Abstract
Background
Lymphatic filariasis (LF) is a neglected tropical disease for which more than a billion people in 73 countries are thought to be at-risk. At a global level, the efforts against LF are designed as an elimination program. However, current efforts appear to aim for elimination in some but not all endemic areas. With the 2020 goal of elimination looming, we set out to develop plausible scale-up scenarios to reach global elimination and eradication. We predict the duration of mass drug administration (MDA) necessary to reach local elimination for a variety of transmission archetypes using an existing model of LF transmission, estimate the number of treatments required for each scenario, and consider implications of rapid scale-up.
Methodology
We have defined four scenarios that differ in their geographic coverage and rate of scale-up. For each scenario, country-specific simulations and calculations were performed that took into account the pre-intervention transmission intensity, the different vector genera, drug regimen, achieved level of population coverage, previous progress toward elimination, and potential programmatic delays due to mapping, operations, and administration.
Principal Findings
Our results indicate that eliminating LF by 2020 is unlikely. If MDA programs are drastically scaled up and expanded, the final round of MDA for LF eradication could be delivered in 2028 after 4,159 million treatments. However, if the current rate of scale-up is maintained, the final round of MDA to eradicate LF may not occur until 2050.
Conclusions/Significance
Rapid scale-up of MDA will decrease the amount of time and treatments required to reach LF eradication. It may also propel the program towards success, as the risk of failure is likely to increase with extended program duration.
Author Summary
Lymphatic filariasis (LF) is a disease caused by filarial worms transmitted by different types of mosquitos that can lead to massive disability, including elephantiasis and hydrocele. LF has no significant zoonotic reservoir and is thought to be a potentially eradicable disease through once yearly treatment distributed by mass drug administration (MDA). In this study, we set out to determine how many treatments and over how much time it might take to globally eliminate and eradicate LF under different levels of treatment intensities. We created a model that took into account country-specific and disease-specific variables, and found that if the current intensity of MDA is maintained, 3,409 million treatments distributed over the next 37 years will be required. However, if treatment is rapidly expanded to the entire at-risk population in all endemic countries, eradication could be achieved with 4,159 million treatments and in less than half the time. While our estimates suggest more time may be needed to reach LF elimination than what is currently projected, with continued commitment, eradicating LF is within reach.
Introduction
Lymphatic filariasis (LF) is a neglected tropical disease (NTD) primarily prevalent in poor populations in 73 countries [1]. LF is caused by infection with Wuchereria bancrofti, Brugia malayi, or B. timori transmitted by a variety of mosquito genera [2]. Infection with the filarial nematodes can damage the lymphatic vessels, the main clinical manifestations being lymphedema, hydrocele, and elephantiasis [3]. In addition to disfigurement and disability, people affected by LF face stigma, social adversity, and economic hardship [4–6].
LF is spread by mosquitoes that take up circulating microfilarae (mf) in the peripheral blood of infected humans [7]. Administration of albendazole with ivermectin or diethylcarbamazine citrate (DEC) has been shown to reduce circulating mf to such low levels that transmission cannot be sustained [8]. For this reason, LF is one of six diseases considered to be potentially eradicable [9]. Accordingly, in 1997 the World Health Assembly (WHA) adopted resolution WHA 50.29, which calls for the elimination of LF as a public health problem and, in 2000, the World Health Organization (WHO) established the Global Program to Eliminate Lymphatic Filariasis (GPELF). The GPELF aims to eliminate LF in all endemic countries by 2020 through annual mass drug administration (MDA) maintained over multiple years [8]. The program benefits through donations from Merck & Co. and GlaxoSmithKline (GSK), which have pledged to provide enough ivermectin and albendazole, respectively, to achieve elimination, as well as from Eisai, which in 2010, pledged 2.2 billion DEC tablets [10, 11].
The GPELF has scaled up rapidly and is among the fastest growing disease elimination programs in the world [12]. By the end of 2013, 56 LF-endemic countries had carried out MDA, of which 15 are now undertaking post-MDA surveillance. In 2013 alone, more than 410 million anti-filarial treatments were distributed under the GPELF. However, the program is not without its challenges: mapping is incomplete in 12 countries, 14 countries requiring MDA are yet to begin, and many of the other endemic countries are targeting relatively small proportions of their at-risk populations [13]. Issues with compliance, contraindications of ivermectin and DEC in areas with hyper Loa loa-endemicity, and interruptions in funding also plague the program [14, 15]. At a global level, the efforts against LF could be considered a global elimination program (elimination of infection in some but not all countries) as the name suggests, or an eradication program (permanent reduction to zero of the worldwide incidence of infection) as implied by the stated aims of the program [13, 16, 17].
In order to assist decision makers in determining whether efforts for LF should be scaled up to try to achieve eradication, it has been proposed to use an analytic and deliberate methodology to produce evidence-based guidance on the rationale for investing [18, 19]. As part of this endeavor, we herein predict the duration of MDA necessary to reach local elimination for a variety of transmission archetypes using an existing model of LF transmission, outline plausible scale-up scenarios leading to global elimination and eradication, and estimate the number of treatments required under each scenario. Potential delays in implementation, previous progress, and different intensities of infection and transmission are also taken into account. Studies on the economic and financial costs, the impact on disease burden, and cost-effectiveness of these scenarios are to be published as companion papers.
Methods
We have defined four hypothetical scenarios that differ in their geographic coverage and rate of scale-up. The global elimination scenario represents the case whereby countries continue with current practices. As such, it serves as the comparator against all other scenarios. The other three scenarios aim at reaching LF eradication through varying levels of MDA scale-up. Key assumptions and differences between the scenarios are outlined in Table 1. The number of years that each endemic country exceeded the minimum effective coverage rate of 65% in previous rounds of MDA, as well as the geographic coverage and rates of scale-up are provided in Table 2 (countries without previous rounds of MDA for LF) and Table 3 (countries that previously carried out MDA for LF). All scenarios were assumed to begin in 2014 and run until the final round of MDA has been distributed in each country under consideration. Though coverage rates above 65% are considered to be the lowest threshold necessary to be effective, the average programmatic coverage for countries that had previously achieved effective coverage was over 80%. Therefore, we presume that prospective MDA will continue to be performed at higher levels, and therefore assume MDA coverage to be fixed at 85%.
