Abstract
The generation of humanized mouse models in which immune deficient mice are engrafted with human tissues allows for the direct in vivo investigation of human-restricted viruses. These humanized mouse models have been developed and improved over the past 30 years. It is now possible to achieve high levels of human cell engraftment producing human myeloid and lymphoid lineage cells. Humanized mouse models have been increasingly utilized in the study of human cytomegalovirus (HCMV), a human-specific beta-herpesvirus that infects myeloprogenitor cells and establishes a life-long latency in the infected host. This review focuses on the strengths and limitations of the current humanized mouse models used to study HCMV replication, pathogenesis and treatment.
Introduction
Although most Human Cytomegalovirus (HCMV) infections are asymptomatic in immune competent individuals, the virus remains a significant cause of morbidity and mortality in bone marrow and solid organ transplant recipients. The strict species specificity of HCMV and the lack of a suitable animal model system have impeded our understanding of viral pathogenesis and the development of antiviral therapies. Over the last two decades, humanized mouse models in which immune deficient mice are engrafted with human tissues has opened the door for the direct in vivo investigation of viruses with growth restricted to human cells. Advancements relating to xenograft tolerance and xenograft tissue function have allowed high levels of human chimerism, especially with respect to immune cells and liver tissue. Due to the critical role that immune cells play in the latency, persistence, and/or in the pathobiology of many human herpesviruses, the field of herpesvirus research has benefited tremendously over the last decade from the continued improvements in human immune system (HIS) mouse technology. HIS mice are generated from immunodeficient mice in which the murine immune cell compartments, most notably the bone marrow, are depleted, typically by irradiation, and reconstituted with human hematopoietic progenitor cells (HPCs). This review will focus on the use of humanized mouse models to study mechanisms of HCMV latency, reactivation and treatment.
Overview on HCMV
Human cytomegalovirus is the prototypical betaherpesvirus and a ubiquitous opportunistic pathogen infecting the majority of the world’s population. HCMV infection is usually asymptomatic in healthy individuals, but viral infection causes severe disease in immunocompromised adults and birth defects in newborns (Table 1) [1]. Additionally, HCMV has been implicated as a possible cofactor in the development of vascular diseases such as atherosclerosis, transplant vascular sclerosis, and coronary restenosis after angioplasty surgery [2] (Table 1).
Table 1.
Human Cytomegalovirus-associated diseases.
| Category | Patient | Diseases |
|---|---|---|
| Infection in normal host | Healthy, adult | Mononucleosis-like syndrome |
| Congenital infection | Healthy, fetal | Intrauterine growth retardation, hepatosplenomegaly, thrombocytopenia, petechiae, chorioretinitis, and hepatitis; CNS involvement (microcephaly, encephalitis, seizures, and focal neurological signs) |
| Infection in the immunocompromised host | Solid organ and hematopoietic stem cell transplant | CMV syndrome, gastrointestinal disease (esophagitis, colitis), pneumonitis, hepatitis, allograft vasculopathy (heart transplant) |
| AIDS | Encephalitis, radiculopathy, pneumonitis, gastrointestinal disease, retinitis | |
| Contribution to chronic vascular disease | Atherosclerotic vascular disease and autoimmune vasculitides |
A characteristic of HCMV infection is the ability of the virus to spread to and persist within multiple host organs [1]. HCMV infects a variety of cells types, including hematopoietic and stromal cells of the bone marrow, endothelial cells, epithelial cells, fibroblasts, neuronal cells, and smooth muscle cells [3,4]. Of the hematopoietic lineage cells, which comprise all hematopoietic stem cell-derived myeloid and lymphoid lineages, the myeloid cell lineage is the most important with respect to HCMV latency, reactivation and persistence [5,6]. Monocytes are the primary targets for infection in the blood and are non-permissive for viral gene expression [7,8,9]. Macrophages, however, are productively infected in patients with HCMV disease [3], and in vitro studies have confirmed that macrophages and monocyte-derived dendritic cells are permissive for HCMV replication [3,10]. HCMV dissemination is proposed to occur, therefore, after infected monocytes migrate into tissues and differentiate into permissive macrophages [11]. Significant evidence indicates that latently infected peripheral blood monocytes (PBMCs) are generated from latently infected HPCs of the bone marrow [6]. HCMV-infected HPCs transiently expressed a subset of viral genes that largely become undetectable by 10 days after infection [5]. Nevertheless, viral genomes are maintained at approximately 5–10 copies per cell in the absence of viral replication during long-term culture. HCMV replication can be reactivated by co-culture of both CD34+ and CD33+ progenitor cells with human fibroblasts [5,6,12]. Although these primitive HPCs have the capacity to mature into a number of cell lineages, latent HCMV DNA is strictly associated with myelomonocytic lineage cells in healthy hosts [13]. This suggests that either latent infection of myeloid stem cells promotes maturation into the myelomonocytic lineage or that only cells of the myelomonocytic lineage are capable of maintaining the latent viral genome.
