Abstract
Using the structure-activity relationship emerging from previous reports, and guided by pharmacokinetic properties, new AIMs have been prepared with both improved efficacy against human glioblastoma cells and cell permeability as determined by fluorescent confocal microscopy. We present our first unambiguous evidence for telomeric G4-forming oligonucleotide anisotropy by NMR resulting from direct interaction with AIMs, which is consistent with both our G4 melting studies by CD, and our working hypothesis. Finally, we show that AIMs induce apoptosis in SNB-19 cells.
Keywords: isoxazole, anthracene, pyrrole, G-quadruplex, glioblastoma, tumor paint
Gliomas represent 78% of new brain and CNS tumors in the United States each year and have a median survival rate of only 12–15 months due to limited treatment options.1 There are difficulties in developing efficacious anticancer compounds that have favorable pharmacokinetic properties and cross the blood-brain barrier. In addition, surgical resection of gliomas has a poor success rate as a result of the inability to distinguish between cancerous and healthy tissue. Techniques using fluorescent compounds in the visualization of tumors during surgery, referred to as tumor paint, are actively being developed.2–4 We have recently reported on the synthesis, bioactivity and structure-activity relationship (SAR) of fluorescent anthracenyl isoxazole amides (AIMs, 1),5 as well as their dimeric analogues (2) 6 depicted in Chart 1. The AIMs have shown promising activity in the National Cancer Institute’s 60-cell line screening protocol (NCI 60) and could be exploited for tumor imaging. There are potential advantages to such agents that could be used both as tumor paint and exhibit antitumor activity.
Chart 1.

Structures of AIMs.
It is often stated that as many as half of all investigational new drugs fail because of poor pharmacokinetic properties.7,8 From our previous reports on the SAR of AIMs, it became apparent that the presence of two dimethylamino propyl groups, or “double tail,” led to increased efficacy in each example studied. We considered the hypothesis that the enhanced activity was attributed to increased bioavailability arising from increased water solubility. We also noted that C(10) groups bearing lone pairs or ð-density, chloro or phenyl respectively, appeared to be superior as well. The combination of these two factors has not been previously studied; for this report, we synthesized, characterized, and studied three novel double tail AIMs substituted with bromo-, chloro- and phenyl at the anthracene’s 10 position (Chart 1). Antitumor activity, cellular penetration of the AIMs and induction of apoptosis were studied in SNB-19 glioblastoma cells, and structural studies were carried out using NMR, circular dichroism spectroscopy (CD), x-ray crytallography and computational modeling.
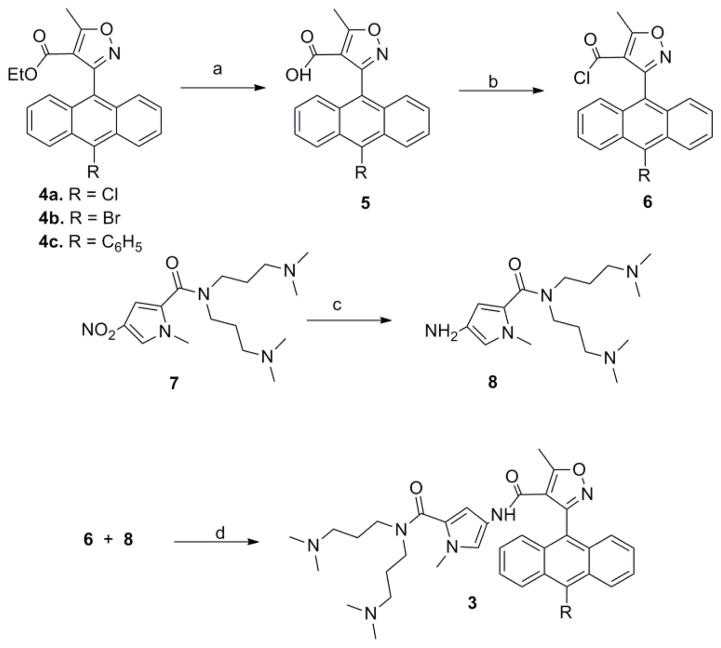
Compounds 3a, 3b and 3c (Chart 1) were prepared by the reaction of the previously reported isoxazole esters,9,10 which were converted to the corresponding acid chlorides.11 The acyl chlorides were then condensed with the bis-dimethylamino propyl pyrrole amine6,11,12 under modified Schotten-Baumann conditions (Scheme 1 and Supplementary Data). Purification was accomplished via preparative TLC on silica gel eluting with 10% ammonium hydroxide in methanol. Full characterization of the new AIMs is described in the Supplementary Data.
