Abstract
Oncogenic, activating mutations in KRAS initiate pancreatic cancer. There are, however, two other Ras family members, Nras and Hras, which can be activated in the presence of oncogenic Kras. The role of these wild-type Ras proteins in cancer remains unclear, as their disruption has been shown to enhance or inhibit tumorigenesis depending upon the context. As pancreatic cancer is critically dependent upon Ras signaling, we tested and now report that loss of Hras increases tumor load and reduces survival in an oncogenic Kras-driven pancreatic adenocarcinoma mouse model. These effects were traced to the earliest stages of pancreatic cancer, suggesting that wild-type Hras may suppress tumor initiation. In normal cells, activated Ras can suppress proliferation through p53-dependent mechanisms. We find that the tumor suppressive effects of Hras are nullified in a homozygous mutant p53 background. As such, loss of wild-type Hras fosters the earliest stages of pancreatic cancer in a p53-dependent manner.
Introduction
It is estimated that approximately 1.5% of Americans will develop pancreatic cancer, primarily pancreatic ductal adenocarcinoma (PDAC). The vast majority of these individuals will succumb to this disease, with a five-year survival rate of only 6.7% [1]. Thus, it is critical to elucidate the signaling pathways underlying this incredibly deadly cancer. Activating mutations in KRAS are the most common genetic alteration found in human PDAC, being present in over 90% of all cases. In fact, the Ras pathway is argued to be engaged in essentially all PDAC [2].
KRAS, and the other Ras family members NRAS and HRAS, encode highly related small GTPases. Normally, stimulation of guanine nucleotide exchange factors [GEFs] promotes the conversion of Ras from an inactive, GDP-bound state to an active, GTP-bound state. Ras-GTP binds to and activates effector proteins that engage the MAPK, PI3K, RalGEF, and other signaling pathways involved in cellular growth and survival. GTPase-activating proteins (GAPs) then enhance hydrolysis of GTP and revert Ras back to an inactive state [3].
The somatic mutations detected in the KRAS gene inactivate the endogenous or GAP-stimulated GTPase activity of the encoded protein, resulting in constitutively GTP-bound and activated Kras [3]. In addition to being commonly detected in PDAC [2], these mutations are also found in the earliest stages of this disease [4] and, when introduced into the murine Kras gene, induce early stage pancreatic cancer that can progress to frank PDAC [5]. As such, oncogenic mutations in KRAS are thought to be the driver mutations in PDAC.
Although the involvement of the oncogenic mutant form of Kras in pancreatic cancer is well described, the remaining wild-type Ras isoforms may also be activated. The first hints in this regard were the findings that targeting the negative regulator RasGAP to the plasma membrane [6] or reducing wild-type Nras expression [7] dampened oncogenic Hras signaling. Indeed, wild-type Nras and Hras have been found to be activated downstream of oncogenic Kras in a manner dependent upon S-nitrosylation [8] or expression of the GEF son-of-sevenless [SOS] [9]. Moreover, wild-type Ras proteins are still susceptible to activation by growth factors, even in the presence of oncogenic Kras [10].
Wild-type Ras proteins can have divergent effects depending on the setting. On one hand, they can inhibit tumorigenesis. Specifically, oncogenic Ras can induce a senescent growth arrest when expressed in normal cells [11] and inhibit tumor formation [12], suggesting a tumor suppressive role for the wild-type Ras proteins. In agreement, carcinogen-induced oncogenic mutations in Kras or Hras in lung and skin tumors of mice are often accompanied by loss of the reciprocal wild-type allele [13–15]. Similarly, loss of heterozygosity of the oncogenic RAS gene has been reported in human cancers [16]. Moreover, mice lacking one allele of wild-type Kras [15], or both alleles of either Hras or Nras, develop more Kras mutation-positive lung tumors [17]. A tumor-suppressive role for wild-type Nras was also observed in a mouse model of thymic lymphomas driven by oncogenic Nras [18]. Thus, wild-type Ras proteins can be tumor suppressive, especially in settings of early tumorigenesis.
Wild-type Ras proteins can also foster tumorigenesis. Specifically, mice lacking one or both alleles of wild-type Hras or Nras develop fewer carcinogen-induced Hras mutation-positive skin tumors [17]. Knocking down the expression of wild-type Ras proteins [8, 19], or reducing their activation by eNOS [8] or SOS [9], inhibits cell viability, transformation, and/or tumorigenic growth of KRAS mutation-positive human cancer cell lines. Conversely, stimulation of wild-type Ras proteins by EGF promotes proliferation of such cells [10]. Thus, particularly in models of more advanced disease, wild-type Ras proteins can enhance tumorigenesis.
Given that wild-type Ras proteins can be activated downstream of oncogenic Kras, yet give rise to opposite effects on tumorigenesis depending on the context, it is important to ascertain their role in the cancer most frequently associated with KRAS mutations, namely pancreatic. Thus, we assessed the consequence of disrupting the endogenous, wild-type Hras gene on the development of oncogenic Kras-driven tumorigenesis in the pancreas of mice. We report that loss of wild-type Hras promotes tumorigenesis in this model, suggesting a tumor suppressive role for wild-type Hras at the early stages of pancreatic cancer.