Development of Humanized Mouse Models to Study HCMV
The strict species specificity of HCMV and the lack of surrogate CMV animal models have driven the development of humanized mouse models in which mice are engrafted with human cells or tissues capable of supporting local HCMV infection. The original humanized mouse models, first reported in 1988, involved SCID (severe combined immunodeficient) mice engrafted with either human peripheral blood leukocytes (SCID-hu-PBL model) [14] or with human fetal thymic and liver tissues (SCID-hu Thy/Liv model) [15]. Mocarski et al. utilized a SCID-hu Thy/Liv mouse model to assess the ability of the Toledo strain of HCMV to replicate within human fetal tissue implants [16]. In a separate study, Brown et al. utilized a SCID-hu Thy/Liv mouse model to evaluate and compare the replicative capacity of a low-passage Toledo strain of HCMV and high-passage, laboratory-adapted HCMV strains AD169 and Towne [17]. These early humanized mouse models had several limitations including lack of long-term human cell engraftment, low diversity in types of cells engrafted, lack of distribution of human cells in the mouse and inability to generate human immune responses.
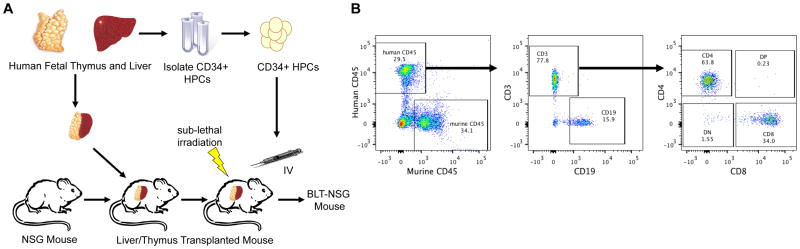
Over the past decade, a second generation of humanized mouse models has been developed in which immune deficient mice have been engrafted with primary HPCs with the goal of recapitulating a functional human immune system. The biggest breakthrough occurred with the development of immune deficient mice with a mutation in interleukin-2 receptor γ-chain locus (IL-2 γc−/−). These mice exhibited a severe impairment of mouse B, T, and NK cell development allowing greater retention of HPC allografts [18,19]. Three main mouse strains have been developed with the IL-2 γc−/− mutation including NOD.Cg-PrkdcscidIl2rgtm1Wjl (NSG mice), NOD.Cg-PrkdcscidIl2rgtm1Sug (NOG mice) and strains based on C;129S4-Rag2tm1FlvIl2rgtm1Flv (RG mice). Each of these mouse strains exhibit differences in human immune system cell development in which NSG mice support higher levels of HSC engraftment and T cell development in comparison to RG mice and greater HSC engraftment in the bone marrow in comparison to NOG mice [19,20,21]. Analysis of human hematopoietic cells demonstrated that these mice reconstituted monocytes, macrophages and B cells as well as limited T cells. The limited maturation of the T cells is believed to be due to education of these cells in the mouse thymus in the context of mouse MHC-I and II. This limitation was overcome with the development of humanized mice that have been reconstituted with human fetal bone marrow/liver/thymus tissue (BLT) [22]. BLT mice exhibit improved systemic reconstitution of human hematopoietic cells including myeloid lineage cells, NK cells and CD4+ and CD8+ T cells due, in part, to the presence of human thymic epithelium (Figure 1). NSG mice reconstituted with human CD34+ HPCs have been used to examine HCMV latency and reactivation as described below. Newer models using BLT mice are being developed to examine not only HCMV latency and reactivation but also immune responses to the virus.