Scheme 1.
Synthesis of AIMs. a: 4, THF, MeOH, KOH(aq) b: 5, SOCl2 c: 7, Pd/C, H2, MeOH d: 6, 8, CH2Cl2, TEA
A single crystal x-ray diffractometry (sc-xrd) of 4c (Figure 1.A) shows the isoxazole of the AIM orients orthogonal to the 3-aryl group, with an observed dihedral angle between the isoxazole and the anthryl mean planes of 88.11°. This stereoelectronic effect was also previously observed in our crystallographic studies of isoxazole-3-anthracenes9,13–17 and isoxazole-3-anthroquinones.18 The isoxazole and ester are largely co-planar with the dihedral angle between the isoxazole mean plane and plane containing the ester carbonyl and ether atom being 10.89°. Two slightly different independent ethyl ester conformations were observed in the structure solution, both with the ethyl group endo to the anthracene; these were taken into account in order to arrive at the final value of R = 0.040. Full sc-xrd parameters and atomic coordinates are given in the Supplementary Data.
Figure 1.
A. Single crystal - x-ray diffractometry of 4c. B. SYBYL X docking of 3a (Gold) with telomeric G4 crystal structure, PDB accession number 1KF1. Interaction energy = −41.3 kcal/mole. C. Autodock Vina25 result of 3c with solution structure of telomeric G4, PDB accession number 2JSM.
Our working hypothesis is that AIMs exert their antitumor activity by binding a specific cellular target,6,9 that is, G-quadruplex (G4) DNA.19,20 The topology of the AIM by sc-xrd is similar to that observed in our computational docking study of the AIMs with human telomeric G4; Figure 1.B illustrates AIM 3a docked with coordinates from PDB accession 1KF1,21 whereas Figure 1.C shows the low energy interaction calculated for the solution conformation of the G4, PDB accession number 2JSM,22–24 docked with 3c. We considered a number of potential G4 coordinates and ligand binding modes; however, the lowest energy was calculated for a unique edge-to-face interaction of the AIM isoxazolyl-3-aryl moiety with the G-tetrad in both cases, which is in contrast to our predictions calculated with earlier programs, which suggested face-to-face π-stacking.6,9
Patel has noted that the telomeric G4 can adopt a variety of topologies in solution dependent on the conditions (counterion of either Na+ or K+), and the specific reading frame of the single strand telomeric repeat sequence. His group has provided evidence that there are two main physiologically relevant conformations present in K+ solution, and that (TTAGGG)4 predominantly adopts Form 1.23 Form 1 possesses a (3+1) G-tetrad core, in which three of the G-tracts are positioned in one direction along the primary sequence, in opposition to the fourth, which requires two double-chain reversals. The natural telomeric sequence d(T2AG3)4 formed a G4 in solution as verified by comparison with previously published spectra of the same sequence.23 We studied the addition of compound 3a to human telomeric G4 DNA which gives rise to select significant changes as evidenced by NMR spectroscopy (Figure 2). Binding induces an upfield shift of key guanine imino proton signals in the region of 10–12 ppm. Tentative assignments of the imino signals can be made by comparison to the studies of analogous sequences by Patel.23 The signals corresponding to the imino protons of G(16) and G(24) are up-field of the remaining protons of the oligo, and are both clearly shifted up-field upon addition of AIM 3a, from 10.99 and 10.94 to 10.89 and 10.87, respectively. Another unambiguous signal is that of G(4), which shifts from 11.86 to 11.74. While in general the trend is for shifts in the up-field direction, not all of the other signals appear to be as greatly influenced by addition of the AIM. Absolute assignment of specific proton shifts by 2D-NOESY is ongoing, however, this observation that only select imino signals experience anisotropy is consistent with an edge-to-face interaction, analogous to that shown in Figure 1.B.
