Summary
Background
The recurrence of port-wine stain (PWS) blood vessels by pulsed dye laser (PDL)-induced angiogenesis is a critical barrier that must be overcome to achieve a better therapeutic outcome.
Objectives
To determine whether PDL-induced angiogenesis can be suppressed by topical axitinib.
Methods
The mRNA expression profiles of 86 angiogenic genes and phosphorylation levels of extracellular signal regulated kinases (ERKs), phosphorylated protein kinase B (AKT) and ribosomal protein S6 kinase (p70S6K) in rodent skin were examined with or without topical axitinib administration after PDL exposure.
Results
The PDL-induced increased transcriptional levels of angiogenic genes peaked at days 3–7 post-PDL exposure. Topical application of 0.5% axitinib effectively suppressed the PDL-induced increase in mRNA levels of the examined angiogenic genes and activation of AKT, P70S6K and ERK from days 1 to 7 post-PDL exposure. After topical administration, axitinib penetrated into rodent skin to an approximate depth of 929.5 μm.
Conclusions
Topical application of 0.5% axitinib can systematically suppress the PDL-induced early stages of angiogenesis via inhibition of the AKT/mammalian target of rapamycin/p70S6K and Src homology 2 domain containing transforming protein-1/mitogen-activated protein kinase kinase/ERK pathway cascades.
Port-wine stain (PWS) is a congenital, progressive vascular malformation of human skin involving the superficial vascular plexus. PWS occurs in an estimated 3–5 children per 1000 live births.1–3 In childhood, PWSs are flat red macules, but lesions tend to darken progressively to purple and, by middle age, often become raised as a result of the development of vascular nodules.4,5 Recently, a low-frequency allelic mutation (c.548G→A, p.R183Q) in the guanine nucleotide-binding protein G alpha subunit q has been identified in PWS skin.6 We have also found consecutive activation of c-Jun N-terminal kinases and extracellular signal regulated kinases (ERKs) in both infantile and adult PWS.7 Taken together, these studies have begun to elucidate the molecular mechanisms underlying the pathogenesis of PWS.
Pulsed dye laser (PDL) is the current treatment of choice for PWS.8,9 However, if the ultimate standard required is complete blanching of the lesion, the degree of PWS blanching achieved following PDL can be variable and unpredictable, with an average treatment success rate below 10% owing to blood vessel recurrence.10–12 The regeneration and revascularization of blood vessels post-PDL treatment is a critical barrier that must be overcome in order to achieve an adequate PWS therapeutic outcome.13 Recent data suggest that activation of angiogenesis pathways induced by PDL in PWS contributes to this process.13 Thus, we hypothesize that a better PWS therapeutic outcome might be achieved with PDL combined with the administration of anti-angiogenesis agents. In our previous studies, we have demonstrated that topical rapamycin (RPM) can suppress the PDL-induced angiogenesis in rodent skin and that systemic administration of RPM post-PDL enhances the blanching response in patients with PWS.13,14 However, multiple signalling pathways are generally activated during PDL-induced angiogenesis; thus, a multitarget inhibitor, such as axitinib, may produce a better anti-angiogenesis effect than RPM, which mainly blocks the protein kinase B (AKT)/mammalian target of rapamycin (mTOR)/ribosomal protein S6 kinase (P70S6K) pathway.15,16 Axitinib, a U.S. Food and Drugs Administration-approved anti-angiogenesis agent for the second-line treatment of patients with advanced renal cell carcinoma, can inhibit many angiogenic tyrosine kinases, including vascular endothelial growth factor (VEGF) receptors (VEGFRs) 1–3, platelet-derived growth factor receptor and stem cell growth factor receptor.17 In this study, we attempted to combine PDL with topical administration of axitinib in order to evaluate its effectiveness in suppressing PDL-induced angiogenesis in rodent skin.
