detection of intranuclear clusters of Sm antigens with monoclonal anti-Sm antibodies by immunoelectron microscopy. J Cell Physiol. 1984 Nov;121(2):449–451. doi: 10.1002/jcp.1041210226. [DOI] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984 Jan;98(1):358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Spliceosomes and snurposomes. Science. 1991 Jun 14;252(5012):1499–1500. doi: 10.1126/science.1828621. [DOI] [PubMed] [Google Scholar]
- Gogstad G. O., Krutnes M. B. Measurement of protein in cell suspensions using the Coomassie brilliant blue dye-binding assay. Anal Biochem. 1982 Nov 1;126(2):355–359. doi: 10.1016/0003-2697(82)90527-9. [DOI] [PubMed] [Google Scholar]
- Hardin J. H., Spicer S. S., Greene W. B. The paranucleolar structure, accessory body of Cajal, sex chromatin, and related structures in nuclei of rat trigeminal neurons: a cytochemical and ultrastructural study. Anat Rec. 1969 Aug;164(4):403–431. doi: 10.1002/ar.1091640403. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):305–308. doi: 10.1073/pnas.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Andres M. A., Berciano M. T., Maquiera E. Organization of nucleoli and nuclear bodies in osmotically stimulated supraoptic neurons of the rat. J Comp Neurol. 1991 Jun 15;308(3):329–339. doi: 10.1002/cne.903080302. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Hervás J. P., Santa-Cruz M. C., Villegas J., Crespo D. The "accessory body" of Cajal in the neuronal nucleus. A light and electron microscopic approach. Anat Embryol (Berl) 1983;166(1):19–30. doi: 10.1007/BF00317942. [DOI] [PubMed] [Google Scholar]
- Nyman U., Jiang W. Q., Mellqvist E., Pettersson I., Ringertz N. Intranuclear localization of a new snRNP-related antigen. Exp Cell Res. 1991 Dec;197(2):307–313. doi: 10.1016/0014-4827(91)90437-y. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Reed R., Griffith J., Maniatis T. Purification and visualization of native spliceosomes. Cell. 1988 Jun 17;53(6):949–961. doi: 10.1016/s0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Reimer G., Pollard K. M., Penning C. A., Ochs R. L., Lischwe M. A., Busch H., Tan E. M. Monoclonal autoantibody from a (New Zealand black x New Zealand white)F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis Rheum. 1987 Jul;30(7):793–800. doi: 10.1002/art.1780300709. [DOI] [PubMed] [Google Scholar]
- Schultz M. C. Three structures associated with the nucleolus in male rat germinal cells: round body, coiled body, and "nubecula" and general presence of round body at male meiosis. Am J Anat. 1990 Sep;189(1):11–23. doi: 10.1002/aja.1001890103. [DOI] [PubMed] [Google Scholar]
- Takasaki Y., Deng J. S., Tan E. M. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981 Dec 1;154(6):1899–1909. doi: 10.1084/jem.154.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner-Capodano A. M., Mauchamp J., Stahl A., Lissitzky S. Nucleolar budding and formation of nuclear bodies in cultured thyroid cells stimulated by thyrotropin, dibutyryl cyclic AMP, and prostaglandin E2. J Ultrastruct Res. 1980 Jan;70(1):37–51. doi: 10.1016/s0022-5320(80)90020-9. [DOI] [PubMed] [Google Scholar]
- Wheeler W. J., Hsu T. C., Tousson A., Brinkley B. R. Mitotic inhibition and chromosome displacement induced by estradiol in Chinese hamster cells. Cell Motil Cytoskeleton. 1987;7(3):235–247. doi: 10.1002/cm.970070306. [DOI] [PubMed] [Google Scholar]
- Wu Z. A., Murphy C., Callan H. G., Gall J. G. Small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins in the amphibian germinal vesicle: loops, spheres, and snurposomes. J Cell Biol. 1991 May;113(3):465–483. doi: 10.1083/jcb.113.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991 Jan;10(1):207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Turnbull D., Mullins J. M., McIntosh J. R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980 Apr;126(2):397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]