Figure 2.
NMR Spectroscopy of human telomeric G4 DNA (TTAGGG)4 with AIM 3a (bottom, 1:1 ratio) and alone (top) at pH 6 and 50 °C. Labels (#) indicate the Form 1 signals as assigned by Patel,22,23 and the Gs are numbered in analogy to those assigned for Form 1 (TAGGG[TTAGGG]3TT).24
One would expect a consequence of G4 binding to be stabilization of G4 DNA as reflected by an increase in the melting temperature (Tm). Therefore we carried out thermal melting studies using circular dichroism. CD of the telomeric sequence evidences a 3+1 hybrid G4, and a statistically significant increase in Tm of 3.27° and 3.26° was observed at 295 nm for both 3a and 3c, respectively (***p<0.0009) (Figure 3).
Figure 3.

Representative CD spectra and telomeric G4 melting curves in the presence of AIMs.
Growth inhibition of SNB-19 human glioblastoma cells was determined using the MTT colorimetric assay as previously described.26 IC50 values were determined from semi-log plots of percent of control versus concentration. Consistent with previous observations,6,11 the use of SNB-19 cells as a prescreen was a good indicator of antitumor activity in the NCI 60. Compound 3c represents the most active antitumor compound in this series to date, and it is the first of the series with a submicromolar IC50 (Table 1). Compound 3a was assigned the designation NSC 748994 in the NCI Developmental Therapeutics Program and evaluated at both single dose and five dose screens in their 60 cell line protocol (see Supplementary Data). The overall mid-graph mean point (which approximates the average – log GI50) of 3a is 5.71, which compares favorably to several agents currently used in general medical practice, such as fluorouracil (3.5), bleomycin (5.2) and rubidazone (5.6).27
Table 1.
Antitumor activity of 1 “single tail”, AIM dimer 2, and “double tail” compounds 3a-c against human glioma SNB-19 cells.
Because of the paramount importance of pharmacokinetic properties, summarized as absorption, distribution, metabolism, elimination and toxicity (ADMET), numerous computational tools have been developed to help synthetic medicinal chemists estimate the germane properties in order to design molecules with better ADMET properties as early as possible in the discovery paradigm. Lipinski’s guidelines for oral bioavailability are one example,8 and a useful graphical representation of such parameters is the pentagonal plot or so-called Symyx radar graph (Figure 4.A and B). Parameters germane to oral bioavailability were calculated using the Symyx Draw v3.1 program. The pentagonal plot takes into consideration: log P - the oil water partition coefficient, PSA – polar surface area, nRotb – the number of rotatable bonds, MW – molecular weight, and WS – water solubility. The color of the pentagon corresponds to the goodness of fit to the Lipinski guidelines for oral bioavailability. Green = 0–1 violations, Yellow = 2 violations, Orange = 3–4 violations, Red = 5 violations. As can be seen in Figure 4.A and 4.B, the “double tail” AIM 3a has only 1 violation (green) whereas the AIM dimer 2 has 4 violations (orange), suggesting much improved bioavailability for AIM 3a. The Symyx Draw Log P calculation is based on Ghose’s AlogP method.28 The Polar Surface area computation is based on Ertl’s fragment method.29 One caveat worth mentioning, is that Bodor has critiqued fragment methods due to their lack of consideration of the 3D conformation of the molecules in question.30,31 In the current case it should be recalled that the AIMs are distinguished in every crystal structure and computation to date by a dihedral angle of 74–880° between the mean planes of the isoxazole to the anthracenyl moiety.6,13–17 This conformation would be expected to have lower surface area and hence lower lipophilicity than an alternative conformation which is planar and conjugated. These properties may improve bioavailability and allow the AIMs to permeate cell membranes as well as the blood brain barrier.
