Abstract
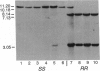
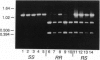
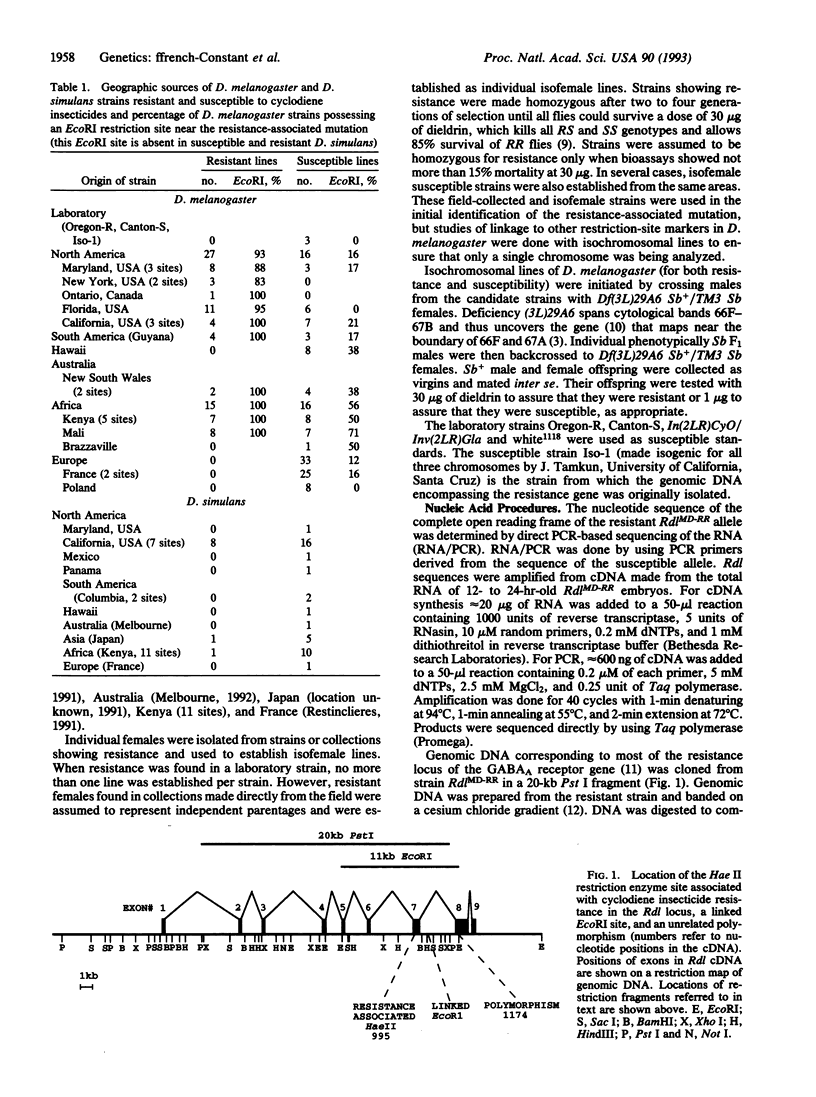
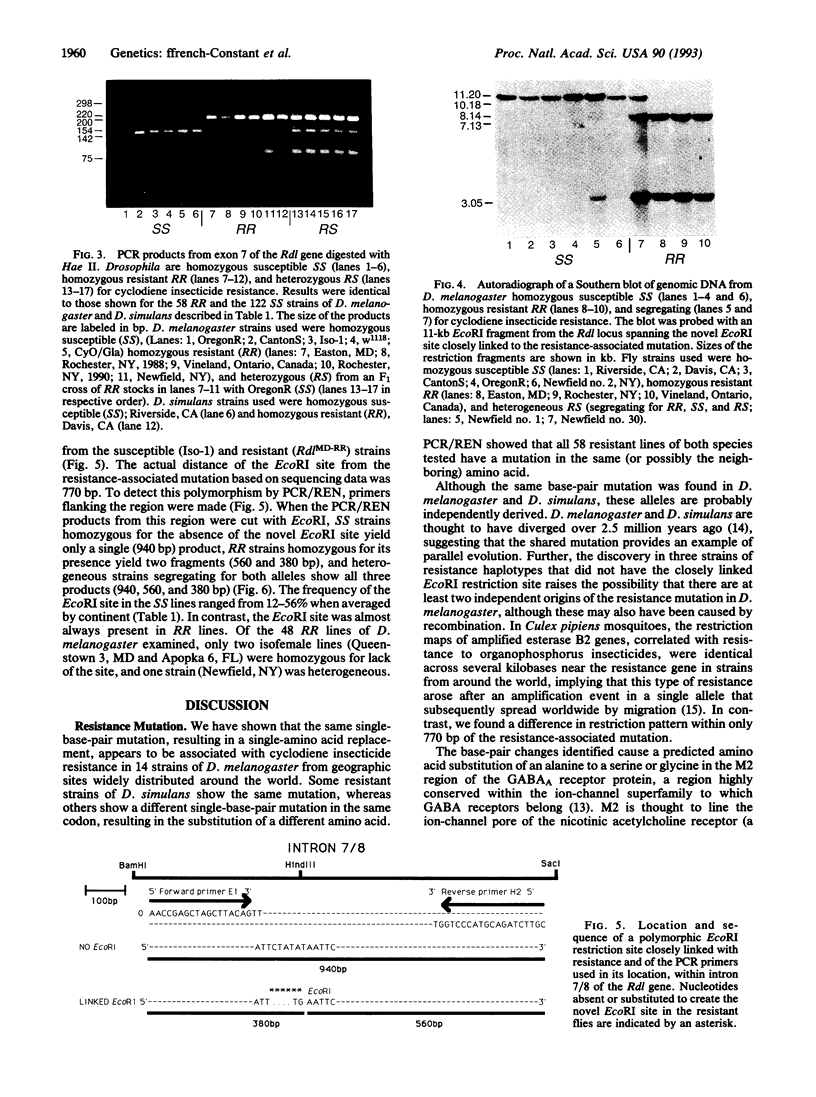
Resistance to cyclodiene insecticides, documented in at least 277 species, is perhaps the most common kind of resistance to any pesticide. By using cyclodiene resistance to localize the responsible gene, a gamma-aminobutyric acid type A receptor/chloride ion-channel gene was previously cloned and sequenced from an insecticide-susceptible Drosophila melanogaster strain. We now describe the molecular genetics of the resistance allele. A single-base-pair mutation, causing a single-amino acid substitution (Ala-->Ser) within the second membrane-spanning region of the channel, was found to be the only consistent difference between resistant and susceptible strains of D. melanogaster. Some resistant strains of Drosophila simulans show the same mutation, whereas others show an alternative single-base-pair mutation in the same codon, resulting in the substitution of a different amino acid (glycine). These constitute single-box-pair mutations in insects that confer high levels of resistance to insecticides. The presence of the resistance mutations was then tested in a much larger set of strains by the PCR and subsequent digestion with a diagnostic restriction endonuclease. Both resistance-associated mutations cause the loss of a Hae II site. This site was invariably present in 122 susceptible strains but absent in 58 resistant lines of the two species sampled from five continents. PCR/restriction endonuclease treatment was also used to examine linkage of an EcoRI polymorphism in a neighboring intron in D. melanogaster, which was found associated with resistance in all but 3 of 48 strains examined. These PCR-based techniques are widely applicable to examination of the uniqueness of different resistance alleles in widespread populations, the identification of resistance mechanisms in different species, and the determination of resistance frequencies in monitoring.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. M., Brogdon W. G. Improved detection of insecticide resistance through conventional and molecular techniques. Annu Rev Entomol. 1987;32:145–162. doi: 10.1146/annurev.en.32.010187.001045. [DOI] [PubMed] [Google Scholar]