Table 1. Key features of the proposed scenarios for global elimination and eradication of LF.
| Global Elimination (comparator) | Eradication I | Eradication II | Eradication III | |
|---|---|---|---|---|
| Intervention | MDA | MDA | MDA | MDA |
| Coverage rate | 85% | 85% | 85% | 85% |
| Countries considered | All LF endemic countries that have previously conducted MDA ¥ | All LF endemic countries ¥ , including all countries co-endemic with L. loa | All LF endemic countries ¥ , including all countries co-endemic with L. loa | All LF endemic countries ¥ , including all countries co-endemic with L. loa |
| Rate of scale-up | Countries with previous MDA continue at same rate as historically | Countries with previous MDA continue at same historical rate, countries without previous progress begin at an ‘average’ rate of MDA scale-up (schedule II) | Schedule I: All countries add 20% of their at-risk populations to the MDA schedule annually | All countries treat 100% of their at-risk populations annually |
¥Assuming country requires MDA
Table 2. Countries without previous rounds of MDA for LF.
| Country | Primary vector | Treatment α | At-risk population, 2012 ¤ | Population growth rate, 2012 ¥ | Scale-up schedule ± | Delay § |
|---|---|---|---|---|---|---|
| Angola | Anopheles | IVM + ALB | 12,090,000 | 3.1% | -/2/1/0 | 4 |
| Brunei Darussalam | Culex * | DEC + ALB | 15,000 | 1.4% | -/2/1/0 | 1 |
| Chad | Anopheles | IVM + ALB | 7,270,000 | 3.0% | -/2/1/0 | 4 |
| Central African Republic | Anopheles | IVM + ALB | 3,300,000 | 3.1% | -/2/1/0 | 4 |
| Equatorial Guinea | Anopheles | IVM + ALB | 420,000 | 2.8% | -/2/1/0 | 1 |
| Eritrea | Anopheles | DEC + ALB | 3,577,000 | 3.3% | -/2/1/0 | 4 |
| Gabon | Anopheles | IVM + ALB | 1,290,600 | 2.4% | -/2/1/0 | 1 |
| Guinea | Anopheles | IVM + ALB | 6,067,135 | 2.6% | -/2/1/0 | 1 |
| New Caledonia | Aedes | DEC + ALB | 12,378 | 1.6% | -/2/1/0 | 1 |
| Palau | Aedes | DEC + ALB | 20,044 | 0.7% | -/2/1/0 | 1 |
| Republic of the Congo | Anopheles | IVM + ALB | 2,600,000 | 2.6% | -/2/1/0 | 1 |
| São Tomé and Príncipe | Anopheles | DEC + ALB | 410,000 | 2.7% | -/2/1/0 | 1 |
| South Sudan | Anopheles | IVM + ALB | 1,659,558 | 4.3% | -/2/1/0 | 4 |
| Sudan | Anopheles | IVM + ALB | 19,893,779 | 2.1% | -/2/1/0 | 4 |
| The Democratic Republic of Congo | Anopheles | IVM + ALB | 49,140,000 | 2.7% | -/2/1/0 | 4 |
| The Gambia | Anopheles | IVM + ALB | 1,200,000 | 3.2% | -/2/1/0 | 1 |
| Zambia | Culex | DEC + ALB | 8,780,000 | 3.2% | -/2/1/0 | 4 |
| Zimbabwe | Culex | DEC + ALB | 6,000,000 | 2.7% | -/2/1/0 | 4 |
*Treatment durations for Culex spp. were used for countries in which primary vector species was unknown.
αTreatment assumed to occur once annually using diethylcarbamazine citrate (DEC) and albendazole (ALB), or in areas co-endemic with onchocerciasis, ivermectin (IVM) and albendazole (ALB)
¤ Preventive Chemotherapy Databank Lymphatic Filariasis [Internet]. WHO. 2015 [cited 2015 January 20]. Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/lf/en/.
¥ United Nations, Department of Economic and Social Affairs, Population Division (2013). World Population Prospects: The 2012 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.227.
± Refers to MDA schedules assumed to be used by these countries for the purposes of our analysis for the global elimination scenario, eradication I, eradication II, and eradication III scenarios, respectively. In schedule I, two deciles (20%) of the at-risk population are added to the MDA schedule annually. In schedule II, one decile is added annually. In schedule III, one decile is added every 2 years, and in schedule IV, one decile is added every 3rd year (see: Rate of Scale-Up and History of Control). ‘-‘ refers to a continued absence of an MDA program. ‘0’ refers to instantaneous scale-up.
§A 4-year delay was assumed for countries that have not completed LF mapping, while a 1-year delay was assumed for those that have completed mapping but have not previously carried out MDA.
Table 3. Countries that previously carried out MDA for LF.