Materials and Methods
Mouse pancreatic cancer models
Kras LSL-G12D/+, Trp53 LSL-R172/+, and Pdx-1-Cre tg/+ mice were obtained from Jackson Labs and as a kind gift from David Kirsch. Hras -/- mice were a kind gift from the NCI and Eugenio Santos. Kras LSL-G12D/+ ;Hras +/- and Pdx-1-Cre tg/+ ;Hras +/- mice were bred to generate Hras +/+ and Hras -/- KC (LSL-Kras G12D/+ ;Pdx-1-Cre tg/+) littermates. Kras LSL-G12D/+ ;Trp53 LSL-R172/+ ;Hras +/+ and Pdx-1-Cre tg/+ ;Hras +/+ mice were bred to generate Hras +/+ KPC (LSL-Kras G12D/+ ;Trp53 LSL-R172/+ ;Pdx-1-Cre tg/+) mice. Kras LSL-G12D/+ ;Trp53 LSL-R172/+ ;Hras -/- and Pdx-1-Cre tg/+ ;Hras -/- mice were bred to generate Hras -/- KPC mice. Kras LSL-G12D/+ ;Trp53 LSL-R172/+ ;Hras +/+ and Pdx-1-Cre tg/+ ;Trp53 LSL-R172/+ ;Hras +/+ mice were bred to generate Hras +/+ KPPC (LSL-Kras G12D/+ ;Trp53 LSL-R172/ LSL-R172H ;Pdx-1-Cre tg/+) mice. Kras LSL-G12D/+ ;Trp53 LSL-R172/+ ;Hras -/- and Pdx-1-Cre tg/+ ;Trp53 LSL-R172/+ ;Hras -/- mice were bred to generate Hras -/- KPPC mice. The described mice were monitored for health and weight three times per week and were euthanized at either the indicated fixed time points or upon reaching a moribundity endpoint. Moribundity endpoints were defined as weight loss exceeding 15% of total body weight, indications of abdominal ascites or swelling, or signs of pain or distress. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. A protocol for this study was specifically approved by the Institutional Animal Care and Use Committee at Duke University (protocol A279-13-11).
Mouse PDAC cell lines
Pancreatic tumor tissue from three Hras +/+ and three Hras -/- KPC mice was minced in collagenase V (Sigma-Aldrich) for 30 minutes at 37°C, and resultant cells were cultured in Dulbecco's Modified Eagle's Media (DMEM) + 10% FBS for at least 4 passages.
Quantification of normal pancreatic acinar area
The percentage of normal acinar area was quantified at 8 and 36 week time-points in KC mice in a blinded study of 4–5 randomly selected high-power fields of H & E-stained pancreatic section per mouse. The amount of acinar area per section was expressed as a percentage of the total area of tissue in the field, determined using Adobe Photoshop freehand selection tool.
Grading of pancreatic lesions
H&E stained sections were blindly reviewed by pathologists (S. Tang, M. Mastrodomenico, and D. Cardona) at 4, 8, and 36 week time points. Each lobule of the pancreas from each section was examined, and the highest grade lesion (ADM or PanIN) from each lobule was identified, and the percent of lobules with the highest grade of each type of lesion was determined based upon the total number of lobules counted.
ADM immunoflourescent staining
Pancreatic specimens from KC mice were snap frozen in liquid nitrogen and 8 uM-thick sections were cut using a cryotome. The samples were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton-X-100. Samples were blocked in normal donkey serum then incubated with 1:100 dilution of the primary antibodies Goat Anti-Amylase C-20 and sc-12821 Rabbit Anti-CK-19 H-60 (Santa Cruz Biotechnology Inc.) overnight at 4°C. Samples were then incubated with the secondary antibodies Alexa fluor 488 Donkey anti-rabbit IgG (Invitrogen A-21206) or Alexa-fluor 594 Donkey anti-goat IgG (Invitrogen A-11058) at a 1:500 dilution for 1 hour at room temperature, then mounted with ProLong Gold antifade reagent with DAPI (Life Technologies P36931). Total number of lesions with cells co-staining for amylase and CK-19 were quantified per high power field in merged images from a Zeiss Axio Imager widefield fluorescence microscope in a blinded fashion.
Senescence-associated β-galactosidase staining
Snap-frozen pancreatic samples were stained as described by Debacq-Chainiaux et al [20] and 5 randomly selected, high-power, images from an Olympus Vanox X microscope were quantified using Image J software in a blinded fashion. Color thresholding was used to determine the total amount of staining per high power field, and the freehand selection tool was used to select only the lesions, where color thresholding was applied to calculate the percentage of positive staining area from only the lesions.
Ki67, CC3, and p16 immunohistochemistry
Heat-induced antigen retrieval was performed on formalin-fixed, paraffin-embedded sections, followed by staining with an anti-Ki67 primary antibody at a 1:50 dilution (Dako M7249), an anti-CC3 primary antibody at a 1:100 dilution (Cell Signaling, D175), or anti-p16 primary antibody (BD Biosciences, 551153) at a 1:100 dilution. Peroxidase-based detection was performed using Vectastain Elite ABC Kit (Vector labs). The total number of Ki67-positive staining cells per field in each of 5 randomly selected, high-power fields was counted in a blinded fashion, as well as the number of lesions with at least one positive-staining cell and quantified as a percentage of the total number of lesions. For CC3 and p16, 5 randomly selected, high-power fields were quantified in a blinded fashion using Image J software. Color thresholding was used to determine the total amount of CC3 and p16 staining per high-power field, and the freehand selection tool was used to select only the lesions, where color thresholding was applied to calculate the percentage of positive staining area from only the lesions.
Hras-GTP analysis
Early passage (within 5 passages from adaption to culture) Hras -/- KPC cell lines were stably infected with retroviruses derived from pBabePuro (vector control) or pBabePuroFLAG-Hras encoding wild-type mouse Hras cDNA, and selected with puromycin, as previously described [21]. Stable lines were then tested within 2 passages for Hras GTP levels by affinity capture with the RBD of Raf followed by immunoblot for HRAS with an α-HRAS antibody (Santa Cruz, sc520), as previously described [21].
PCR of Kras alleles
DNA was isolated from pancreatic tissue, facial papillomas, or vulvar tumors and amplified by PCR to detect the wild-type and recombined Kras alleles as previously described [5].
Statistics
Statistical analyses were performed using GraphPad Prism v5 (GraphPad Software). A 2-sided, unpaired t-test was used to compare the amount of normal acinar tissue remaining in the pancreata in Hras +/+ and Hras -/- KC and KPPC mice, and to compare levels of Ki67, p16, ADM, SA-β-gal, and CC3 staining in each cohort. A 2-sided unpaired t-test was also used to compare the levels of each type of graded lesions at the specified time-points and the number of facial papillomas between cohorts. These results were then confirmed by Mann-Whitney test using the same software. Kaplan-Meier survival curves were generated for each of the KPC and KPPC cohorts, as well as for the development of facial and vulvar papillomas over time in the KPC cohort, and P-values were calculated using the log-rank (Mantel-Cox) test.