Materials and methods
Animals
All experiments were conducted under a protocol approved by the institutional animal care and use committee of the University of California, Irvine. Adult male Sprague–Dawley rats with an initial bodyweight of 100–150 g were used. Topical axitinib was prepared following the same protocol as described previously.13
Laser irradiation
Laser exposure was performed on the abdominal side of rodent skin. Sites were irradiated with a 585-nm PDL (Candela, Wayland, MA, U.S.A.): pulse duration was 0.45 ms and energy density was 8 J cm−2 delivered on a 2-mm spot diameter. Each animal had three sites in designated areas (1.5 cm × 2.0 cm) side-by-side on the skin: control (no treatment), PDL only and PDL + axitinib. The PDL-only group also received a topical vehicle composed of exactly the same ointment as the topical axitinib formulation but without axitinib. Topical axitinib or vehicle ointment was applied daily for 1, 3, 5, 7, 10 and 14 days post-PDL exposure. Animals were then euthanized and four biopsy samples (4 mm in diameter) were taken from each site. Three to four animals were in each group for each time point post-PDL exposure.
RNA extraction and real-time reverse transcription polymerase chain reaction array analysis
The procedures for total RNA, protein extraction and real-time polymerase chain reaction (RT-PCR) array were carried out as reported previously with minor modifications.13 For the RT-PCR array, the conditions were one cycle at 95 °C for 2 min followed by 45 cycles of 15 s at 95 °C and 60 s at 60 °C in a 384-well format. Relative quantification of RT-PCR was based upon the amplification efficiency of the target and reference genes, and the cycle number at which fluorescence crossed a prescribed background level cycle threshold (Ct).
Immunohistochemistry and immunoblot
Skin biopsies (4 mm) from each site were fixed in 10% buffered formalin and embedded in paraffin. Antigen retrieval was performed in 10 mmol L–1 sodium citrate buffer (pH 6.0) at 90 °C for 12 h. Antibodies against β-actin (SC-81178), phosphor-ERK (pERK; E-4, SC-7383), phospho-P70S6K (SC-8416), P70S6K (SC-8418), AKT (SC-8312) and phospho-AKT (SC-7985-R) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The procedures for immunostaining and immunoblot assays were performed as described previously.7,13
Results
Inhibitory effects of topical axitinib on expression profiles of angiogenic factors induced by pulsed dye laser
We attempted to address the inhibitory effects of topical axitinib on PDL-induced angiogenesis. At days 1, 3, 5 and 7 post-PDL exposure, the numbers of angiogenic genes with increased mRNA levels by a factor of two or higher in the PDL + axitinib groups were 27, 13, 31 and 22, respectively, compared with 46, 57, 48 and 81 out of 86 genes in the control group at each corresponding treatment time, suggesting topical axitinib rendered a systematic, but not complete, inhibition on PDL-induced angiogenesis (Fig. 1). On days 10 and 14 post-PDL exposure, the expression levels of angiogenic genes returned to normal baseline control levels (Fig. 1). Thus, axitinib exhibited a profound overall inhibition through days 1–7, but not days 10–14 post-PDL exposure.
Fig 1.
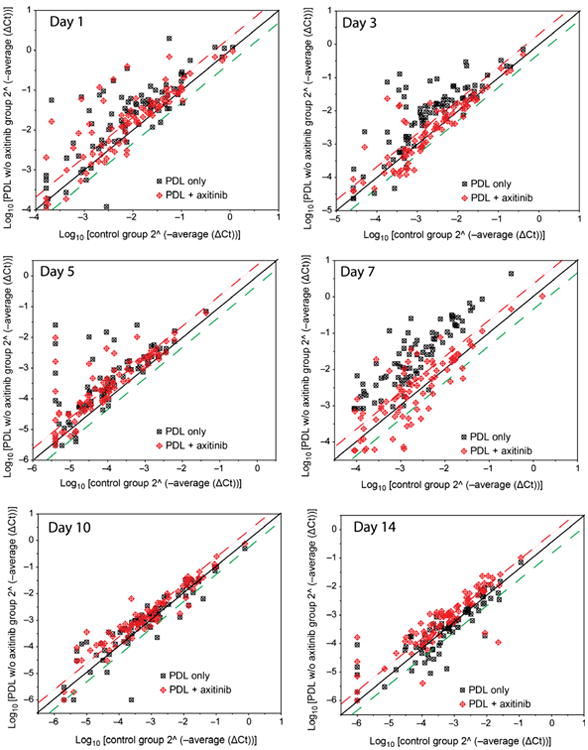
Scatter plots showing the expression profiles of 86 angiogenic factors with or without topical axitinib administration through days 1–14 after PDL exposure. The threshold was set at two times control. The squares above the upper threshold line (red) represent the angiogenic genes in treatment groups that had twofold or more increased mRNA levels compared with the normal control group. The squares below the lower threshold line (green) represent the angiogenic genes in treatment groups that had twofold or more decreased mRNA levels compared with the normal control group. The squares between both red and green threshold lines represent the angiogenic genes in treatment groups showing either increased or decreased mRNA levels within twofold compared with the normal control group values.