| Country | Primary vector | Treatment α | At-risk population, 2012 ¤ | Population growth rate, 2012 ¥ | Previous effective years ¤ | Scale-up schedule ± |
|---|---|---|---|---|---|---|
| >50% targeted | ||||||
| Burkina Faso | Anopheles | IVM + ALB | 16,779,208 | 2.9% | 11 | 1/1/1/0 |
| Cameroon | Anopheles | IVM + ALB | 17,091,469 | 2.5% | 5 | 1/1/1/0 |
| Côte d'Ivoire | Anopheles | IVM + ALB | 14,000,000 | 2.3% | 1 | 1/1/1/0 |
| Comoros | Culex | DEC + ALB | 514,110 | 2.4% | 5 | 1/1/1/0 |
| Egypt | Culex | DEC + ALB | 536,443 | 1.7% | 11 | 1/1/1/0 |
| Fiji | Aedes | DEC + ALB | 529,984 | 0.8% | 7 | 1/1/1/0 |
| French Polynesia | Aedes | DEC + ALB | 274,544 | 1.1% | 10 | 1/1/1/0 |
| Ghana | Anopheles | IVM + ALB | 11,925,399 | 2.2% | 11 | 1/1/1/0 |
| Haiti | Culex | DEC + ALB | 10,732,356 | 1.4% | 10 | 1/1/1/0 |
| India | Culex | DEC + ALB | 617,170,000 | 1.3% | 15 | 1/1/1/0 |
| Kenya | Culex * | DEC + ALB | 3,421,741 | 2.7% | 3 | 1/1/1/0 |
| Lao PDR | Culex * | DEC + ALB | 132,644 | 1.9% | 2 | 1/1/1/0 |
| Liberia | Anopheles | IVM + ALB | 3,600,000 | 2.7% | 0 | 1/1/1/0 |
| Malawi | Anopheles | IVM + ALB | 14,807,685 | 2.9% | 5 | 1/1/1/0 |
| Mali | Anopheles | IVM + ALB | 16,166,882 | 3.0% | 7 | 1/1/1/0 |
| Mozambique | Anopheles | IVM + ALB | 17,114,949 | 2.5% | 3 | 1/1/1/0 |
| Nepal | Culex | DEC + ALB | 15,755,990 | 1.2% | 10 | 1/1/1/0 |
| Niger | Anopheles | IVM + ALB | 12,467,592 | 3.8% | 4 | 1/1/1/0 |
| Philippines | Aedes | DEC + ALB | 29,383,286 | 1.7% | 9 | 1/1/1/0 |
| Samoa | Aedes | DEC + ALB | 186,649 | 0.8% | 5 | 1/1/1/0 |
| Sierra Leone | Anopheles | IVM + ALB | 6,667,687 | 1.9% | 5 | 1/1/1/0 |
| Thailand | Aedes | DEC + ALB | 73,495 | 0.3% | 11 | 1/1/1/0 |
| Tuvalu | Aedes | DEC + ALB | 10,373 | 0.2% | 4 | 1/1/1/0 |
| Uganda | Anopheles | IVM + ALB | 14,464,244 | 3.4% | 5 | 1/1/1/0 |
| 30–50% targeted | ||||||
| Dominican Republic | Culex | DEC + ALB | 249,803 | 1.3% | 6 | 2/2/1/0 |
| Guyana | Culex | DEC + ALB | 690,869 | 0.6% | 2 | 2/2/1/0 |
| Indonesia | Culex | DEC + ALB | 113,283,453 | 1.2% | 7 | 2/2/1/0 |
| Myanmar | Culex | DEC + ALB | 41,666,403 | 0.8% | 9 | 2/2/1/0 |
| Timor Leste | Anopheles | DEC + ALB | 1,180,067 | 2.9% | 3 | 2/2/1/0 |
| United Republic of Tanzania | Culex | IVM + ALB | 45,173,251 | 3.0% | 11 | 2/2/1/0 |
| 20–30% targeted | ||||||
| Bangladesh | Culex | DEC + ALB | 77,230,000 | 1.2% | 14 | 3/3/1/0 |
| Benin | Anopheles | IVM + ALB | 3,747,913 | 2.7% | 11 | 3/3/1/0 |
| Guinea Bissau | Anopheles | IVM + ALB | 1,582,496 | 2.4% | 1 | 3/3/1/0 |
| Malaysia | Anopheles | DEC + ALB | 1,266,123 | 1.7% | 7 | 3/3/1/0 |
| Nigeria | Anopheles | IVM + ALB | 108,526,381 | 2.8% | 5 | 3/3/1/0 |
| <20% targeted | ||||||
| Brazil | Culex | DEC | 1,700,000 | 0.9% | 4 | 4/4/1/0 |
| Ethiopia | * Culex | IVM + ALB | 30,000,000 | 2.6% | 4 | 4/4/1/0 |
| Kiribati | Culex | DEC + ALB | 103,058 | 1.5% | 5 | 4/4/1/0 |
| Madagascar | Anopheles | DEC + ALB | 18,602,379 | 2.8% | 6 | 4/4/1/0 |
| Micronesia | Aedes | DEC + ALB | 11,241 | 0.1% | 1 | 4/4/1/0 |
| Papua New Guinea | Anopheles | DEC + ALB | 5,602,188 | 2.2% | 1 | 4/4/1/0 |
| Senegal | Anopheles | IVM + ALB | 5,314,600 | 2.9% | 3 | 4/4/1/0 |
*Treatment durations for Culex spp. were used for countries in which primary vector species was unknown.
αTreatment assumed to occur once annually using diethylcarbamazine citrate (DEC) and albendazole (ALB), or in areas co-endemic with onchocerciasis, ivermectin (IVM) and albendazole (ALB)
¤ Preventive Chemotherapy Databank Lymphatic Filariasis [Internet]. WHO. 2015 [cited 2015 January 20]. Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/lf/en/.
¥ United Nations, Department of Economic and Social Affairs, Population Division (2013). World Population Prospects: The 2012 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.227.
± Refers to MDA schedules assumed to be used by these countries for the purposes of our analysis for the global elimination scenario, eradication I, eradication II, and eradication III scenarios, respectively. In schedule I, two deciles (20%) of the at-risk population are added to the MDA schedule annually. In schedule II, one decile is added annually. In schedule III, one decile is added every 2 years, and in schedule IV, one decile is added every 3rd year (see: Rate of Scale-Up and History of Control). ‘0’ refers to instantaneous scale-up.
Scenario Development
Scenarios were developed by first reviewing the WHO preventive chemotherapy (PCT) databank to assess progress made towards LF elimination as of 2012 [13]. The scenarios were further refined, with key assumptions agreed upon in a series of technical advisory group meetings, including stakeholders from WHO, Centers for Disease Control and Prevention (CDC), funders, pharmaceutical companies, and program managers from endemic countries.
In the global elimination scenario, countries that have not yet started will not start, and countries that have started continue according to their assigned level of scale-up (see: Rate of scale-up). In the eradication I scenario, countries that have already started MDA continue as in the global elimination scenario and countries that have not yet started implement MDA following an ‘average’ level of scale-up. The eradication II scenario represents the case in which all countries scale-up MDA more quickly (fast). Eradication III serves as the ‘best case’ scenario, whereby all endemic countries provide MDA to their entire at-risk populations immediately. Thus, this analysis provides insight into the differences in the amount of time and treatments required to extend elimination efforts to all endemic countries (eradication I), increase MDA intensity (eradication II) and, most ideally, scale-up instantaneously (eradication III).