Results
Increased number of PanIN lesions in the absence of wild-type Hras
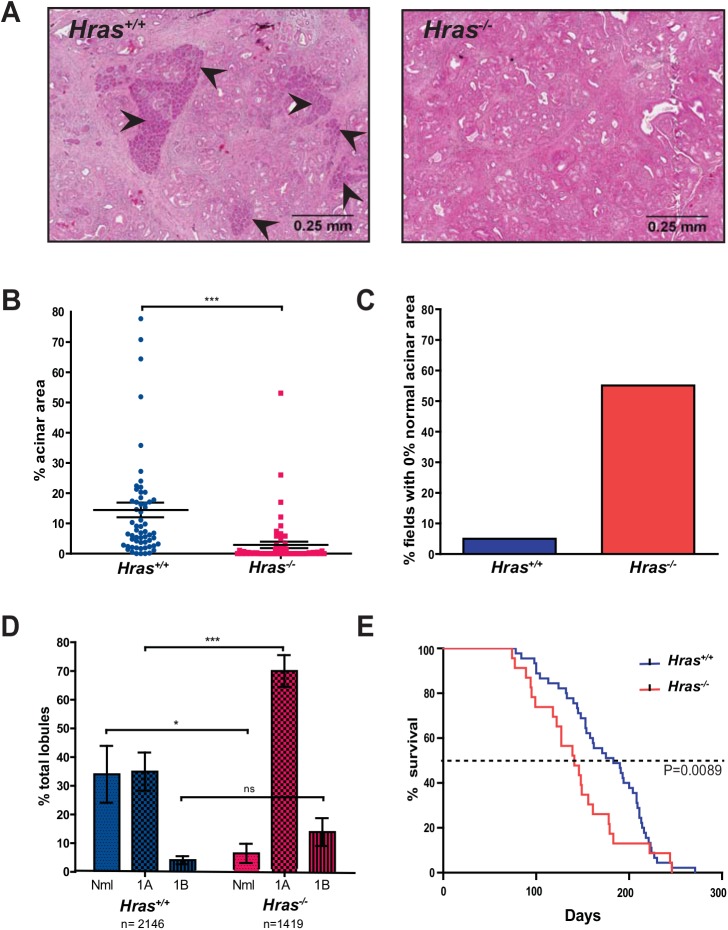
To assess the role of wild-type Ras proteins on oncogenic Kras-driven pancreatic tumorigenesis in vivo, mice were generated with either homozygous null Hras -/- [22] or wild-type Hras +/+ alleles in a K ras LSL-G12D/+ ;Pdx-1- C re tg/+ (KC) background [5]. We chose Hras, as it is activated downstream of oncogenic Kras in PDAC cell lines [8–10], and of the three Ras genes, is the only one that is not embryonic lethal in a background like that of KC mice which encodes only one functional Kras allele [22]. We chose the KC model because the activation of an endogenous oncogenic (G12D) Kras in the developing pancreas of these mice results in the development of pre-invasive pancreatic intraepithelial neoplasias (PanINs) that progress at a low frequency to PDAC through a series of histological changes that resemble those seen in human patients [5]. Cohorts of 12 Hras +/+ and Hras -/- KC mice were generated and then euthanized at 36 weeks of age, a time when a spectrum of PanIN lesions are easily detected [5]. H & E staining of pancreatic sections revealed a frank loss of normal tissue and expansion of PanIN lesions in the Hras -/- KC mice (Fig 1A). Quantification of the amount of normal acinar tissue revealed that Hras +/+ KC mice had an average of 14.5% normal tissue/field, whereas Hras -/- KC mice had 2.9%, a significant decrease of nearly 5-fold in Hras -/- KC mice (Fig 1B). This effect was more evident when the percentage of sections lacking normal acinar tissue was quantified. 5% of sections in Hras +/+ KC mice lacked normal acinar tissue whereas this value was 55.1% in Hras -/- KC mice, an increase of over 11-fold in Hras -/- KC mice (Fig 1C). Pathological grading of 2146 pancreatic lobes from Hras +/+ KC mice and 1419 lobes fromHras -/- KC mice revealed 27.4% fewer lobes with no lesions and 30% more lobes with the highest grade being PanIN 1A in Hras -/- KC mice (Fig 1D). Thus, wild-type Hras suppresses the tumorigenic activity of oncogenic Kras during pancreatic tumorigenesis.
Fig 1. Mice lacking wild-type Hras develop more PanIN lesions and have reduced survival in oncogenic Kras-driven models of pancreatic cancer.
(A) Representative H & E stained sections (arrowheads: normal acinar cells), (B) quantification of % total normal acinar area remaining per field (at 4x magnification, 4–5 fields from 12 mice, bar: mean ± S.E.M.), (C) % of fields (at 4x magnification) with no normal acinar tissue (based on the data from B), and (D) % of lobules with the indicated highest grade lesion (Nml: normal, 1A: PanIN-1A, 1B: PanIN-1B, bar: mean ± S.E.M.) of pancreata isolated from Hras +/+ versus Hras -/- KC mice at 9 months of age. (E) Kaplan-Meier curve of Hras +/+ (n = 45) versus Hras -/- (n = 23) KPC mice. ns: not significant. *P<0.05. ***P<0.0001.
Decreased survival in the absence of wild-type Hras
Admittedly, the above approach did not determine the effect of Hras loss on the commonly diagnosed stage of PDAC [23] or on survival, the most clinically relevant endpoint. To overcome these shortcomings, we generated Hras +/+ and Hras -/- mice in a K ras LSL-G12D/+ ;Tr p 53 LSL-R172H/+ ;Pdx-1- C re tg/+ [KPC] setting [24]. These mice are based on the KC genetic background, but contain an additional inducible dominant-negative Trp53 R172H allele, which promotes PDAC that pathologically resembles the human disease [24]. Cohorts of 45 Hras +/+ and 23 Hras -/- KPC mice were generated and monitored regularly. Mice were euthanized upon reaching humane moribundity endpoints to determine their lifespan. This analysis revealed that the median survival in Hras +/+ KPC mice was 183 days, but 141 days in Hras -/- KPC mice, a significant reduction of nearly a quarter in Hras -/- KPC mice (Fig 1E). Thus, loss of wild-type Hras not only potentiates early oncogenic Kras-driven pancreatic tumorigenesis, but is also associated with a reduction in lifespan in mice developing PDAC.