Figure 1. BLT-NSG mouse model to examine HCMV latency, reactivation and immune response to the virus.
A) To generate BLT (bone marrow, liver, thymus) mice, fetal liver and thymus fragments are implanted under the renal capsule in irradiated adult immunodeficient mice and hematopoietic progenitor cells (HPCs) derived from the same fetal liver are injected intravenously. B) Beginning at 8 weeks the mice are assessed for human cell engraftment by flow cytometry using antibodies specific for human CD45 and cell type specific markers for B-cells (CD19) and T-cells (CD3, CD4 and CD8).
HCMV Latency and Reactivation in Humanized Mice
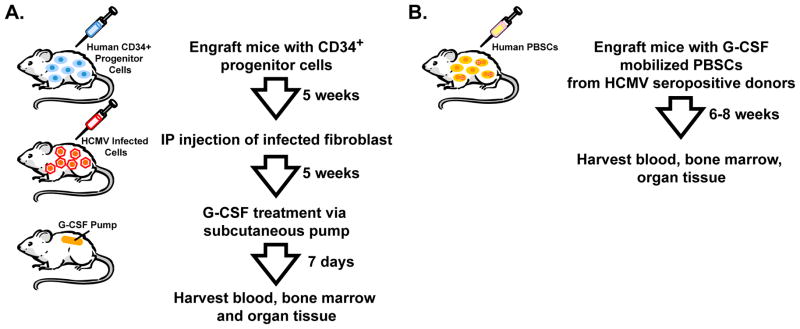
As discussed above, myeloid lineage cells play an integral role in viral latency, persistence, dissemination to organ tissue, and pathogenesis. Smith et al. developed a HU-NSG mouse model for HCMV latency and reactivation [23]. In this model, 7 to 10 week old NSG mice were sub-lethally irradiated and engrafted with CD34+ cord blood cells (Figure 2A). At 8 weeks post-engraftment, approximately 5% of PBMCs were human monocytes (huCD45+/huCD33+/huCD14+), which is approximately half of the percentage of monocytes comprising the PBMC of healthy humans [23]. In testing multiple routes of HCMV infection, the authors report that intraperitoneal (IP) injection of HCMV-infected fibroblasts at 4 weeks post-engraftment was the only route of infection leading to detectable viral DNA in organ tissue. Granulocyte-colony stimulating factor (G-CSF) treatment has been suggested to mobilize latently infected cells, which would promote viral spread to the peripheral blood and organ tissue triggering HCMV reactivation [24]. Indeed, G-CSF treatment of HU-NSG mice robustly increased the percentage of monocytes comprising the PBMCs to approximately 24% [23]. G-CSF mobilization in HCMV-infected HU-NSG mice correlated with HCMV spread to the peripheral blood, spleen, liver and kidney in all mice tested and with detectable HCMV spread to the lung, submandibular salivary gland, and bladder in some mice [23]. Moreover, early and late HCMV transcripts were present in the liver tissue of all HCMV-infected, G-CSF mobilized HU-NSG mice and absent in HCMV-infected, non-mobilized NSG mice. Immunofluorescence staining of liver tissue revealed that HCMV early and late proteins co-localized exclusively with human monocyte and macrophage markers [23].
Figure 2. HU-NSG mouse models for HCMV latency and reactivation.
A) NSG mice are engrafted with human CD34+ progenitor cells isolated from cord blood. At 5 weeks post-engraftment, the mice are infected with HCMV-infected fibroblasts (1×107) by intraperitoneal (IP) injection. At 5 weeks post-infection, HCMV mRNA expression associated with lytic infection is absent in these mice, which is suggestive of a latent HCMV infection. To induce viral reactivation the mice are treated with G-CSF via an osmotic pump for 7 days and then tissues are harvested and analyzed for viral DNA/RNA. B) NSG mice are engrafted with G-CSF mobilized peripheral blood stem cells (PBSC) from HCMV-seropositive donors. Mice are sacrificed at 6–8 weeks after transplantation or sooner if they appear sick. Peripheral blood, spleen, and bone marrow are analyzed for engraftment by flow cytometry and for HCMV DNA by quantitative PCR.