- Eldefrawi A. T., Eldefrawi M. E. Receptors for gamma-aminobutyric acid and voltage-dependent chloride channels as targets for drugs and toxicants. FASEB J. 1987 Oct;1(4):262–271. doi: 10.1096/fasebj.1.4.2443413. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Mortlock D. P., Shaffer C. D., MacIntyre R. J., Roush R. T. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Roush R. T. Gene mapping and cross-resistance in cyclodiene insecticide-resistant Drosophila melanogaster (Mg.). Genet Res. 1991 Feb;57(1):17–21. doi: 10.1017/s0016672300028986. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Roush R. T., Mortlock D., Dively G. P. Isolation of dieldrin resistance from field populations of Drosophila melanogaster (Diptera: Drosophilidae). J Econ Entomol. 1990 Oct;83(5):1733–1737. doi: 10.1093/jee/83.5.1733. [DOI] [PubMed] [Google Scholar]
- Fournier D., Bride J. M., Hoffmann F., Karch F. Acetylcholinesterase. Two types of modifications confer resistance to insecticide. J Biol Chem. 1992 Jul 15;267(20):14270–14274. [PubMed] [Google Scholar]
- Harvey R. J., Vreugdenhil E., Zaman S. H., Bhandal N. S., Usherwood P. N., Barnard E. A., Darlison M. G. Sequence of a functional invertebrate GABAA receptor subunit which can form a chimeric receptor with a vertebrate alpha subunit. EMBO J. 1991 Nov;10(11):3239–3245. doi: 10.1002/j.1460-2075.1991.tb04887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Raymond M., Callaghan A., Fort P., Pasteur N. Worldwide migration of amplified insecticide resistance genes in mosquitoes. Nature. 1991 Mar 14;350(6314):151–153. doi: 10.1038/350151a0. [DOI] [PubMed] [Google Scholar]
- Yarbrough J. D., Roush R. T., Bonner J. C., Wise D. A. Monogenic inheritance of cyclodiene insecticide resistance in mosquitofish, Gambusia affinis. Experientia. 1986 Jul 15;42(7):851–853. doi: 10.1007/BF01941551. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant R. H., Rocheleau T. Drosophila cyclodiene resistance gene shows conserved genomic organization with vertebrate gamma-aminobutyric acidA receptors. J Neurochem. 1992 Oct;59(4):1562–1565. doi: 10.1111/j.1471-4159.1992.tb08475.x. [DOI] [PubMed] [Google Scholar]