Increased number of skin papillomas in the absence of wild-type Hras
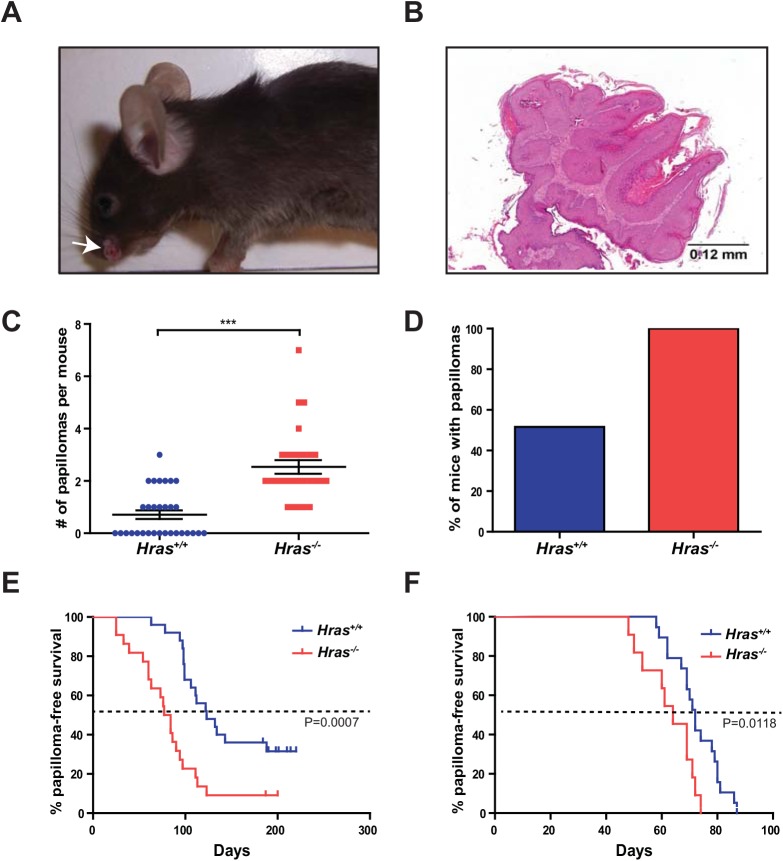
In addition to PanIN lesions, KC mice are prone to develop skin papillomas in the face and vulvar tissues due to Cre expression by Pdx-1 in these tissues [5, 25–27]. The development of papillomas in the same KC mice provides a well-controlled system to assess the role of wild-type Hras in a second independent tissue type [25]. To this end, we monitored the number of facial papillomas developing in cohorts of 24 Hras +/+ and 27 Hras -/- KC mice. Visual inspection revealed an obvious increase in the number of facial lesions (Fig 2A) of papilloma pathology (Fig 2B) in Hras -/- KC mice. Quantification revealed that there was an average of 0.7 facial papillomas/mouse in the Hras +/+ KC cohort, but 2.5 in the Hras -/- KC cohort, a significant increase of nearly 4-fold in Hras -/- KC mice (Fig 2C). Furthermore, while only about half the Hras +/+ KC mice developed a papilloma by the study endpoint, all the Hras -/- KC mice developed at least one papilloma (Fig 2D). PCR of DNA from a subset of these papillomas revealed the expected recombination of the LSL-Kras G12D allele (S1 Fig). Perhaps most telling, the average time when these lesions first appeared in Hras +/+ KPC mice was 123 days, but 80.5 days in Hras -/- KPC mice, a significant decrease of 42.5 days in Hras -/- KPC mice (Fig 2D). Similarly, vulvar papillomas also appeared, on average, 8 days earlier in Hras -/- KPC mice (Fig 2E). Taken together, these results suggest that loss of Hras promotes oncogenic Kras-driven skin tumorigenesis, potentially at a very early stage such as initiation.
Fig 2. Mice lacking wild-type Hras develop more oncogenic Kras-driven skin tumors.
(A) Representative photograph (white arrow) and (B) H & E stained section of facial papillomas that develop in KC mice. (C) Number of facial papillomas per Hras +/+ (n = 31) versus Hras -/- (n = 28) KC mice (bar: mean ± S.E.M.). (D) % of total Hras +/+ and Hras -/- KC mice (from C) that developed facial papillomas. (E) % facial papilloma-free survival of Hras +/+ (n = 45) versus Hras -/- (n = 23) KPC mice. (F) % vulvar papilloma-free survival of Hras +/+ (n = 19) versus Hras -/- (n = 11) female KPC mice. ***P<0.0001.