A follow-up study by Hakki et al. examined the possibility of increased HCMV transmission during peripheral blood stem cell (PBSC) transplantation (Figure 2B). In this study, the authors isolated G-CSF mobilized PBSCs from HCMV-seropositive human donors and transplanted them to HCMV-negative humanized mice. Viral DNA was detected in bone marrow, liver and spleen following transplantation, indicating that transmission and dissemination occurred in the mice and that mobilized human CD34+ cells were a source of virus [25].
Of equal importance to our understanding of the earliest cell lineage reservoir of viral latency is identifying the roles of specific HCMV gene products in the establishment and maintenance of latency and in reactivation. Umashankar et al. recently utilized the HCMV HIS model developed by Smith et al. to extend in vitro findings regarding the role of the UL133-UL138 locus in modulating cell type-specific outcomes of infection to an in vivo model of viral reactivation and spread [26]. Petrucelli et al. previously determined that disruption of the UL133-UL138 locus results in a loss of latency phenotype in HPCs infected in vitro [27]. Umashankar et al. report that HU-NSG mice infected with UL133-UL138null HCMV exhibited increased viral replication and dissemination following G-CSF-induced stem cell mobilization in comparison to those mice infected with the wild type HCMV clinical isolate TB40E [26]. These results suggest an important role for the UL133-UL138 locus in HCMV reactivation and dissemination in vivo and demonstrate the utility of HIS mice as a model to study the role of specific viral gene products in vivo.
Humanized Mouse Models to Study HCMV Pathogenesis
HCMV infection has also been associated with transplant coronary arteriosclerosis [2]. An immunodeficient mouse model with transplanted human artery tissue was developed and examined for the impact of HCMV infection upon immune rejection of the graft [28]. Human internal mammary artery tissue was obtained and infected with HCMV, then transplanted as infrarenal aortic interposition grafts into the abdominal cavity of immunodeficient mice. One week later, allogenic human PBMCs derived from single blood donors were transferred IP into mice and analyzed for graft rejection. HCMV infection was confirmed by detection of viral DNA and antigens. In addition, vascular lesions and immune cell infiltrates were more pronounced in animals receiving HCMV-infected arterial grafts compared to uninfected grafts [28].
HCMV infection of the fetus and premature infants is a major global health problem [29]. Maidij et al. generated a SCID-hu lung mouse model that closely recapitulates the different stages of human lung development in utero [30]. The authors show that HCMV efficiently replicated in the lung implants forming large viral lesions. Their findings highlight that congenital and neonatal HCMV infection can adversely impact lung development, leading to pneumonia and acute lung injury. Tabata et al. used the same model to transplant human placental villi and they reported that in xenografts, HCMV infected cytotrophoblasts had a severely diminished capacity to invade and remodel resident arteries [31].
A chimeric severe combined immunodeficient/urokinase type plasminogen activator under the control of an albumin promoter (SCID/Alb-uPA) mouse model transplanted with human hepatocytes has recently been proposed by Kawahara et al. for immune and therapeutic evaluation of acute HCMV infection in the liver [32]. The authors demonstrated the establishment of HCMV infection in human liver chimeric mice by monitoring the plasma levels of HCMV over the time and looking at the characteristic CMV histopathological intranuclear findings (“owl’s eyes”) in human hepatocytes. Moreover, they found that adoptive therapy of human liver NK cells prior to viral inoculation reduced plasma HCMV viral loads, suggesting that human liver NK cells have the potential to inhibit HCMV replication.
Finally, SCID-hu Thy/Liv and retinal transplant models have been used to study viral genetics. Using a SCID-hu Thy/Liv mouse model, Wang et al. demonstrated that the 15-kbp DNA segment present in Toledo and all virulent HCMV strains but deleted in AD169 and other attenuated strains is required for in vivo replication [33]. Humanized mice using retinal tissue transplants also support HCMV replication in graft tissue glial cells [34]. HCMV UL27 mutants were inoculated into these tissues in order to determine if the UL27 gene is necessary for in vivo replication and it was determined to be non-essential [35].
