Abstract
Background
Extended thromboprophylaxis after hospital discharge following cancer surgery has been shown to reduce the incidence of venous thromboembolism (VTE); however, this practice has not been universally adopted. We conducted a population-based analysis to determine the proportion of patients with symptomatic VTE diagnosed within 90 days after initial discharge following major abdominopelvic cancer surgery who might have benefited from extended thromboprophylaxis.
Methods
We used the Manitoba Cancer Registry to identify patients who underwent major abdominopelvic cancer surgery between 2004 and 2009. The proportion in whom VTE was diagnosed during the initial hospital stay was determined by accessing the Hospital Separations Abstracts. The proportion in whom VTE was diagnosed after discharge was determined by examining repeat admissions within 90 days and by accessing Drug Programs Information Network records for newly prescribed anticoagulants. Detailed tumour and treatment-specific data allowed calculation of VTE predictors.
Results
Of 6612 patients identified, 106 (1.60%) had VTE diagnosed during the initial stay and 96 (1.45%) presented with VTE after discharge. Among patients in whom VTE developed after discharge, 33.3% had a pulmonary embolus, 24% had deep vein thrombosis, and 6.3% had both. Predictors of presenting with VTE after discharge within 90 days of surgery included advanced disease, presence of other complications, increased hospital resource utilization, primary tumours of noncolorectal gastrointestinal origin and age younger than 45 years. The development of VTE was an independent predictor of decreased 5-year overall survival.
Conclusion
The cumulative incidence of VTE within 90 days of major abdominopelvic oncologic surgery was 3.01%, with about half (1.45%) having been diagnosed within 90 days after discharge.
Abstract
Contexte
La thromboprophylaxie prolongée après le congé hospitalier suite à une chirurgie pour cancer a permis de réduire l’incidence de la thrombo-embolie veineuse (TEV); or, cette pratique n’a pas été universellement adoptée. Nous avons procédé à une analyse de population afin de déterminer la proportion de patients qui ont reçu un diagnostic de TEV symptomatique dans les 90 jours suivant leur congé à la suite d’une chirurgie majeure pour cancer abdomino-pelvien et qui auraient pu bénéficier d’une thromboprophylaxie prolongée.
Méthodes
Nous avons utilisé le registre du cancer du Manitoba pour recenser les patients ayant subi une chirurgie majeure pour cancer abdomino-pelvien entre 2004 et 2009. La proportion de patients chez qui une TEV a été diagnostiquée au cours du séjour hospitalier initial a été calculée à partir des sommaires d’hospitalisation préparés au congé du patient. La proportion de patients chez qui la TEV a été diagnostiquée après le congé provient de l’examen des dossiers de réadmission dans les 90 jours et du réseau provincial d’information sur les programmes de médicaments pour les anticoagulants nouvellement prescrits. L’analyse des données détaillées sur les tumeurs et les traitements a permis d’établir les prédicteurs de la TEV.
Résultats
Sur 6612 patients recensés, 106 (1,60 %) ont reçu un diagnostic de TEV durant leur séjour initial et 96 (1,45 %), après leur congé. Parmi les patients chez qui la TEV est survenue après le congé, 33,3 % ont souffert d’une embolie pulmonaire, 24 %, d’une thrombose veineuse profonde et 6,3 %, des deux. Les prédicteurs de la TEV consécutive au congé hospitalier dans les 90 jours suivant une chirurgie incluaient : maladie avancée, présence d’autres complications, utilisation accrue des ressources hospitalières, tumeur primitive d’origine gastro-intestinale non colorectale et âge < 45 ans. La TEV s’est révélée être un prédicteur indépendant d’une plus brève survie globale à 5 ans.
Conclusion
L’incidence cumulative des TEV dans les 90 jours suivant une chirurgie majeure pour cancer abdomino-pelvien a été de 3,01 %, environ la moitié des cas (1,45 %) ayant été diagnostiqués dans les 90 jours suivant le congé.