Loss of wild-type Hras affects early pancreatic tumorigenesis
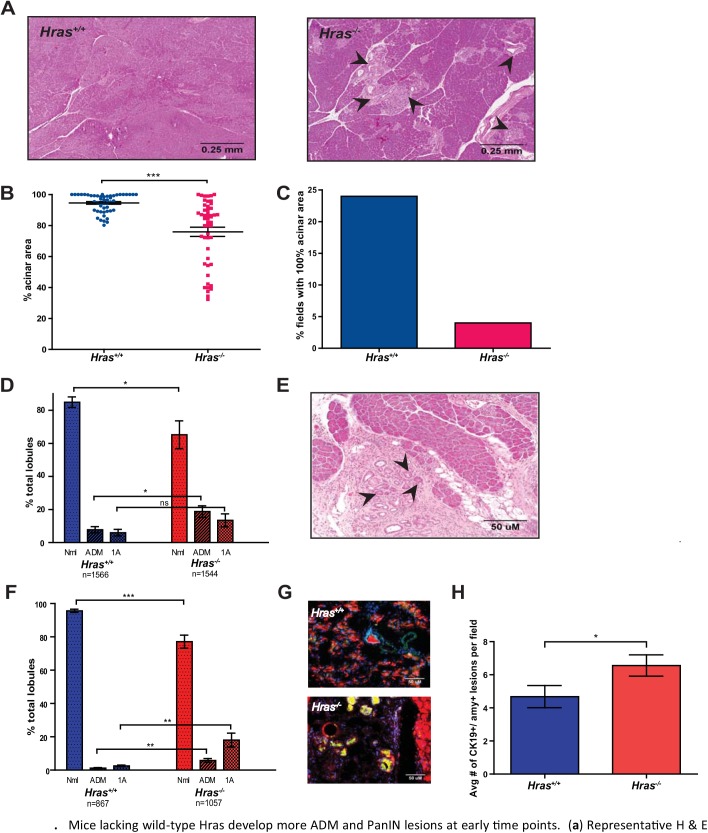
The above observations point towards the Hras null background affecting an early stage of tumorigenesis, such as initiation. To assess whether this may explain the observed increase in pancreatic lesions in KC mice and reduced lifespan in KPC mice upon loss of wild-type Hras, pancreatic tumorigenesis was analyzed at 8 weeks of age, when KC mice typically exhibit fewer and lower grade lesions [5]. H & E staining of pancreatic sections again revealed a reduction in normal tissue and expansion of PanIN lesions in the Hras -/- KC mice (Fig 3A). Quantification of the amount of normal acinar tissue revealed that Hras +/+ KC mice had an average of 94.6% normal tissue/field, whereas Hras -/- KC mice had 75.9%, a significant reduction by nearly 20% in Hras -/- KC mice (Fig 3B). This effect was particularly evident when the analysis was repeated by quantitating the number of sections with all the normal acinar tissue remaining. 24% of the sections in Hras +/+ KC mice had all normal acinar tissue, compared to 4% in Hras -/- KC mice, a reduction of 6-fold in Hras -/- KC mice (Fig 3C). Pathological grading of 1566 pancreatic lobes from Hras +/+ KC mice and 1544 pancreatic lobes from Hras -/- KC mice revealed 18.7% more lobes with no lesions and a trend towards more PanIN 1A lesions in Hras -/- KC mice (Fig 3D). Interestingly, pathological grading also revealed a significant increase of over 2-fold in the number of lobules with acinar-to-ductal metaplasia (ADM) as the highest-grade lesion in Hras -/- KC mice (Fig 3D). ADM (e.g. Fig 3E) is one of the earliest changes in the pancreas of PDAC mouse models, and has been suggested to be a possible precursor to PanIN lesions [28, 29].
Fig 3. Mice lacking wild-type Hras develop more ADM and PanIN lesions at early time points.
(A) Representative H & E stained section (arrowhead: PanIN lesion), (B) quantification of % total normal acinar area remaining per field (at 4x magnification, 5 fields from 10 mice, bar: mean ± S.E.M.), (C) % of fields with all normal acinar tissue (from B), and (D) % of lobules with the indicated highest grade lesion (Nml: normal, ADM: acinar-to-ductal metaplasia, 1A: PanIN-1A, bar: mean ± S.E.M.) of pancreata isolated from Hras +/+ versus Hras -/- KC mice at 8 weeks of age. (E) Representative H & E stained section of a pancreas from an 8-week old KC mouse (arrowhead: acinar-to-ductal metaplasia). (F) % of lobules with the indicated highest grade lesion (Nml: normal, ADM: acinar-to-ductal metaplasia, 1A: PanIN-1A bar: mean ± S.E.M.) of pancreata isolated from Hras +/+ versus Hras -/- KC mice at 4 weeks of age. (G) Representative pancreatic section from a 4-week old Hras +/+ versus Hras -/- KC mouse immunostained for cells (DAPI, blue) and markers of acinar (amylase, red) and ductal (CK-19, green) cells to highlight ADM lesions (co-staining, yellow). (H) Number of amylase+/CK-19+ positive (ADM) cells per field (at 4x magnification, (at 4x magnification, 5 fields from 10 mice) from pancreata isolated from Hras +/+ versus Hras -/- KC mice at 4 weeks of age (bar: mean ± S.E.M.). *P<0.05. ***P<0.0001.
Given the increase in the early grade lesions at 8 weeks in Hras -/- KC mice, we explored the impact of the Hras null genotype at 4 weeks of age when typically there is little or no evidence of pancreatic lesions in KC mice. Pathological grading of 867 lobes from Hras +/+ KC mice revealed, as expected, nearly all the lobes (95.6%) lacked any lesion (Fig 3F). In contrast, there was a significant increase of nearly 5-fold in the number of lobes with highest grade of ADM lesions and nearly 7-fold in the number of lobes with PanIN 1A lesions in the Hras -/- KC mice (Fig 3F). This difference was not recapitulated in culture, as the number of ADM events was similar between cultured Hras -/- versus Hras +/+ acinar cells when LSL-Kras G12D was activated by Ad-Cre (not shown). However, we independently and directly validated the increase in ADM lesions in vivo in the Hras -/- KC mice by quantitating the number of cells co-staining for immunoflourescent markers of acinar (amylase) and ductal (CK-19) cells (Fig 3G). Quantification revealed an average of 4.7 amylase/CK-19 co-staining pancreatic lesions/field in Hras +/+ KC mice, but 6.6 in Hras -/- KC mice, a significant increase in Hras -/- KC mice (Fig 3H). There were no differences in the amount of Ki67 (a marker of cellular proliferation), CC3 (a marker of apoptosis), SA-β-gal or p16 (markers of senescence) positive-staining lesions between Hras +/+ and Hras -/- KC mice (S2A–S2D Fig), suggesting that the difference imparted by the loss of Hras had already occurred by the time lesions were detected. Taken together, these data suggest that Hras suppresses early pancreatic tumorigenesis, perhaps at the stage of initiation.