Assumptions Regarding Interventions and Loiasis Co-endemicity
An important assumption underlying this study is that annual MDA using DEC with albendazole, or, in onchocerciasis-endemic countries, ivermectin and albendazole, will be sufficient to reduce circulating mf enough to interrupt the transmission cycle of LF if maintained for an appropriate number of years. Therefore, hardly predictable features that could undermine success, including systematic non-compliance with MDA, but particularly events such as civil unrest and humanitarian emergencies (e.g. earthquakes in Haiti and Nepal; Ebola epidemic in West Africa) that could compromise the health system’s capacity, could not be accounted for. We also assume that countries undertake MDA without interruption.
Administration of ivermectin to communities with high prevalence (>40%) of L. loa is contraindicated, as the microfilaracidal actions of the drug poses an unjustifiably high risk of causing severe adverse events. As such, the WHO provisionally recommends the LF program to instead treat these areas with albendazole monotherapy distributed bi-annually and vector control [20]. Here we assume that this strategy will be equally efficacious as annual albendazole-ivermectin, and thereby assume the number of years of MDA required in areas co-endemic with L. loa to be equivalent to the number of years required with albendazole-ivermectin.
Rate of Scale-Up and History of Control
The GPELF advises LF endemic countries to conduct MDA for 4–6 years [8]. This duration only holds at a country level if all endemic areas are treated simultaneously. To incorporate scaling-up of geographic coverage for each scenario, we divided each country’s at-risk population into deciles, and assumed MDA to start in subsequent deciles after varying durations according to four schedules of scale-up. In schedule I (fast), 20% of the at-risk population is added to the MDA schedule annually. In schedule II (average), one decile is added each year, in schedule III (slow) one decile is added every two years and in schedule IV (very slow) this period is three years.
In the global elimination scenario, scale-up is based upon the proportion of the at-risk population each country previously targeted. In order to be allocated to schedule I, the at-risk population targeted in the most recent round of MDA had to exceed 50%. Schedule II has been assigned to countries previously targeting 30–50%, schedule III to those targeting 20–29.9%, and schedule IV to those targeting <20%. Rather than attempting to recreate the progress of each country exactly, we used these categories to incorporate a range of scale-up levels encountered. Previous progress made towards local elimination was further taken into account by counting the number of previously effective years of MDA, which was considered as any year in which program coverage within the targeted area (regardless of the at-risk population targeted) exceeded 65%. We then subtracted the number of effective years previously achieved from the number of years of MDA deemed necessary (see below: Transmission Archetypes; Table 4) in order to determine the number of years of MDA remaining.
Table 4. Estimates of the number of annual MDA rounds needed to reach local LF elimination by transmission archetypes, based on sets of 500 simulations using EpiFil and assuming 85% coverage.
| Primary vector | Treatment α | Baseline MF prevalence | |||
|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | ||
| Anopheles spp. | DEC + ALB | 6 | 6 | 7 | 7 |
| IVM + ALB | 7 | 9 | 11 | 11 | |
| Culex spp. | DEC + ALB | 9 | 10 | 11 | 11 |
| IVM + ALB | 11 | 13 | 15 | 15 | |
αTreatment assumed to occur once annually using diethylcarbamazine citrate (DEC) and albendazole (ALB), or in areas co-endemic with onchocerciasis, ivermectin (IVM) and albendazole (ALB)
The number of rounds corresponds to the minimum at which at least 97.5% of simulations went to elimination.
Delays
For all scenarios, we assume that countries that have finished mapping but not begun MDA have a 1-year delay, whereas countries that have not completed mapping nor begun MDA have a 4-year delay. While countries face challenges of different magnitudes and require different durations to map, the 4-year delay assumed corresponds to the average number of years that mapping took in countries with available data to support the calculation [13].
Prevalence Data
To account for heterogeneity in transmission intensity within countries, we obtained paired baseline circulating filarial antigenaemia prevalence, measured through immunochromatographic tests (ICTs), and mf prevalence data from sentinel site surveys from program countries across the AFRO region. As specified by the WHO, these surveys involve collecting fingertip blood, between 10 p.m. and 2 am. from at least 300 participants aged five years and above [21]. We gained additional access to ICT prevalence data from mapping studies in 17 African countries. The relationship between mf and antigenaemia prevalence was estimated using the non-parametric regression proposed by Passing and Bablock, which assumes linearity and uncertainties in both variables [22]. The regression equation calculated from the paired prevalence data was then used to infer mf prevalence from the ICT mapping data.
We determined the percentage of the at-risk population that fell into prevalence quartiles: <5%, 5–10%, 10.1–15%, >15%, for each country that provided district level prevalence data. To account for uncertainties in this approach, we took 500 random draws from a multinomial distribution with probabilities based on weighted averages from the dataset and assumed these to be the possible ranges of pre-intervention prevalence distributions for all countries in our analysis.
Transmission Archetypes
It has been theoretically demonstrated that the required duration of MDA is region-specific and dependent on various factors, including drug regimen and level of coverage, vector species, and pre-intervention transmission intensity [23–25]. In order to broadly capture the heterogeneous transmission patterns of LF, we defined transmission archetypes (Table 4). In addition to prevalence levels and drug regimens, we accounted for differences in transmission between Anopheles spp. and Culex spp., which notably differ in their mf-density dependent likelihood of becoming infected [26]. Predicting regional anopheline- or culicine-mediated LF transmission has been shown to require different model formulations and parameterizations [27]. For our analysis we made several simplifications: we assumed transmission of W. bancrofti by Aedes spp. was similar to transmission efficacy by Culex spp., while transmission of Brugia spp. was assumed to be comparable to W. bancrofti transmission by Anopheles spp. Where the primary vector was unclear, infection by Culex spp. was assumed in order to avoid underestimating the number of MDA rounds required.
Modeling the Number of MDA Rounds Required to Reach Local Elimination
The duration of MDA required to eliminate LF was predicted for the transmission archetypes using a deterministic model of LF transmission, EpiFil [28]. The model used for the current analysis has been described in detail, validated against multiple data sets for both transmission settings with Anopheles spp. and Culex spp., and used extensively to predict LF intervention outcomes [28–31]. Details on model structure, equations, and the approach to obtaining parameter estimates are provided in Supporting Text 1: LF model description.