Major abdominal cancer surgery is a risk factor for venous thromboembolism (VTE).1–4 The risk persists after hospital discharge and after discontinuation of the usual perioperative thromboprophylaxis.5–11 Only a few studies have evaluated the efficacy and safety of prolonged thromboprophylaxis with low molecular weight heparin (LMWH) after discharge from hospital in patients undergoing surgery for abdominal or pelvic cancer. In both the FAME8 and ENOXACAN II12 trials, substantial numbers of patients were left unaccounted for. Much of the benefit in the ENOXACAN II study was seen in distal deep vein thromboses (DVT) picked up on routine venography.12 A third study failed to show a protective effect of prolonged thromboprophylaxis with LMWH.13 A recent Cochrane meta-analyis,14 however, did show a benefit of extended prophylaxis in terms of both proximal and symptomatic VTE.
Despite these trials, the practice of providing extended thromboprophylaxis after major abdominal oncologic surgery has not been universally adopted. There is still controversy regarding the clinical significance of an occult, radiographically detected DVT and the additional cost of extended thromboprophylaxis.
The primary objective of our study was to determine the proportion of patients who underwent major abdominal or pelvic surgery for cancer and in whom VTE was subsequently diagnosed postdischarge within 3 months of surgery. These patients presenting with late VTEs after their initial hospital stay are presumably the population that could benefit most from extended prophylaxis. A significant number would lend justification to adopting the practice of extended thromboprophylaxis. Secondary objectives included determining the characteristics and predictors of VTE.
Methods
Study design
This study was a population-based review of the incidence of VTE up to 3 months postdischarge for patients who underwent major abdominal or pelvic surgery for cancer between January 2004 and December 2009. We used administrative data from Manitoba Health, the government agency responsible for providing universal health insurance for all citizens living in the province of Manitoba, Canada. The University of Manitoba’s Health Research Ethics Board approved our study.
Population
All adult patients who underwent major abdominal or pelvic surgery for cancer in the Province of Manitoba between January 2004 and December 2009 were considered eligible. The starting year 2004 was chosen because this was the first year that the Manitoba Cancer Registry (MCR) began to collect detailed tumour-node-metastasis (TNM) staging for each entry in the registry. Patients were included if the surgery was done under general anesthetic and the length of hospital stay (LOS) was at least 2 days. Only patients with solid-organ cancers were included. Patients with bloodborne malignancies, such as leukemia and lymphomas, were excluded from the analysis.
Procedure
Patient-specific information was retrieved using several administrative databases maintained by Manitoba Health.
Manitoba Cancer Registry
The MCR is maintained by CancerCare Manitoba (CCMB), the provincially mandated central cancer agency to which patients are referred for consideration of chemotherapy or radiation therapy. The MCR receives reports on all cases of cancer in Manitoba, whether the patients are treated at CCMB or not. Using this registry, we identified patients and collected detailed demographic data, including age, sex and postal codes of the patients’ home addresses. The MCR data sources provided detailed tumour-specific information, including the histological diagnosis and the TNM status at the time of diagnosis.
Medical Claims (Physician Billing) Database and Hospital Separations Abstracts
The Medical Claims and Hospital Separations Abstracts databases contain patient-specific information about contacts with the health care system. We used the Medical Claims Database to obtain information about consultations and services provided to patients both in and out of hospital. We used these records to identify the health care provider, location and type and date of surgery. It also provided detailed information regarding subsequent contacts with the health care system because of subsequent diagnoses of VTE. In addition, the Hospital Separations Abstracts provided admission dates, discharge dates and information on up to 16 diagnoses (ICD-9-CM) and 12 procedures (ICD-9-CM). From these data, we calculated patient comorbidity according to the Charlson score, which allowed this variable to be controlled in the analyses.15 This also allowed us to determine in-hospital mortality associated with VTE.
Manitoba Health Registry
The Manitoba Health Registry contains information on every Manitoban covered by the province’s health care insurance plan. This provided up-to-date vital statistics for each patient, including information on the last date of coverage and the reason for cancellation (e.g., moved or died). Also, it provided further information regarding the mortality associated with VTE.