Wild-type Hras is activated in PDAC
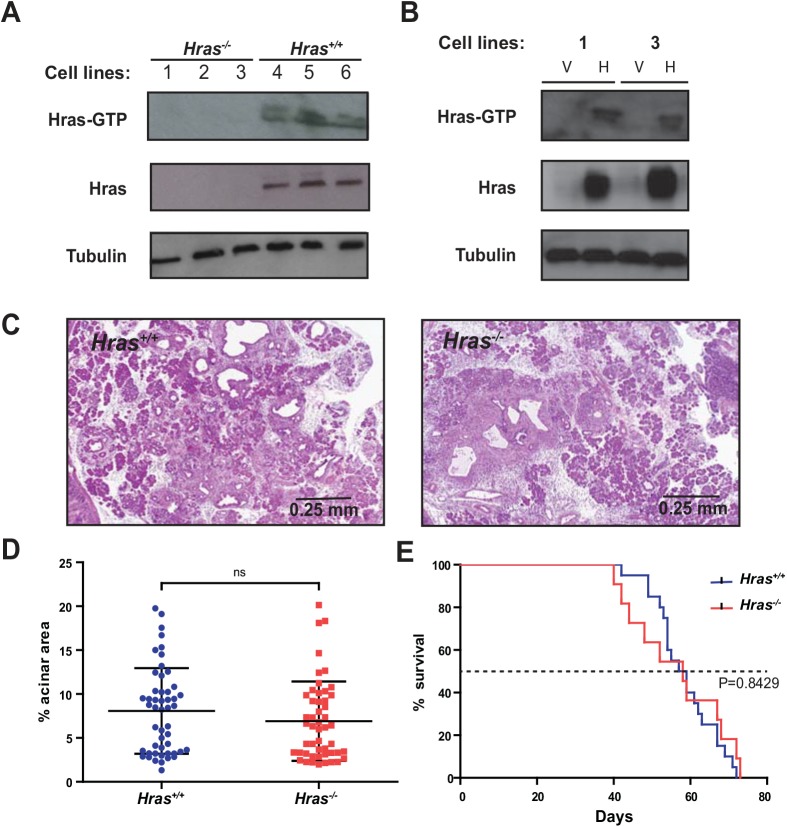
Wild-type Ras can be activated in the presence of a mutant oncogenic allele in cancer cell lines [8–10, 19]. In pancreatic tissue at 8 weeks of age, when there are few PanIN lesions in Hras +/+ KC mice (Fig 3A and 3B), Hras was not detected at appreciable levels (not shown). To evaluate the status of Hras in later stages of disease, PDAC cell lines were established from three different Hras +/+ and Hras -/- KPC mice. GTP-bound Hras was then affinity captured with Raf-RBD followed by immunoblotting with an anti-Hras antibody to detect the protein. In all three Hras +/+ KPC cell lines, Hras-GTP was recovered. In contrast, this pool of active Hras was completely absent in the PDAC tumor cells derived from the three Hras -/- KPC mice (Fig 4A). Moreover, this pool of active Ras was restored upon re-expressing wild-type Hras in two Hras -/- KPC cell lines at early passage (Fig 4B), although this effect was lost as the cells were passaged (not shown). Thus, loss of Hras removes a pool of active Ras from pancreatic tumor cells.
Fig 4. Wild-type Hras is activated in KPC cell lines and Hras +/+ and Hras -/- KPPC mice exhibit similar tumor burden and lifespan.
Immunoblot analysis of Hras and Hras-GTP in PDAC cell lines derived from (A) Hras -/- (cell lines 1–3) and Hras +/+ (cell lines 4–6) KPC mice or (B) PDAC cell lines 1 and 3 derived from Hras -/- KPC mice stably infected with a retrovirus encoding no transgene (vector, V) or wild-type Hras (H). Tubulin serves as a loading control. (C) Representative H&E stained sections from pancreata of Hras +/+ versus Hras -/- KPPC mice at 14 days of age. (4x magnification) (D) Quantification of the % normal acinar area remaining per field (at 4x magnification, 5 fields from 10 mice) from Hras +/+ versus Hras -/- KPPC mice (bar: mean ± S.E.M). (E) Kaplan-Meier survival curve of Hras +/+ (n = 20) versus Hras -/- (n = 11) KPPC mice. ns: not significant.
Loss of wild-type Hras has no overt effect on pancreatic tumorigenesis in a homozygous mutant p53 background
The effect on pancreatic tumorigenesis upon losing Hras in a homozygous Trp53 R172H background, which has been reported to suppress a loss of pancreatic cells when the LSL-Kras G12D allele is activated [30], was next determined. Cohorts of 10 Hras +/+ versus Hras -/- mice in a Kras LSL-G12D/+ ;Trp53 LSL-R172H/LSL-R172H ;Pdx-1-Cre tg/+ (KPPC) background were euthanized at a fixed time point of 14 days of age, and the amount of normal acinar tissue remaining in the pancreas was assessed (Fig 4C). Quantification of H & E stained pancreatic sections revealed no significant difference in the amount of normal tissue remaining between the two cohorts (Fig 4D). Cohorts of 20 Hras +/+ and 11 Hras -/- KPPC mice were also allowed to age until moribundity endpoints, revealing a nearly identical median survival of 50 and 47.5 days, respectively (Fig 4E). With the caveat that the KPPC background may be so aggressive as to mask any effect on early tumorigenesis caused by the loss of Hras, these data are consistent with a model whereby a homozygous mutant p53 background can negate the tumor-suppressive effects of wild-type Hras on both pancreatic tumorigenesis and survival.
Discussion
We report that a homozygous null Hras background led to more and higher grade pre-invasive PanIN lesions in the KC mouse model of early pancreatic cancer. In agreement, reducing the levels of wild-type Ras signaling also promotes tumorigenesis in other models of pre-invasive cancer [13, 15, 17, 18]. Multiple lines of evidence point towards these phenotypes being a manifestation of the loss of Hras at a very early stage of pancreatic tumorigenesis. First, the percentages of proliferation, apoptosis, and senescence marker-positive PanIN lesions was similar between Hras -/- and Hras +/+ KC mice at an early time point, suggestive of a difference prior to the detection of these lesions. Second, skin papillomas were detected earlier and at a higher frequency in the Hras -/- background, consistent with an effect on tumor initiation. In agreement, there were more ADM and PanIN1A lesions in Hras -/- KC mice at early time points, both of which have been argued to reflect an initiating event [31]. We attempted to assess if this effect occurred at an even earlier time point by assaying for recombination of LSL-EGFP as a surrogate marker for cells with an activated KrasG12D allele, but mosaic staining for GFP precluded any meaningful analysis (not shown). Collectively, these results suggest that the loss of Hras enhances the earliest stages of pancreatic tumorigenesis, perhaps even initiation. Taking this concept one step further, perhaps epigenetic differences in levels of Ras proteins may influence whether an oncogenic mutation in KRAS leads to pancreatic tumorigenesis. As KPC mice had a reduced lifespan in the Hras -/- background, such early effects of wild-type Ras proteins may have lasting repercussions.