For all transmission archetypes, we ran 500 simulations of once-yearly MDA of varying total durations, drawing from a range of parameter estimates. The lowest number of rounds at which the 95th percentile range of the simulations resulted in an mf prevalence below 1% 50 years after the start of the MDA program was taken as a conservative measure of the number of rounds required to ensure elimination.
Calculating the Number of Future Treatments Required
Population at-risk figures were taken from the WHO PCT database for 2012 and adjusted for population growth using country-specific 2012 United Nations estimates [13, 32]. MDA coverage rates were assumed to be 85% for all countries. Except for areas co-endemic with L. loa, treatments are assumed to occur annually. Based on the pre-intervention prevalence distributions, we developed 500 estimates of the number of treatments needed for each country and scenario. Results are reported as the mean number of treatments by region and scenario, along with 95% credible intervals (CI).
Results
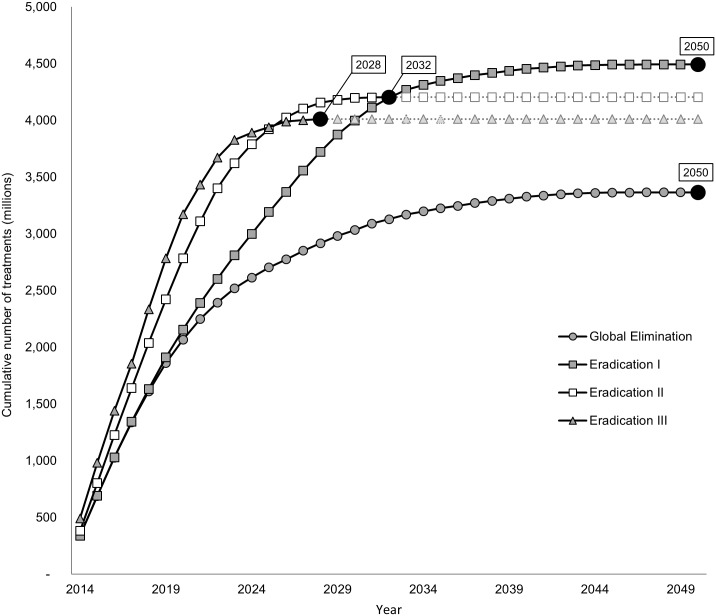
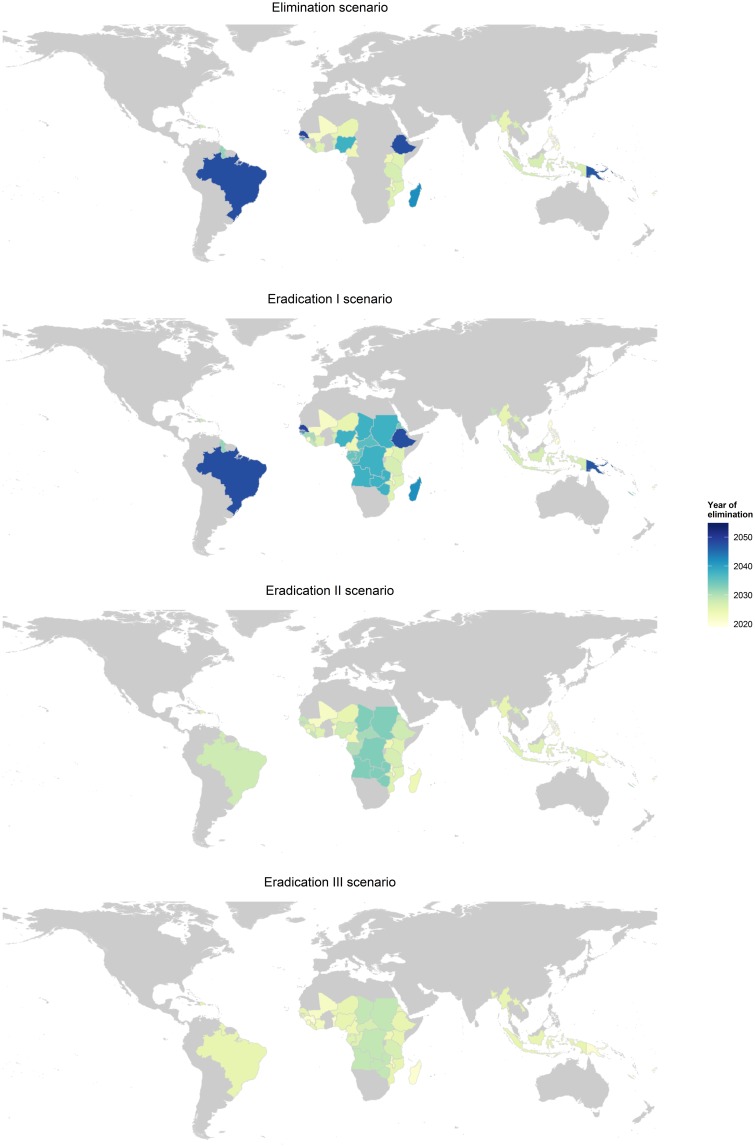
Our results indicate that interrupting LF transmission in all countries by 2020 is unlikely, though if MDA is drastically scaled-up and expanded, the final round of MDA to eradicate LF could be carried out by 2028 (eradication III; Fig 1). If scale-up continues at the current rate, as modeled in our global elimination and eradication I scenarios, the last round of MDA will not be given until 2050, largely due to slow scale-up in areas where transmission occurs through Culex spp. The eradication II scenario reaches the last round of MDA by 2032. As this scenario assumes that all countries add 20% of their at-risk populations to MDA annually, the last countries to reach local elimination are those that were delayed due to mapping, and whose vector and treatment combination included Anopheles spp. and ivermectin or Culex spp. and DEC, including: Angola, Chad, the Democratic Republic of Congo, South Sudan, Sudan, Zambia, and Zimbabwe. Fig 2 provides a visual representation of the impact different intensities of scale-up and expansion have on time to reach local elimination for each country.
Fig 1. Cumulative number of treatments by year.
The line with circular markers represents the global elimination (comparator) scenario. As highlighted in the text boxes, both the global elimination and eradication I scenario are estimated to conclude MDA after 37 years of MDA. Eradication II, the intensified scale-up scenario, sees the last round of MDA to occur by 2032, after 19 years of MDA. Eradication III is estimated to require 15 years of MDA, concluding in 2028.