The Use of Humanized Mice to Test HCMV Antivirals
Current drugs that have been licensed in the United States for treatment of cytomegalovirus infection include foscarnet (FOS), cidofovir (CDV), ganciclovir (GCV), and the GCV oral prodrug valganciclovir (VGCV). The desire for drugs with improved efficacy, oral availability, and reduced toxicity and the emergence of drug resistance HCMV strains drive the development both of analogues of these drugs and new classes of anti-viral drugs with activity against HCMV.
It has been suggested that the SCID-hu Thy/Liv model and the retinal implant SCID-hu model should be used concurrently to evaluate novel antiviral drugs against HCMV, because both models may represent different drug bioavailability characteristics. For example, studies where GCV and CDV treatments were compared between retinal implant and SCID-hu Thy/Liv mice revealed interesting outcomes. While both GCV and CDV completely or very substantially reduced viral titers by >3–5 logs in SCID-hu Thy/Liv between days 14 and 28 post-infection, only CDV but not GCV treatment had the same significant impact on viral replication in the retinal implant SCID-hu mice [36]. These findings support the notion that the blood-eye barrier impedes the bioavailability of these drugs in retinal tissue. Kern et al. have also evaluated the efficacy of other potential anti-HCMV therapeutics including benzimidazole D- and L-ribonucleosides using both the SCID-hu retinal implant and a SCID-hu Thy/Liv implant models [37].
Bravo et al. developed a cellular implant SCID-hu model system in which SCID mice are subcutaneously implanted with Gelfoam implants containing HCMV-infected human foreskin fibroblasts [38]. At 14 days post-implantation, the Gelfoam implant became encapsulated by a tissue membrane and vascularized, resulting in the bioavailability of CDV and GCV within the implant and a significant reduction in HCMV titer in mice receiving GCV and CDV treatment initiated at either day 0 or day 7 post-implantation [38]. Lischka et al. utilized a similar approach to test the in vivo efficacy of the anti-cytomegalovirus compound AIC246 (3,4-dihydro-quinazoline-4-yl-acetic acid) [39]. The authors determined that AIC246 exhibited antiviral activity comparable to VGCV at the ED50 level and that AIC246 surpassed the in vivo activity of VGCV at the ED90 level. Weber et al. developed a model system using the culture of human cells within polyvinylidine fluoride hollow fibers, which can be implanted IP or subcutaneous in SCID mice [40]. Weber et al. demonstrated with this model system that a novel non-nucleosidic compound 3-hydroxy-2,2-dimethyl-N-[4(([5-(dimethylamino)-1-naphthyl]sulfonyl)amino)-phenyl] pronanamide (BAY 38-4766) exhibited antiviral activity against HCMV comparable to GCV [40]. Use of the newer HIS models, including the BLT and SCID/Alb-uPA models, would provide increased utility for evaluating novel therapeutics, both for efficacy and side effects, specifically those involving immune perturbation and liver toxicity, respectively.
Conclusions and Future Prospects
HCMV is implicated in a variety of diseases (Table 1), however only a fraction have been recapitulated in humanized mice (Table 2). Efforts to reproduce the remaining diseases in humanized mice need to be made. With respect to the current state of HIS mouse technology, one can envision that optimization of the HCMV HIS mouse model developed by Smith et al. could address a number of key questions regarding HCMV latency in the near future [23]. While it is known that CD34+ stem cells support HCMV latency, CD34+ stem cells are a heterogeneous population comprised of pluripotent stem cells, lymphoid lineage committed stem cells, and myeloid lineage committed stem cells. Although an effort has been made to better define the precise stem cell populations harboring latent HCMV, reproducing the conditions by which stem cell infection, latency, and reactivation occur in vivo is technically challenging and subject to experimental bias. Therefore, the CD34+ progenitor cell phenotype harboring latent HCMV is still uncertain. HIS mice capable of supporting latent HCMV infection such as that reported by Smith et al. will undoubtedly be valuable tools in delineating the defined latent stem cell phenotype.