While it remains to be determined how wild-type Hras suppresses early pancreatic tumorigenesis, a number of mechanisms are possible. One possibility is that the loss of Hras may reduce the pool of active Ras to a level below that which triggers a growth arrest in response to activation of oncogenic Kras, increasing the chances that an oncogenic mutation in Kras will initiate tumorigenesis. More specifically, normal cells can be sensitive to high levels of Ras signaling, resulting in senescence rather than proliferation, which can inhibit tumor formation [32, 33]. In this regard, pancreatic cells expressing oncogenic Kras have been reported to be selectively lost at two to six weeks of age, apparently a consequence of high Kras signaling [30]. As such, changes that reduce this signaling may promote tumor initiation. In this regard, we demonstrated that loss of Hras reduced the pool of active Ras in pancreatic cancer cells. Bi-allelic loss of wild-type p53 has been shown to rescue the early loss of pancreatic cells upon activation of oncogenic Kras [30]. In agreement, both tumor burden and lifespan were similar between the Hras +/+ and Hras -/- genotypes in a homozygous mutant p53 background of pancreatic cancer. Admittedly, there was no difference in β-galactosidase staining, a common marker for senescence, between Hras +/+ and Hras -/- KC mice at 8-weeks of age, and endogenous Hras was not detected by immunoblot in the pancreatic tissue at this time point. A number of other mechanisms are most certainly possible. For example, loss of Hras may reduce apoptosis or increase proliferation. However, as with the case of senescence markers, there was no difference in Ki67, cleaved caspase 3, or TUNEL staining between Hras +/+ and Hras -/- KC mice at 8-weeks of age, arguing either against this model or pointing towards an effect at an earlier time point. Alternatively, rather than the loss of Hras reducing the amplitude of Ras signaling, it is also possible that this loss changes the spectrum of this signaling. More specifically, Ras isoforms exhibit differences in their subcellular localizations, which may result in engagement of effector proteins in different locations within the cells [34]. Loss of Hras may thus reduce the diversity of this signaling. It has also been suggested that wild-type Ras proteins may sequester effectors from the oncogenic protein, thereby reducing the amplitude or altering the signaling output, which may manifest as increased tumorigenesis when wild-type Hras is deleted [35]. The loss of Hras during development may lead to an increase in the type of cells that ultimately give rise to pancreatic cancer, due to increased proliferation, decreased apoptosis or senescence, or altered differentiation. Finally, it is even possible that the loss of Hras in the stroma underlies the increase in pancreatic lesions observed in Hras -/- KC mice.
We fully recognize that the tumor-suppressive effect of wild-type Hras may very well be context dependent. Indeed, loss of Nras has been shown to promote oncogenic Kras-driven lung tumorigenesis, yet inhibit oncogenic Hras-driven skin tumorigenesis [17]. Similarly, two oncogenic alleles of Nras were found to be more tumorigenic than one allele in a mouse model of CMML and JMML [36]. The negative impact of wild-type Ras proteins on tumorigenesis may also be negated during tumor progression, for example, when cells become resistant to higher levels of Ras signaling. Wild-type Ras proteins may even promote more malignant stages of tumorigenesis, as shRNA-mediated knock down of wild-type Ras proteins has been shown to inhibit the tumor growth of KRAS mutation-positive cancer cell lines isolated from late stage disease [8–10, 19]. Nevertheless, we find that Hras is clearly tumor suppressive in pancreatic cancer, at least at early stages of this disease, which could have important ramifications on the susceptibility of developing pancreatic cancer upon oncogenic insult.
Supporting Information
Representative PCR amplification using primers specific for the wild-type Kras allele or the LSL-Kras G12D allele following Cre-excision (loxed allele) shows successful recombination in the pancreata (panc) and facial papillomas (pap), but not the negative control tails. DNA was isolated from the tissues of 9-month old Hras +/+ and Hras -/- mice.
(PDF)
Representative stained sections and quantification of % positive-staining lesions for (A) Ki67 as a marker for proliferation, (B) CC3 as a marker for apoptosis, (C) p16 and, (D) SA-β-gal as markers for senescence. For each, the % of positive-staining lesions was quantified in 10 random high-power fields from 5 mice of each cohort. (bar: mean ± S.E.M.). ns = not significant. Line = 50 μM.