Figure 4.
Radar graphs summarizing pharmacokinetic properties of AIM dimer 2 and 3a Compound 2 (A, orange) lies outside of the optimum parameters for bioavailability in almost all respects, while the “double tail” of structure 3a (B, green) confers a much improved cLogP and AlogD (7.4), the latter value is 1.14. Fluorescent Confocal Microscopy of SNB-19 human glioma cells and 2 (C) and 3a (D), each at 1 μM concentration and 40x magnification.
The cellular distribution of the AIMs was determined by taking advantage of their fluorescent properties. AIMs 2 and 3a were added to cultured SNB-19 human glioblastoma cells and visualized by confocal microscopy (Olympus FV1000 confocal laser scanning microscope using selective laser excitation at 405 nm). Images were processed with Nuance 1.6.2.368 alpha software (Cambridge Research Institute). Both the AIM dimer 2 (Figure 4.C) and the new double tail analog 3a (Figure 4.D) permeate the cell membrane of the human glioma cells, and punctate binding is observed with 3a suggesting the induction of apoptosis by this compound.32 Annexin V flow cytometry confirmed that 3a does indeed induce apoptosis in SNB-19 cells (Figure 5). Also promising is the fact that the apoptosis is not accompanied by a substantial increase in necrosis. Both early and late apoptosis were significantly increased at 5 μM concentration.
Figure 5.
Annexin V flow cytometry shows that 3a induces apoptotic cell death in SNB-19 human glioblastoma cells after 24-h exposure. *p<0.05, ***p<0.0001.
In summary, the new AIMs described here exhibit potent antitumor activity in cell culture and fall in the nanomolar regime for the first time against the SNB-19 human glioma cell line. The AIMs possess useful spectroscopic properties, penetrate into tumor cells, and confocal microscopy exhibits several of the critical hallmarks of apoptosis. Also presented is our first unambiguous evidence for telomeric G4-forming oligonucleotide anisotropy by NMR resulting from direct interaction with AIMs, which is consistent with both our G4 melting studies by CD and our working hypothesis. Finally, we show that AIMs induce apoptosis in SNB-19 cells. This study illustrates the use of SAR to guide the design of molecules with favorable pharmacokinetic properties relatively early in the discovery process. We continue to apply new chemistry to the discovery of new biology, and will report on our progress in due course. The potential to capitalize on AIM fluorescence for tumor imaging will be the focus of a future study.
Supplementary Material
Acknowledgments
We thank the Nationals Institutes of Health for grants NINDS 7R15- NS038444-04 (NRN), P20 RR015583 (NRN, MPG), P20RR017670 (HDB), P30 NS 055022 (KCR, NRN), NIH/NCATS UL1 TR001082 (DSB, PRR) and the ALSAM Foundation Skaggs Scholars Program (PRR, HDB, NRN).
Footnotes
Dedicated to the memory of Professor Albert I. Meyers.