Expression profiles of some key angiogenic factors
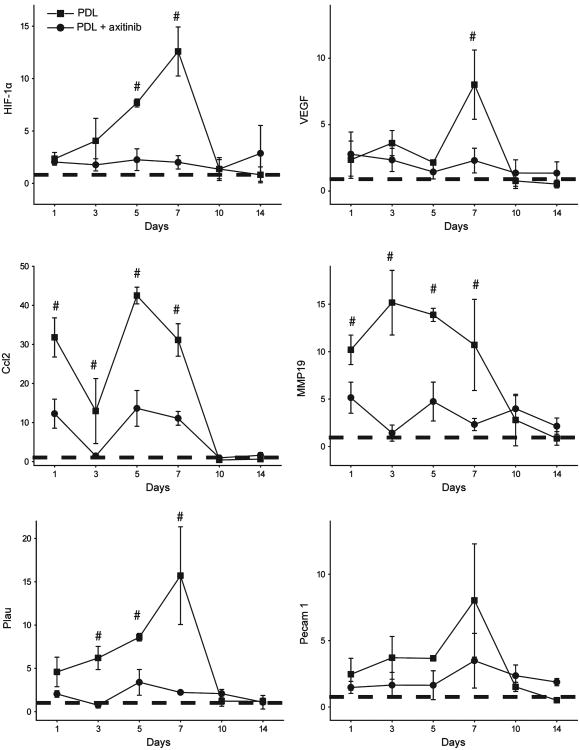
Both VEGF and hypoxia-inducible factor (HIF)-1α showed increased mRNA levels from day 1 and reached peak expression levels at day 7 post-PDL exposure. Topical axitinib resulted in significant inhibition of the expression of HIF-1α on days 5 and 7 (n = 3; P < 0.05) and VEGF on day 7 post-PDL exposure (n = 3; P < 0.05) (Fig. 2).
Fig 2.
Topical application of axitinib significantly suppressed PDL-induced mRNA levels of angiogenic factors. The relative mRNA levels of hypoxia-inducible factor (HIF)-1α, vascular endothelial growth factor (VEGF), chemokine (C-C motif) ligand 2 (Ccl2), matrix metalloproteinase (MMP)19, plasminogen activator urokinase (Plau) and platelet endothelial cell adhesion molecule (Pecam) 1 were plotted against days post-PDL exposure. The data are presented as mean ± SD and show the fold changes of mRNA levels of target genes (y-axes) compared with the normal controls (dashed lines). #P < 0.05 in the PDL only group compared with the PDL + axitinib group (paired t-test).
Topical axitinib resulted in strong inhibition of some angiogenic factors throughout the course of PDL-induced angiogenesis, such as chemokine (C-C motif) ligand 2 (Ccl2), matrix metalloproteinase 19 (MMP19) and plasminogen activator urokinase (Plau). The mRNA levels of Ccl2, MMP19 and Plau increased 31.0-, 10.0- and 4.6-fold, respectively, compared with normal skin on the first day post-PDL in the PDL-only group. Their expression levels peaked on days 3, 5 and 7, respectively, post-PDL exposure in the PDL-only group. Topical axitinib elicited significant inhibitory effects on PDL-induced increases in the mRNA levels of all these genes on days 1–7 post-PDL exposure (n = 3; P < 0.05) (Fig. 2).