(PDF)
Acknowledgments
We thank David Kirsch and Eugenio Santos for reagents, Tso-Pang Yao, David Kirsch, Douglas Marchuk, Blanche Capel, and members of the Counter laboratory for helpful discussions, and Tom Ribar, David Hsu, Owen Sansom, Jennifer Morton, Corinne Linardic, Zuowei Su, Susan Reeves, and Steven Conlon for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Cancer Institute, USA (http://www.cancer.gov) grant CA123031. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Surveillance, Epidemiology, and End Results [SEER] Program. National Cancer Institute, 2013. Available: www.seercancer.gov. [Google Scholar]
- 2. Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008; 321: 1801–1806. 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011; 11: 761–774. 10.1038/nrc3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014; 111: 817–822. 10.1038/bjc.2014.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003; 4: 437–450. [DOI] [PubMed] [Google Scholar]
- 6. Huang DC, Marshall CJ, Hancock JF. Plasma membrane-targeted ras GTPase-activating protein is a potent suppressor of p21ras function. Mol Cell Biol. 1993; 13: 2420–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamilton M, Wolfman A. Ha-ras and N-ras regulate MAPK activity by distinct mechanisms in vivo. Oncogene. 1998; 16: 1417–1428. [DOI] [PubMed] [Google Scholar]
- 8. Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008; 452: 646–649. 10.1038/nature06778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeng HH, Taylor LJ, Bar-Sagi D. Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat Comun. 2012; 3: 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young A, Lou D, McCormick F. Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov. 2013; 3: 112–123. 10.1158/2159-8290.CD-12-0231 [DOI] [PubMed] [Google Scholar]
- 11. Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010; 10: 51–517. 10.1038/nrc2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007; 9: 493–505. [DOI] [PubMed] [Google Scholar]
- 13. Balmain A, Brown K, Bremner R. The interplay between ras oncogenes and tumor suppressor genes in experimental carcinogenesis. Immunology Ser. 1990; 51: 75–788. [PubMed] [Google Scholar]
- 14. Hegi ME, Devereux TR, Dietrich WF, Cochran CJ, Lander ES, Foley JF, et al. Allelotype analysis of mouse lung carcinomas reveals frequent allelic losses on chromosome 4 and an association between allelic imbalances on chromosome 6 and K-ras activation. Cancer Res. 1994; 54: 6257–6264. [PubMed] [Google Scholar]
- 15. Zhang Z, Wang Y, Vikis HG, Johnson L, Liu G, Li J, et al. Wildtype Kras2 can inhibit lung carcinogenesis in mice. Nat Genet. 2001; 29: 25–33. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Zhang Z, Dai Z, Plass C, Morrison C, Wang Y, et al. LOH of chromosome 12p correlates with Kras2 mutation in non-small cell lung cancer. Oncogene. 2003; 22: 1243–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. To MD, Rosario RD, Westcott PM, Banta KL, Balmain A. Interactions between wild-type and mutant Ras genes in lung and skin carcinogenesis. Oncogene. 2013; 32: 4028–4033. 10.1038/onc.2012.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diaz R, Ahn D, Lopez-Barcons L, Malumbres M, Perez de Castro I, Lue J, et al. The N-ras proto-oncogene can suppress the malignant phenotype in the presence or absence of its oncogene. Cancer Res. 2002; 62: 4514–4518. [PubMed] [Google Scholar]
- 19. Grabocka E, Pylayeva-Gupta Y, Jones MJ, Lubkov V, Yemanaberhan E, Taylor L, et al. Wild-type H- and N-Ras promote mutant K-Ras-driven tumorigenesis by modulating the DNA damage response. Cancer Cell. 2014; 25: 243–256. 10.1016/j.ccr.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated β-galactosidase [SA-βgal] activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009; 4: 1798–1806. 10.1038/nprot.2009.191 [DOI] [PubMed] [Google Scholar]
- 21. de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997; 14: 623–625. [DOI] [PubMed] [Google Scholar]
- 22. Esteban LM, Vicario-Abejon C, Fernandez-Salguero P, Fernandez-Medarde A, Swaminathan N, Yienger K, et al. Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol Cell Biol. 2001; 21: 1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011; 378: 607–620. 10.1016/S0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005; 7: 469–483. [DOI] [PubMed] [Google Scholar]
- 25. Lampson BL, Kendall SD, Ancrile BB, Morrison MM, Shealy MJ, Barrientos KS, et al. Targeting eNOS in pancreatic cancer. Cancer Res. 2012; 72: 4472–4482. 10.1158/0008-5472.CAN-12-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazur PK, Gruner BM, Nakhai H, Sipos B, Zimber-Strobl U, Strobl LJ, et al. Identification of epidermal Pdx1 expression discloses different roles of Notch1 and Notch2 in murine Kras[G12D]-induced skin carcinogenesis in vivo. PLoS One. 2010; 5: e13578 10.1371/journal.pone.0013578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gades NM, Ohash A, Mills LD, Rowley MA, Predmore KS, Marler RJ, et al. Spontaneous vulvar papillomas in a colony of mice used for pancreatic cancer research. Comp Med. 2008; 58: 271–275. [PMC free article] [PubMed] [Google Scholar]
- 28. Shi G, DiRenzo D, Qu C, Barney D, Miley D, Konieczny SF. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene. 2013; 32: 1950–1958. 10.1038/onc.2012.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, et al. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010; 70: 7114–7124. 10.1158/0008-5472.CAN-10-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci USA. 2010; 107: 246–251. 10.1073/pnas.0908428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011; 121: 4572–4578. 10.1172/JCI57131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997; 88: 593–602. [DOI] [PubMed] [Google Scholar]
- 33. Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: Senescence in premalignant tumours. Nature. 2005; 436: 642 [DOI] [PubMed] [Google Scholar]
- 34. Henis YI, Hancock JF, Prior IA. Ras acylation, compartmentalization, and signaling nanoclusters (Review). Mol Membr Biol. 2009; 26: 80–92. 10.1080/09687680802649582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh A, Sowjanya AP, Ramakrishna G. The wild type Ras road ahead. FASEB J. 2005; 19: 161–169. [DOI] [PubMed] [Google Scholar]
- 36. Xu J, Haigis KM, Firestone AJ, McNerney ME, Li Q, Davis E, et al. Dominant role of oncogene dosage and absence of tumor suppressor activity in Nras-driven hematopoietic transformation. Cancer Discov. 2013; 3: 993–1001. 10.1158/2159-8290.CD-13-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative PCR amplification using primers specific for the wild-type Kras allele or the LSL-Kras G12D allele following Cre-excision (loxed allele) shows successful recombination in the pancreata (panc) and facial papillomas (pap), but not the negative control tails. DNA was isolated from the tissues of 9-month old Hras +/+ and Hras -/- mice.
(PDF)
Representative stained sections and quantification of % positive-staining lesions for (A) Ki67 as a marker for proliferation, (B) CC3 as a marker for apoptosis, (C) p16 and, (D) SA-β-gal as markers for senescence. For each, the % of positive-staining lesions was quantified in 10 random high-power fields from 5 mice of each cohort. (bar: mean ± S.E.M.). ns = not significant. Line = 50 μM.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.