Fig 2. Maps depicting the final year of MDA per country for the four scenarios.
The global elimination scenario does not include countries that have not yet begun MDA.
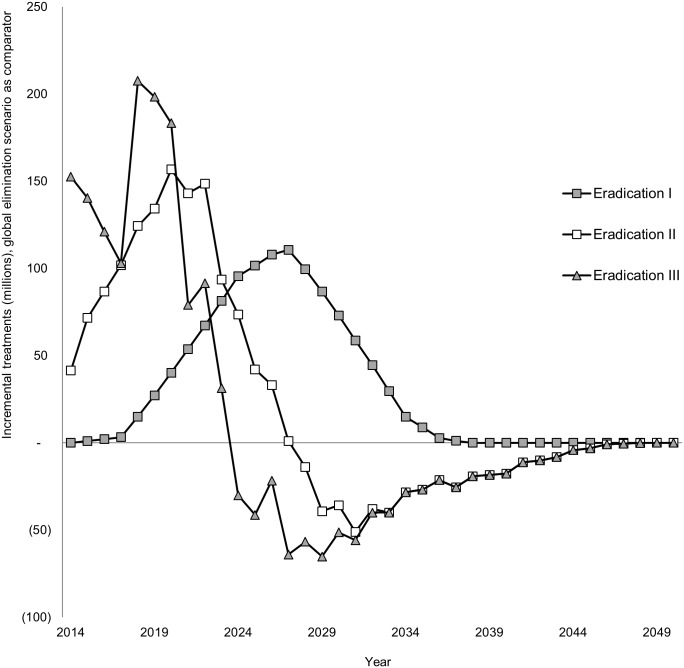
Since the scenarios take into account population growth, rapid scale-up of MDA also decreases the number of treatments required. As depicted in Fig 3, the eradication III scenario initially requires substantially more treatments, but by 2024, the treatments under this scenario are projected to be less than that required under all other scenarios. The global elimination scenario is projected to require approximately 3,409 million treatments (95% CI: 3,185m–3,538 million). Expanding the program to all endemic countries will increase the number of treatments to 4,666 million (95% CI: 4,419m–4,904 million). Scaling up MDA more rapidly, as under the eradication II scenario, results in savings of nearly 300 million treatments compared to the eradication I scenario. Under the most optimistic scenario (eradication III), eradication could be achieved with 4,159 million treatments (95% CI: 3,924m–4,382 million). As shown in Fig 1, this represents nearly 750 million treatments more than the global elimination scenario but 210 million treatments less than the intensified eradication scenario (eradication II). Owing to the largest burden, the AFRO region requires the majority of treatments, followed by Southeast Asia. With the shift from global elimination to eradication, the number of treatments required in the Eastern Mediterranean region increases by more than 380 fold due to treatments required for Sudan, which is not considered under the elimination scenario (Table 5).
Fig 3. Incremental treatment projections by year (global elimination scenario as comparator).
All eradication scenarios see an increase in the number of treatments after 4 years as the result of the imposed delay for countries that have not previously finished mapping or begun MDA. By 2024, the eradication III scenario requires less treatments than the global elimination (comparator) scenario, and from 2028, the eradication II scenario is also projected to require fewer treatments than global elimination.
Table 5. Projected treatment needs (in millions) by WHO region with 95% credible intervals.
| Global elimination (comparator) | Eradication I | Eradication II | Eradication III | |
|---|---|---|---|---|
| AFRO | 2,117 (2,011–2,223) | 3,202 (3,048–3,355) | 2,930 (2,788–3,074) | 2,746 (2,605–2,889) |
| SEAR | 1,148 (1,102–1,190) | 1,148 (1,102–1,190) | 1,141 (1,096–1,183) | 1,139 (1,096–1,181) |
| WPR | 109.3 (104.5–114.0) | 109.7 (104.9–114.4) | 100.1 (95.6–104.7) | 98.55 (94.25–102.94) |
| AMR | 34.66 (33.07–36.27) | 34.66 (33.07–36.27) | 33.43 (31.87–35.00) | 33.10 (31.60–34.62) |
| EMR | 0.3729 (0.3380–0.4095) | 173.0 (165.2–180.9) | 164.1 (156.6–171.5) | 142.0 (134.2–150.2) |
| Total | 3,409 (3,185–3,538) | 4,667 (4,419–4,904) | 4,369 (4,133–4,594) | 4,159 (3,924–4,382) |
Discussion
As not all LF endemic countries are considered under the global elimination (comparator) scenario, any eradication campaign will require a massive increase in treatments. However, if LF is to be eliminated in all endemic countries, then rapid scale-up as soon as possible will lead to increased savings—both in terms of time and treatments. Accelerated MDA may also propel the program towards success, as the risk of failure (due to lapses in funding, donor fatigue, or occurrence of calamitous events) potentially increases with extended program duration [33]. It is conceivable that a decrease in program duration may also decrease the likelihood of drug resistance evolution [34].
Noticeably missing from our analysis is India. While India has the greatest burden of LF [35], it has made substantial progress against the disease, having distributed nearly 3.5 billion antifilarial treatments since 2001 [13]. As such, our model suggests that further rounds may not be necessary for India. However, previous studies have found pockets of systematic non-compliance in India, leading to MDA coverage in those areas to fall below effective coverage [36]. It is therefore possible that transmission of LF may still occur in India. However, in order to remain consistent in our approach, and in recognizing that to provide global estimates we cannot take into account all eventualities, additional treatments for India have not been considered.
We sought data from a number of diverse sources. Due to the inherent structure of the LF program, however, our analysis relies heavily on data that have been collected and reported directly by each country. While this arrangement raises a number of issues, discrepancies in the data could also decrease the validity of our estimates. Inconsistencies in coverage data may affect the number of years required to interrupt transmission, while inaccuracies in at-risk estimations would directly impact the number of treatments projected to reach our scenario endpoints. Whether these issues would result in underestimates or overestimates is dependent upon the direction and magnitude of the error.