Supplementary Data available. Representative experimental procedures, NMR, MS, CD, and HPLC data for all new compounds. Crystal structure coordinates, distances, dihedral angles and intermolecular interactions for 4c and NCI 60 data for 3a. This material is available free of charge via the Internet at http://elesevier.com.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang L, Wei Q, Wang LE, Aldape KD, Cao Y, Okcu MF, Hess KR, El-Zein R, Gilbert MR, Woo SY, Prabhu SS, Fuller GN, Bondy ML. JCO. 2006;24:1627–1632. doi: 10.1200/JCO.2005.04.0402. [DOI] [PubMed] [Google Scholar]
- 2.Smeltzer C, Cannon MJ, Pinson P, Munger JS, West FG, Grissom CB. Org Lett. 2001;3:799–801. doi: 10.1021/ol006825v. [DOI] [PubMed] [Google Scholar]
- 3.McGreevy JM, Cannon M, Grissom CB. J Surg Res. 2003;111:38–44. doi: 10.1016/s0022-4804(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 4.Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 5.Mirzaei YR, Weaver MJ, Steiger SA, Kearns AK, Gajewski MP, Rider KC, Beall HD, Natale NR. Tetrahedron. 2012;68:10360–10364. doi: 10.1016/j.tet.2012.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han X, Li C, Mosher MD, Rider KC, Zhou P, Crawford RL, Fusco W, Paszczynski A, Natale NR. Bioorg Med Chem. 2009;17:1671–1680. doi: 10.1016/j.bmc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy T. Drug Discovery Today. 1997;2:436–444. [Google Scholar]
- 8.Lipinski CA. Drug Discovery Today: Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Li C, Rider KC, Blumenfeld A, Twamley B, Natale NR. Tetrahedron Lett. 2002;43:7673. [Google Scholar]
- 10.Han X, Twamley B, Natale NR. J Heterocycl Chem. 2003;40:539. [Google Scholar]
- 11.Gajewski MP, Beall H, Schnieder M, Stranahan SM, Mosher MD, Rider KC, Natale NR. Bioorg Med Chem Lett. 2009;19:4067–4069. doi: 10.1016/j.bmcl.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiwaki E, Tanaka S, Lee H, Shibuya M. Heterocycles. 1988;27:1945–1952. [Google Scholar]
- 13.Mosher MD, Natale NR, Vij A. Acta Crystallogr Sect C: Cryst Struct Commun. 1996;C52:2513. [Google Scholar]
- 14.Han X, Twamley B, Natale NR. J Heterocycl Chem. 2003;40:539. [Google Scholar]
- 15.Li C, Twamley B, Natale NR. Acta Crystallogr, Sect E: Struct Rep Online. 2006;E62:o854. doi: 10.1107/S1600536808041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Twamley B, Natale NR. J Heterocycl Chem. 2008;45:259–264. [Google Scholar]
- 17.Li C, Campbell MJ, Weaver MJ, Duncan NS, Hunting JL, Natale NR. Acta Crystallogr, Sect E: Struct Rep Online. 2013;E69:o1804–o1805. doi: 10.1107/S1600536813031395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan NS, Beall HD, Kearns AK, Li C, Natale NR. Acta Crystallogr, Sect E: Struct Rep Online. 2014;E70:o315–o316. doi: 10.1107/S1600536814003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neidle S. Therapeutic Applications of Quadruplex Nucleic Acids. Elsevier/Academic Press; Amsterdam: 2012. [Google Scholar]
- 20.Siddiqui-Jain A, Hurley LH. Nature Chem. 2013;5(3):153–5. doi: 10.1038/nchem.1587. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson GN, Lee MP, Neidle S. Nature. 2002;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- 22.Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJ. J Am Chem Soc. 2006;128:9963–9970. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan AT, Luu KN, Patel DJ. Nucleic Acids Res. 2006;34:5715–5719. doi: 10.1093/nar/gkl726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan AT, Kuryavyi V, Luu KN, Patel DJ. Nucleic Acids Res. 2007;35:6517–6525. doi: 10.1093/nar/gkm706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trott O, Olsen AJ. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keyari CM, Kearns AK, Duncan NS, Eickholt EA, Abbott G, Beall HD, Diaz P. J Med Chem. 2013;56:3806–3819. doi: 10.1021/jm301689x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd MR. Principles & Practices of Oncology. 1989;3:1–12. [Google Scholar]
- 28.Ghose AK, Viswanadhan VN, Wendoloski JJ. J Phys Chem A. 1998;102:3762–3772. [Google Scholar]
- 29.Ertl P, Rohde B, Selzer P. J Med Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 30.Bodor N, Gabanyi Z, Wong CK. J Am Chem Soc. 1989;111:3783–3786. [Google Scholar]
- 31.Bodor N, Buchwald P. J Phys Chem B. 1997;101:3404–3412. [Google Scholar]
- 32.Suen DF, Norris KL, Youle RJ. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.