The suppression of a few angiogenic genes was minimal in response to topical axitinib. For example, the mRNA level of platelet endothelial cell adhesion molecule (Pecam) 1 remained increased 2–7-fold compared with the normal control throughout days 1–10 post-PDL exposure. Topical axitinib showed a mild degree of inhibition during the course of angiogenesis, but it was not statistically significant (Fig. 2).
Suppression of phosphorylation of extracellular regulated kinase, protein kinase B and ribosomal protein S6 kinase by topical axitinib
pERK showed some basal immunoreactive signals in the epidermis and hair follicles in normal control skin (Fig. 3). The number of immunoreactive cells, particularly in blood vessels, increased in the dermis post-PDL (Fig. 3). Application of 0.5% topical axitinib for 3–5 consecutive days completely abolished immunoreactive pERK signals from cells in the epidermis and dermis. Cells in the muscular layers, approximately 929.5 ± 106.1 μm below the skin surface, showed intact immunoreactive pERK signals (Fig. 3), which we concluded was a result of the effective penetration depth of topical axitinib in rodent skin. The immunoreactive pERK signals were partially restored by day 10 and almost completely restored by day 14 in the epidermis and dermis (Fig. 3).
Fig 3.
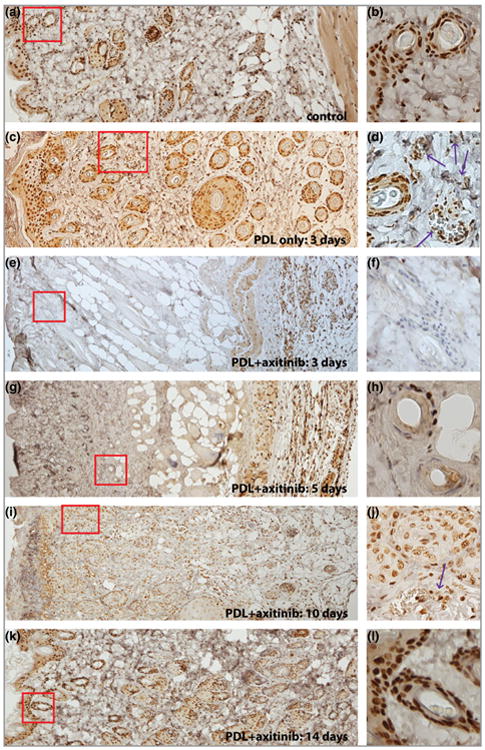
Topical application of axitinib significantly blocked PDL-induced activation of extracellular regulated kinase (ERK). (a, b) Epidermis and hair follicles showed strong basal activity of ERK. (c, d) The activity of ERK was elevated in the photocoagulated blood vessels (indicated by the arrows) in the dermis 3 days post-PDL exposure. (e–h) The activity of ERK in the epidermis and dermis, but not in muscular layers, was blocked by administration of 0.5% topical axitinib for (e, f) 3 or (g, h) 5 days. (i, j) The activity of ERK was partially restored in the dermis, but not the epidermis, 10 days post-PFL exposure. Note the weaker intensity of phosphor-ERK (pERK) immunoreactive signals of hair follicles in (j) compared with the basal activity of ERK in (b). (k, l). The pERK expression pattern in both the epidermis and dermis was back to normal 14 days post-PDL exposure. The images in the right-hand column are higher magnifications of the boxed areas in the corresponding left-hand panels. Red boxes highlight epidermis/dermis or dermis only.
pAKT and pP70S6K were examined by immunoblot. The phosphorylation levels of AKT and P70S6K showed significant increases during the first 3 or 7 days, respectively, and declined to normal levels at day 10 post-PDL exposure (P < 0.05; Fig. 4). Administration of 0.5% topical axitinib significantly blocked PDL-induced pATK and pP70S6K during the first 3 or 7 days post-PDL exposure, respectively (P < 0.05; Fig. 4).