Table 2.
Humanized mouse models of HCMV infection.
| Mouse strain | Human cells and/or tissues transplanted | Human cells reconstituted | Features of HCMV infection reproduced | Reference |
|---|---|---|---|---|
| CB-17/SCID | Fetal thymus, and liver tissue under the kidney capsule | Graft tissue cells, localized | Replication in transplanted human fetal tissues | [16,17,33,36,37] |
| Fetal lung tissue or fetal colon tissue or chorionic villi under the kidney capsule or fetal skin implanted subcutaneously | Graft tissue cells, localized | Replication in transplanted human fetal tissues; lymphoangiogenesis and vascular remodeling | [16,30,31] | |
| Fetal retinal cells implant ocularly | Graft tissue cells, localized | Replication in transplanted human fetal tissues | [36,37,34,35]; | |
| Gelfoam containing neonatal or foreskin fibroblasts | Graft tissue cells, localized | Replication in transplanted human fetal tissues | [38,39,40] | |
| CB-17/SCID/Alb-uPA | Hepatocytes injected into spleen, liver NK cells | Hepatocytes, liver NK cells | Acute HCMV infection of hepatocytes | [32] |
| C57BL/6-Rag-2−/−gc−/− | Mammary artery transplanted as infrarenal aortic graft, PBMCs | Circulating B cells, T cells, monocytes, macrophages; graft tissue cells | HCMV-induced transplant arteriosclerosis | [28] |
| NOD/SCID/IL2Rgcnull | CD34+ cells isolated from cord blood | Circulating and tissue resident B cells, T cells, monocytes, macrophages | Replication, latency and reactivation | [23,26] |
| G-CSF mobilized PBSCs from HCMV seropositive donors | Circulating B cells, T cells, monocytes, macrophages | Reactivation after HSC transplantation | [25] |
As noted with respect to the BLT mouse studies for Epstein-Barr Virus [41] and HIV [42] the potential to evaluate HCMV-specific immune responses exists, thus HCMV BLT mouse models could be utilized to evaluate the efficacy of candidate HCMV vaccines. Given that CMVs are highly immunogenic viruses and that CMVs can re-infect and disseminate in hosts with a prior history of CMV infection, CMV is now considered to be an attractive vaccine vector [43,44]. Tsuda et al. recently developed a replicating murine cytomegalovirus-based vaccine encoding a CD8+ T cell epitope from the nucleoprotein of Zaire Ebolavirus that protected mice from lethal Ebolavirus challenge [43]. Hansen et al. report that rhesus macaques infected with a rhesus cytomegalovirus-based simian immunodeficiency virus (SIV) vaccine vector encoding SIV Gag, Rev/Nef/Tat, and Env were resistant to acquisition of progressive SIV infection following repeated intra-rectal challenge [44].
Improvements in humanized mouse models in recent years have provided a platform to study HCMV. We believe that the newer generation of humanized mice will be a useful tool to not only better define mechanisms of HCMV latency, reactivation and pathogenesis but also to validate HCMV-based vaccine vectors for human pathogens and cancer.
Highlights.