While we avoided underestimating scale-up potential through our eradication III scenario, it is possible that we overestimated the capacity of some countries to scale-up. It is possible that we also overestimated the effectiveness and ability to proceed with rapid scale-up in areas co-endemic with L. loa. While WHO has provisional guidelines for dealing with LF and L. loa co-endemicity, no such areas have been broadly targeted for LF elimination as yet, and thus the effectiveness and feasibility of the strategy remains unclear. At the same time, the mass distribution of long-lasting insecticidal nets (LLINs) in many malaria endemic sites is likely to have a large impact on LF transmission by anophelines [37, 38]. Because the impact remains difficult to quantify, and uncertainty remains regarding the duration LLINs have to remain in place, we have not included this here. The time and treatment estimates in this study are based on data and model formulations and parameterizations currently available to the authors. Many of the assumptions and simplifications inherent to our scenarios are in need of closer investigation. Ideally, models would be fit to specific transmission settings within and between countries, as parameter values have been shown to differ by region [29]. Other aspects equally deserving of more attention, but likewise beyond the scope of this project, are the effectiveness of twice-yearly albendazole in concert with vector control for areas co-endemic with L. loa, and the consequences of mid-program delays, [39, 40]. Care should thus be taken when interpreting these results, particularly at a country-specific level.
Our duration estimates are considerably longer than those proposed under the GPELF, which envisages all endemic countries to reach full geographic coverage by 2016, with post-MDA surveillance in all countries anticipated by 2020 [17]. While this level of scale-up is similar to that proposed under our eradication III scenario, we project the last round of MDA to occur nearly a decade later, in 2028. This divergence arises from differences in the assumed number of rounds of MDA required to interrupt transmission. Depending on baseline prevalence and vector-treatment combinations, our model estimates interruption in transmission to occur after 6–15 rounds of MDA (Table 4). In contrast, the GPELF assumes five years of MDA in all areas [17]. It is worth noting that the durations in this study represent a potentially conservative measure, as they were based on the 95th percentile range of simulations leading to elimination, accounting for the uncertainty in our parameter estimates. This measure was taken to represent the time that could guarantee elimination with a reasonable level of certainty, but does not preclude that shorter durations may be sufficient in many areas. However, the discrepancy between predicted MDA durations and those advocated by GPELF was also evident in previous estimates with both deterministic and stochastic LF transmission models [41]. While aggressive goals for disease elimination and eradication potentially propel campaigns forward, overly optimistic projections could stifle innovations and further investment, ultimately hindering the initiative.
This study adds to the growing body of evidence on the feasibility of eradicating LF. While our estimates suggest more time may be needed to reach LF elimination than what is currently projected, the treatment estimates for our scenarios represent 66–89% of that which has already been distributed under the GPELF. Thus, our analysis indicates that with continued commitment, eradicating LF is within reach.
Supporting Information
(DOCX)
(TIF)
(TIF)
From top to bottom: 5, 10, 15, 20%, using diethylcarbamazine citrate and albendazole (red) or ivermectin and albendazole (blue) combination therapy.
(TIF)
(DOCX)
Acknowledgments
We wish to thank the program managers and the drug donation programs that provided us with prevalence data, as well as Dr. Wilma Stolk and Dr. Jürg Utzinger for reviewing and putting forth valuable comments on earlier versions of the manuscript. We would further like to thank Dr. Nakul Chitnis for his contributions in the model used for this analysis and Ms. Mariabeth Silkey for providing statistical support. This project was implemented with the support of experts belonging to our technical advisory group (alphabetically listed): Jan Agosti, Uche Amazigo, Maria-Gloria Basáñez, Moses Bockarie, Stephen Cochi, Ciro de Quadros, David B. Evans, Amadou Garba, Lance Gordon, Lee Hall, Ian Hastings, Mamoun Homeida, Adrian Hopkins, Julie Jacobson, Dean Jamison, Tom Kanyok, Claudio Lanata, Mike J. Lehane, Julia Lupp, Mwele N. Malecela, David H. Molyneux, Hassane Mahamat, Eric Ottesen, Regina Rabinovich, J. H. F. Remme, Jose Ignacio Santos, Lorenzo Savioli, Pere Simarro, Wilma Stolk, Mark Taylor, Stephen Torr, Hugo Turner, and Laurent Yameogo.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by Eradication Investment Case (EIC) of Onchocheriasis, Lymphatic Filariasis and Human African Trypanosomiasis, and grant #OPP1037660 of the Bill and Melinda Gates Foundation (BMGF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO. Global programme to eliminate lymphatic filariasis: progress report for 2012. Wkly Epidemiol Rec. 2013;88(37): 389–99. Epub 2013/10/01. [PubMed] [Google Scholar]
- 2. de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, Boakye DA. Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors. 2012;5: 259 10.1186/1756-3305-5-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Addiss DG, Dimock KA, Eberhard ML, Lammie PJ. Clinical, parasitologic, and immunologic observations of patients with hydrocele and elephantiasis in an area with endemic lymphatic filariasis. J Infect Dis. 1995;171(3): 755–8. [DOI] [PubMed] [Google Scholar]
- 4. Suma TK, Shenoy RK, Kumaraswami V. A qualitative study of the perceptions, practices and socio-psychological suffering related to chronic brugian filariasis in Kerala, southern India. Ann Trop Med Parasitol. 2003;97(8): 839–45. [DOI] [PubMed] [Google Scholar]
- 5. Perera M, Whitehead M, Molyneux D, Weerasooriya M, Gunatilleke G. Neglected patients with a neglected disease? A qualitative study of lymphatic filariasis. PLoS Negl Trop Dis. 2007;1(2): e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiss MG. Stigma and the social burden of neglected tropical diseases. PLoS Negl Trop Dis. 2008;2(5): e237 10.1371/journal.pntd.0000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Parasites—Lymphatic Filariasis27 April 2015. Available: http://www.cdc.gov/parasites/lymphaticfilariasis/gen_info/faqs.html.
- 8. Fact Sheet: Lymphatic Filariasis [Internet]. Geneva, Switzerland: World Health Organization; 2012. Available: http://www.who.int/mediacentre/factsheets/fs102/en/. [Google Scholar]
- 9. Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep. 1993;42(RR–16): 1–38. [PubMed] [Google Scholar]
- 10. Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75(6): 491–503. [PMC free article] [PubMed] [Google Scholar]
- 11. Sugiyama H, Yamaguchi A, Murakami H. Japanese Corporate Initiatives in Global Health: Case Studies and Policy Recommendations. Tokyo, Japan: 2012. [Google Scholar]
- 12.Liverpool School of Tropical Medicine. Annual Report. Liverpool, U.K.: LSTM, 2007–2008.