Fig 4.
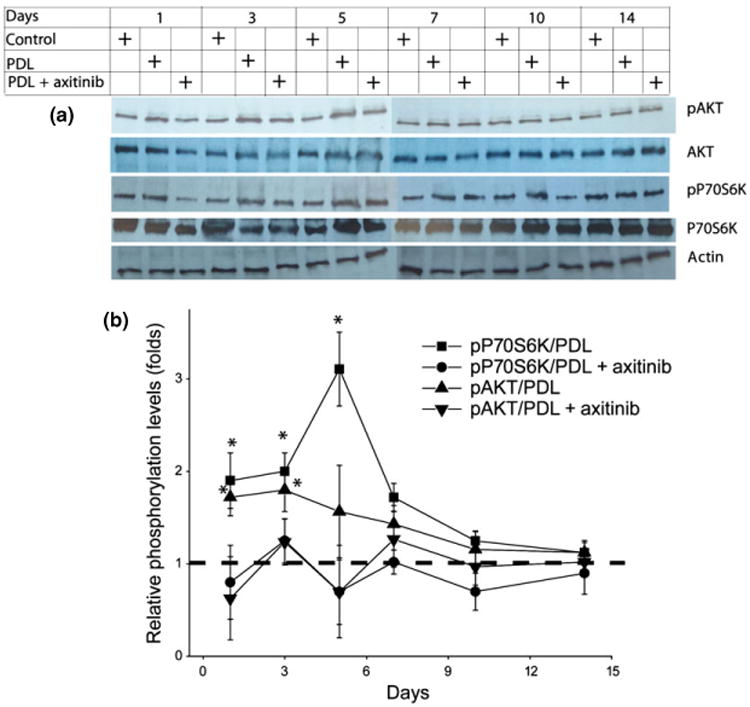
Topical application of axitinib significantly blocked PDL-induced activation of protein kinase B (AKT) and ribosomal protein S6 kinase (P70S6K). (a) The protein levels of AKT and P70S6K, and phosphorylation levels of AKT (Ser473) and P70S6K (Ser411) were determined by immunoblot. (b) The relative phosphorylated AKT (pAKT) and phosphorylated P70S6K (pP70S6K) profiles during the PDL-induced angiogenic course with or without topical axitinib application. The relative pAKT and pP70S6K were quantified to the adjusted AKT and P70S6K protein levels, which were normalized to the actin levels. The pAKT and pP70S6K levels in normal control skin were set as ‘1’ (dashed line). The data are presented as mean ± SD. n = 3 for each time point. *Phosphorylation levels in the PDL-only group were significantly higher than the control and PDL + axitinib groups (P < 0.05). P-values were obtained by a paired t-test.
Discussion
We hypothesized that angiogenesis pathways play critical roles in the pathophysiological process of regeneration and revascularization of PWS blood vessels after PDL exposure. Thus, suppression of angiogenesis by an inhibitor, axitinib, may lead to better PWS therapeutic outcomes. In this study, we determined that topical application of axitinib can significantly suppress PDL-induced angiogenesis, effectively penetrate the stratum corneum, reach the dermis and take action at the site of application.
Pulsed dye laser-induced blood vessel injury can induce an inflammatory response, evident by increases in expression of many chemokines and proinflammatory cytokines, such as Ccl2, which is a small cytokine that recruits monocytes, memory T cells and dendritic cells to the sites of inflammation.18,19 In this study, the expression patterns of Ccl2 suggested that inflammatory/immunomodulatory factors were among the first molecules activated by PDL and played very active roles throughout the angiogenesis processes. Furthermore, molecules related to degradation of extracellular matrix, such as MMP19 and Plau, showed dramatic, acute increases in their mRNA levels in response to laser-induced injury. MMPs are required to degrade the vascular basement membrane and remodel the extracellular matrix to allow endothelial cells to migrate during angiogenesis. In addition, MMPs can enhance the availability and bioactivity of many angiogenic factors, such as VEGF, fibroblast growth factor and transforming growth factor-β.20 Plau, produced by infiltrating lymphocytes, can cooperatively degrade the extracellular matrix and basement membrane, as well as regulate the activities of MMPs directly.21 Our results suggest that MMPs and Plau may be involved in the process of clearing injured blood vessels and preparing the extracellular spaces for regrowth of new blood vessels during angiogenesis.