HCMV is a ubiquitous and opportunistic betaherpesvirus
Development of humanized mice allows for the study of human-restricted viruses
Myeloid lineage cells are critical for HCMV latency, reactivation, and persistence
Humanized mouse models are tools to study HCMV-associated diseases and treatments
Acknowledgments
We would like to thank Andrew Townsend for graphics assistance. This research was supported by grants AI21640 (JAN) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been
highlighted as:
* of special interest
** of outstanding interest
- 1.Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235:288–297. doi: 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- 2.Streblow DN, Dumortier J, Moses AV, Orloff SL, Nelson JA. Mechanisms of Cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing. Curr Top Microbiol Immunol. 2008;325:397–415. doi: 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvis MA, Nelson JA. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr Opin Microbiol. 2002;5(4):403–407. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis MA, Nelson JA. Human cytomegalovirus tropism for endothelial cells: not all the endothelial cells are created equal. J Virol. 2007;81(5):2095–2101. doi: 10.1128/JVI.01422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodrum FD, Jordan CT, High K, Shenk T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc Natl Acad Sci USA. 2002;99(25):16255–16260. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodrum F, Jordan CT, Terhune SS, High KP, Shenk T. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic subpopulations. Blood. 2004;104:687–695. doi: 10.1182/blood-2003-12-4344. [DOI] [PubMed] [Google Scholar]
- 7.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 8.Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68(3):1597–1604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brytting M, Mousavi-Jazi M, Boström L, Larsson M, Lunderberg J, Ljungman P, Ringdén O, Sundqvist VA. Cytomegalovirus DNA in peripheral blood leukocytes and plasma from bone marrow transplant recipients. Transplantation. 1995;60(9):961–965. [PubMed] [Google Scholar]
- 10.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91(1):119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 11.Soderberg-Naucler C, Fish KN, Nelson JA. Growth of human cytomegalovirus in primary macrophages. Methods. 1998;16(1):126–138. doi: 10.1006/meth.1998.0650. [DOI] [PubMed] [Google Scholar]
- 12.Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Sci USA. 1998;95(7):3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68(6):4017–4021. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosier DE. Immunodeficient mice xenografted with human lymphoid cells: new models for in vivo studies of human immunobiology and infectious diseases. J Clin Immunol. 1990;10(4):185–191. doi: 10.1007/BF00918650. [DOI] [PubMed] [Google Scholar]
- 15.McCune JM, Namikawa R, Kaneshima H, Schultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 16.Mocarski ES, Bonyhadi M, Salimi S, McCune JM, Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci U S A. 1993;90(1):104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JM, Kaneshima H, Mocarski ES. Dramatic interstrain differences in the replication of human cytomegalovirus in SCID-hu mice. J Infect Dis. 1995;171(6):1599–1603. doi: 10.1093/infdis/171.6.1599. [DOI] [PubMed] [Google Scholar]
- 18.Shultz LD, Lyons BL, Burzenski LM, Gott B, Che X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human Lymphoid and Myeloid Cell Development in NOD/LtSz-scid IL2Rγnull Mice Engrafted with Mobilized Human Hematopoietic Stem Cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 19.Brehm MA, Wiles MV, Greiner DL, Shultz LD. Generation of improved humanized mouse models for human infectious diseases. J Immunol Methods. 2014;410:3–17. doi: 10.1016/j.jim.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human coed blood engraftment between immunocompromised mouse strains. Blood. 2010;116(2):193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 22.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 23**.Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB, Othieno FA, Streblow DN, Garcia JV, Fleming WH, Nelson JA. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe. 2010;8(3):284–291. doi: 10.1016/j.chom.2010.08.001. The first demonstration that HCMV infects and establishes latency in HU-NSG mice and can be reactivated using granulocyte-colony factor stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess DA, Bonde J, Craft TP, Wirthlin L, Hohm S, Lahey R, Todt LM, Dipersio JF, Devine SM, Nolta JA. Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor. Biol Blood Marrow Transplant. 2007;13(4):398–411. doi: 10.1016/j.bbmt.2006.12.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Hakki M, Goldman DC, Streblow DN, Hamlin KL, Krekylwich CN, Fleming WH, Nelson JA. HCMV infection of humanized mice after transplantation od G-CSF-mobilized peripheral blood cells from HCMV-seropositive donors. Biol Blood Marrow Transplant. 