- 13.Preventive Chemotherapy Databank Lymphatic Filariasis [Internet]. WHO. 2015 [cited 2015 January 20]. Available: http://www.who.int/neglected_diseases/preventive_chemotherapy/lf/en/.
- 14. Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070): 18–22. [DOI] [PubMed] [Google Scholar]
- 15. Alexander ND. Are we nearly there yet? Coverage and compliance of mass drug administration for lymphatic filariasis elimination. Trans R Soc Trop Med Hyg. 2015. Epub 2015/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Molyneux DH, Hopkins DR, Zagaria N. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol. 2004;20(8): 347–51. [DOI] [PubMed] [Google Scholar]
- 17. Ichimori K, King JD, Engels D, Yajima A, Mikhailov A, Lammie P, et al. Global programme to eliminate lymphatic filariasis: the processes underlying programme success. PLoS Negl Trop Dis. 2014;8(12): e3328 10.1371/journal.pntd.0003328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tediosi F, Steinmann P, de Savigny D, Tanner M. Developing eradication investment cases for onchocerciasis, lymphatic filariasis, and human African trypanosomiasis: rationale and main challenges. PLoS Negl Trop Dis. 2013;7(11): e2446 10.1371/journal.pntd.0002446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker DG, Lupp J. Guide for preparing an eradication investment case. 2011.
- 20.Provisional strategy for interrupting lymphatic filariasis transmission in loiasis-endemic countries: report on the meeting on lymphatic filariasis, malaria and integrated vector management. Accra, Ghana: WHO, 5–9 March 2012.
- 21. World Health Organization. Training in monitoring and epidemiological assessment of mass drug administration for eliminating lymphatic filariasis Learner's guide. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 22. Passing H, Bablok. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21(11): 709–20. [DOI] [PubMed] [Google Scholar]
- 23. Stolk WA, de Vlas SJ, Borsboom GJ, Habbema JD. LYMFASIM, a simulation model for predicting the impact of lymphatic filariasis control: quantification for African villages. Parasitology. 2008;135(13): 1583–98. 10.1017/S0031182008000437 [DOI] [PubMed] [Google Scholar]
- 24. Stolk WA, ten Bosch QA, de Vlas SJ, Fischer PU, Weil GJ, Goldman AS. Modeling the impact and costs of semiannual mass drug administration for accelerated elimination of lymphatic filariasis. PLoS Negl Trop Dis. 2013;7(1): e1984 10.1371/journal.pntd.0001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michael E, Malecela-Lazaro MN, Maegga BT, Fischer P, Kazura JW. Mathematical models and lymphatic filariasis control: monitoring and evaluating interventions. Trends Parasitol. 2006;22(11): 529–35. [DOI] [PubMed] [Google Scholar]
- 26. Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Ann Trop Med Parasitol. 2002;96 Suppl 2: S143–52. [DOI] [PubMed] [Google Scholar]
- 27. Stolk WA, de Vlas SJ, Habbema JD. Advances and challenges in predicting the impact of lymphatic filariasis elimination programmes by mathematical modelling. Filaria J. 2006;5: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Norman RA, Chan MS, Srividya A, Pani SP, Ramaiah KD, Vanamail P, et al. EPIFIL: the development of an age-structured model for describing the transmission dynamics and control of lymphatic filariasis. Epidemiol Infect. 2000;124(3): 529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gambhir M, Bockarie M, Tisch D, Kazura J, Remais J, Spear R, et al. Geographic and ecologic heterogeneity in elimination thresholds for the major vector-borne helminthic disease, lymphatic filariasis. BMC Biol. 2010;8: 22 10.1186/1741-7007-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gambhir M, Michael E. Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis. PLoS One. 2008;3(8): e2874 10.1371/journal.pone.0002874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michael E, Bundy DA, Grenfell BT. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112 (Pt 4): 409–28. [DOI] [PubMed] [Google Scholar]
- 32.United Nations, Department of Economic and Social Affairs, Population Division (2013). World Population Prospects: The 2012 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.227.
- 33.WHO, Rotary International, CDC. Global Polio Eradication Initiative (GPEI) Status Report. 29 April 2013.
- 34. Gillespie SH. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrob Agents Chemother. 2002;46(2): 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859): 2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 36. Babu BV, Babu GR. Coverage of, and compliance with, mass drug administration under the programme to eliminate lymphatic filariasis in India: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(9): 538–49. 10.1093/trstmh/tru057 [DOI] [PubMed] [Google Scholar]
- 37. Reimer LJ, Thomsen EK, Tisch DJ, Henry-Halldin CN, Zimmerman PA, Baea ME, et al. Insecticidal bed nets and filariasis transmission in Papua New Guinea. N Engl J Med. 2013;369(8): 745–53. 10.1056/NEJMoa1207594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stone CM, Lindsay SW, Chitnis N. How effective is integrated vector management against malaria and lymphatic filariasis where the diseases are transmitted by the same vector? PLoS Negl Trop Dis. 2014;8(12): e3393 10.1371/journal.pntd.0003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noor AM, Mutheu JJ, Tatem AJ, Hay SI, Snow RW. Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373(9657): 58–67. 10.1016/S0140-6736(08)61596-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giardina F, Kasasa S, Sie A, Utzinger J, Tanner M, Vounatsou P. Effects of vector-control interventions on changes in risk of malaria parasitaemia in sub-Saharan Africa: a spatial and temporal analysis. Lancet Glob Health. 2014;2(10): e601–15. 10.1016/S2214-109X(14)70300-6 [DOI] [PubMed] [Google Scholar]
- 41. Stolk WA, Stone C, de Vlas SJ. Modelling lymphatic filariasis transmission and control: modelling frameworks, lessons learned and future directions. Adv Parasitol. 2015;87: 249–91. 10.1016/bs.apar.2014.12.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TIF)
(TIF)
From top to bottom: 5, 10, 15, 20%, using diethylcarbamazine citrate and albendazole (red) or ivermectin and albendazole (blue) combination therapy.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.