Axitinib is a potent and highly selective inhibitor of VEGFR tyrosine kinases 1, 2 and 3 through competitive binding to the intracellular adenosine triphosphate (ATP) site domain of the receptors.17 Blockage of VEGF/VEGFR pathways leads to suppression of endothelial nitric oxide synthase, AKT and ERK.17 The binding of VEGF to VEGFR2 results in activation of both the PI3/K/AKT/mTOR/P70S6K and Src homology 2 domain containing transforming protein-1 (SHC1)/mitogen-activated protein kinase kinase (MEK)/ERK pathway cascades.22 Application of topical axitinib should inhibit both pathways as the blockage takes place at the ATP binding site of VEGFRs. Indeed, we found that the PDL-induced increase in pP70S6K and pEKR was completely abolished in the dermis after topical application of axitinib. Our results confirmed that topical axitinib could effectively suppress both the PI3/K/AKT/mTOR/P70S6K and SHC1/MEK/ERK pathway cascades during angiogenesis.
Systemic exposure of anti-angiogenesis drugs may cause side-effects and patient morbidity, and might preclude the use of those drugs in infants and young children on whom the effects of systemic exposure have yet to be studied. For example, > 20% of patients may have some adverse effects, including diarrhoea, hypertension, fatigue, nausea, stomatitis and vomiting, following systemic administration of axitinib.23 Thus, development of a topical formulation of axitinib is very important to treat pathology confined to the skin, such as PWS. In this study, we confirmed that topical axitinib was able to suppress systematically PDL-induced angiogenesis in rodent skin. We did not observe any acute adverse effects, such as skin irritation, allergy, persistent erythema, blistering, erosion or abnormal wound healing, or long-term adverse effects, such as scarring or dyspigmentation, with application of topical axitinib post-PDL, which suggests that our topical formulation of axitinib is potentially safe for human use. Our immunohistochemistry data showed that topical axitinib can effectively suppress the activation of ERK in the epidermis and dermis but not in the subcutaneous and muscle layers, through which we estimated the approximate penetration depth of topical axitinib from the skin surface. However, the pharmacokinetics of topical axitinib in rodent skin have yet to be determined and will be the subject of future studies.
In conclusion, we have shown that topical axitinib can systematically suppress PDL-induced angiogenesis via inhibition of AKT/mTOR/P70S6K and SHC1/MEK/ERK pathway cascades. We have also shown the effective penetration depth of topical axitinib in rodent skin. Our data provide essential information on dosage, duration and molecular mechanisms for potential clinical applications of topical axitinib for PWS treatment in combination with PDL exposure.
What's already known about this topic?
The regeneration of blood vessels in port-wine stain (PWS) after pulsed dye laser treatment is a critical barrier that must be overcome to achieve an adequate therapeutic outcome.
Activation of angiogenesis pathways induced by PDL contributes to PWS blood vessel recurrence.
What does this study add?
Topical application of 0.5% axitinib can systematically suppress PDL-induced angiogenesis.
We propose a potentially new therapeutic strategy using PDL + axitinib for the clinical management of patients with PWS.
Acknowledgments
We greatly appreciate the assistance of Amanda Dickson of the Sue & Bill Gross Stem Cell Research Center at the University of California, Irvine, for her expertise on the real-time polymerase chain reaction and histology image acquisition process experiments. We are also grateful to Linda Li at the Beckman Laser Institute and Medical Clinic for help in biopsy tissue processing and immunohistochemistry. Institutional support was provided by the Arnold and Mabel Beckman Foundation and the David and Lucile Packard Foundation. Any views expressed here represent the authors' personal opinions and do not necessarily reflect those of the U.S. Department of Health and Human Services or the United States federal government.