2014;20(1):132–135. doi: 10.1016/j.bbmt.2013.10.019. The first evidence that Hu-NSG mouse model can be used as a tool for studying HCMV transmission and infection using clinical samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Umashankar M, Petrucelli A, Cicchini L, Caposio P, Kreklywich CN, Rak M, Bughio F, Goldman DC, Hamlin KL, Nelson JA, Fleming WH, Streblow DN, Goodrum F. A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection. PLoS Pathog. 2011;7(12):e1002444. doi: 10.1371/journal.ppat.1002444. This study reports the first in vivo evidence of the role played by the UL133–138 locus in HCMV latency and reactivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrucelli A, Rack M, Grainger L, Goodrum F. Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus. J Virol. 2009;83(11):5615–5629. doi: 10.1128/JVI.01989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Abele-Ohl S, Leis M, Wollin M, Mahmoudian S, Hoffmann J, Muller R, Heim C, Spriewald BM, Weyand M, Stamminger T, Ensminger SM. Human cytomegalovirus infection leads to elevated levels of transplant arteriosclerosis in a humanized mouse aortic xenograft model. Am J Transplant. 2012;12(7):1720–1729. doi: 10.1111/j.1600-6143.2012.04018.x. The first description of HCMV pathogenesis (arteriosclerosis) in an in vivo humanized mouse model. [DOI] [PubMed] [Google Scholar]
- 29.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Maidji E, Kosikova G, Joshi P, Stoddart CA. Impaired Surfactant Production by Alveolar Epithelial Cells in a SCID-hu Lung Mouse Model of Congenital Human Cytomegalovirus Infection. J Virol. 2012;23(86):12795–12805. doi: 10.1128/JVI.01054-12. The first demonstration of fetal pathogenesis resulting from HCMV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabata T, Petitt M, Fang-Hoover J, Rivera J, Nozawa N, Shiboski S, Inoue N, Pereira L. Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model. AM J Pathol. 2012;181(5):1540–1559. doi: 10.1016/j.ajpath.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Kawahara T, Lisboa LF, Cader S, Douglas DN, Nourbakhsh M, Pu CH, Lewis JT, Churchill TA, Humar A, Kneteman NM. Human cytomegalovirus infection in humanized liver chimeric mice. Hepatol Res. 2013;43:679–684. doi: 10.1111/j.1872-034X.2012.01116.x. The first description of HCMV pathogenesis in human liver in an in vivo humanized mouse model. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Taylor SL, Leisenfelder SA, Morton R, Moffat JF, Smirnov S, Zhu H. Human cytomegalovirus genes in the 15-kilobase region are required for viral replication in implanted human tissues in SCID mice. J Virol. 2005;79(4):2115–2123. doi: 10.1128/JVI.79.4.2115-2123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidanset DJ, Rybak RJ, Hartline CB, Kern ER. Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. J Infect Dis. 2001;184(2):192–195. doi: 10.1086/322015. [DOI] [PubMed] [Google Scholar]
- 35.Prichard MN, Quenelle DC, Bidanset DJ, Komazin G, Chou S, Srach JC, Kern ER. Human cytomegalovirus UL27 is not required for viral replication in human tissue implanted in SCID mice. Virol J. 2006;3(1):18–20. doi: 10.1186/1743-422X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern ER, Rybak RJ, Hartline CB, Bidanset DJ. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir Chem Chemother. 2001;12(Suppl 1):149–156. [PubMed] [Google Scholar]
- 37.Kern ER, Rybak R, Drach J, Townsend L, Poiron K, Bidanset D. Activities of Benzimidazole D- and L-Ribonucleosides in Animal Models of Cytomegalovirus Infections. Antimicrob Agents Chemother. 2004;48(5):1749–1755. doi: 10.1128/AAC.48.5.1749-1755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bravo FJ, Cardin RD, Bernstein DI. A model of human cytomegalovirus infection in severe combined immunodeficient mice. Antiviral Res. 2007;76(2):104–110. doi: 10.1016/j.antiviral.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, Ruebsamen-Schaeff H, Zimmermann H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother. 2010;54(3):1290–1297. doi: 10.1128/AAC.01596-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber O, Bender W, Eckenberg P, Goldmann S, Haerter M, Hallenberger S, Henninger K, Reefschläger J, Trappe J, Witt-Laido A, Ruebsamen-Waigmann H. Inhibition of murine cytomegalovirus and human cytomegalovirus by a novel non-nucleosidic compound in vivo. Antiviral Res. 2001;49(3):179–189. doi: 10.1016/s0166-3542(01)00127-9. [DOI] [PubMed] [Google Scholar]
- 41.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature medicine. 2006;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 42.Denton WD, Garcia JV. Humanized mouse models of HIV infection. AIDS Rev. 2011;13(30):135–148. [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis. 2011;5(8):e1275. doi: 10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]