Funding sources: Grants were received from the National Institutes of Health (AR063766 to W.T.and AR47551 and AR59244 to J.S.N.), and the American Society for Laser Medicine and Surgery (research grants F03.12 and F01.13 to W.T).
Footnotes
Conflicts of interest: None declared.
References
- 1.Mulliken JB, Young AR. Vascular Birthmarks – Hemangiomas and Malformations. Philadelphia, PA: W.B. Saunders Co.; 1988. [Google Scholar]
- 2.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–22. [PubMed] [Google Scholar]
- 3.Pratt AG. Birthmarks in infants. AMA Arch Derm Syphilol. 1953;67:302–5. doi: 10.1001/archderm.1953.01540030065006. [DOI] [PubMed] [Google Scholar]
- 4.Lever WF, Schaumburg-Lever G. Histopathology of the Skin. 7th. Philadelphia, PA: J.B. Lippincott Co.; 1990. [Google Scholar]
- 5.Geronemus RG, Ashinoff R. The medical necessity of evaluation and treatment of port-wine stains. J Dermatol Surg Oncol. 1991;17:76–9. doi: 10.1111/j.1524-4725.1991.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 6.Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–9. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan W, Chernova M, Gao L, et al. Sustained activation of c-Jun N-terminal and extracellular signal regulated kinases in port wine stain blood vessels. J Am Acad Dermatol. 2014;71:964–8. doi: 10.1016/j.jaad.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–7. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 9.Nelson JS, Milner TE, Anvari B, et al. Dynamic epidermal cooling during pulsed laser treatment of port-wine stain. A new methodology with preliminary clinical evaluation. Arch Dermatol. 1995;131:695–700. [PubMed] [Google Scholar]
- 10.Katugampola GA, Lanigan SW. Five years' experience of treating port wine stains with the flashlamp-pumped pulsed dye laser. Br J Dermatol. 1997;137:750–4. [PubMed] [Google Scholar]
- 11.van der Horst CM, Koster PH, de Borgie CA, et al. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338:1028–33. doi: 10.1056/NEJM199804093381504. [DOI] [PubMed] [Google Scholar]
- 12.Yohn JJ, Huff JC, Aeling JL, et al. Lesion size is a factor for determining the rate of port-wine stain clearing following pulsed dye laser treatment in adults. Cutis. 1997;59:267–70. [PubMed] [Google Scholar]
- 13.Tan W, Jia W, Sun V, et al. Topical rapamycin suppresses the angiogenesis pathways induced by pulsed dye laser: molecular mechanisms of inhibition of regeneration and revascularization of photocoagulated cutaneous blood vessels. Lasers Surg Med. 2012;44:796–804. doi: 10.1002/lsm.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson JS, Jia W, Phung TL, et al. Observations on enhanced port wine stain blanching induced by combined pulsed dye laser and rapamycin administration. Lasers Surg Med. 2011;43:939–42. doi: 10.1002/lsm.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–32. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 17.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–83. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 18.Carr MW, Roth SJ, Luther E, et al. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu LL, Warren MK, Rose WL, et al. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60:365–71. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–79. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekkawy AH, Morris DL, Pourgholami MH. Urokinase plasminogen activator system as a potential target for cancer therapy. Future Oncol. 2009;5:1487–99. doi: 10.2217/fon.09.108. [DOI] [PubMed] [Google Scholar]
- 22.Keefe SM, Cohen MA, Brose MS. Targeting vascular endothelial growth factor receptor in thyroid cancer: the intracellular and extracellular implications. Clin Cancer Res. 2010;16:778–83. doi: 10.1158/1078-0432.CCR-08-2743. [DOI] [PubMed] [Google Scholar]
- 23.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–83. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]