Drug Program Information Network (DPIN) Registry
The DPIN is an online point-of-sale prescription drug system that connects Manitoba Health and pharmacies in the province and generates complete drug profiles for each client. From this registry, patients with new outpatient prescriptions for LMWH were identified and included since there was a high probability that these prescriptions were for VTE. New prescriptions for warfarin or other anticoagulants without an initial period of heparinization with LMWH were not included, since such prescriptions were unlikely to be for VTE and more likely for other indications, such as atrial fibrillation.
From these data, we determined the proportion of patients who underwent major abdominal or pelvic surgery for cancer and in whom VTE was subsequently diagnosed within 90 days after discharge from hospital. We assessed the characteristics of the VTEs and LOS; specific predictors analyzed included age, sex, type of cancer, anatomic location, TNM stage, surgical procedure (laparoscopic, open, or laparoscopic converted to open), the presence of neoadjuvant chemotherapy and/or radiation therapy, the presence of other postsurgical complications, preoperative morbidity as measured by the Charlson comorbidity index,15 hospital volume, surgeon volume and resource utilization band (RUB). An RUB is defined as a variable measuring the expected utilization of health resources, rated on a scale from none to very high, derived from the Johns Hopkins Adjusted Clinical Group (ACG) system.16
Statistical analysis
We analyzed continuous variables using the Mann–Whitney U test and categorical variables using a χ2 or Fisher exact test. Variables found to be significantly associated with morbidity or mortality on univariate analysis were analyzed using logistic regression. Rates of VTE over time were calculated using the Kaplan–Meier method, and we compared groups using the log rank test. We considered results to be significant at p < 0.05.
The sample population was one for convenience. We estimated that incidence of VTE after discharge would be about 1%.8,12,14 In order to be 95% confident that the true incidence is within ± 1%, 381 patients needed to be included in the sample. Therefore our study had more than enough power to reach its primary objective.
Results
There were 6612 patients in the Province of Manitoba who had major abdominal or pelvic surgery for solid-organ cancers between 2004 and 2009. The overall patient characteristics of this cohort are shown in Table 1. Of those 6612 patients, 202 (3.05%) had VTE within 90 days of surgery, either during or after the initial hospital stay. A VTE was diagnosed during the initial hospital stay in 106 patients (1.60%) and after discharge in 96 (1.45%) patients. Of these 96 patients, 64 were readmitted to hospital with a principal diagnosis of a new VTE within 90 days of surgery, and 32 patients received a new prescription for LMWH in the outpatient setting within 90 days of surgery. These latter patients were assumed to have had a VTE treated outside of hospital.
Table 1.
Demographic and predictor characteristics of cohort by VTE status
| Characteristic*† | Group; no. (%) or mean ± SD | ||
|---|---|---|---|
| All patients n = 6612 |
VTE in hospital n = 106 |
VTE postdischarge n = 96 |
|
| Age, yr | 65.50 ± 12.7 | 68.56 ± 13.2 | 63.13 ± 11.9 |
| Sex | |||
| Female | 3096 (46.82) | 44 (41.51) | 37 (38.54) |
| Male | 3516 (53.18) | 62 (58.49) | 59 (61.46) |
| AJCC stage | |||
| I | 1718 (25.55) | 15 (13.64) | 13 (12.62) |
| II | 2287 (34.01) | 27 (24.55) | 19 (18.45) |
| III | 1774 (26.38) | 42 (38.18) | 45 (43.69) |
| IV | 723 (10.75) | 16 (14.55) | 21 (20.39) |
| Missing | 223 (3.32) | 10 (9.09) | 5 (4.85) |
| Grade | |||
| 1 | 682 (10.14) | 6 (5.45) | 10 (9.71) |
| 2 | 3289 (48.16) | 45 (40.91) | 49 (47.57) |
| 3 | 1819 (27.05) | 37 (33.64) | 29 (28.16) |
| 4 | 189 (2.81) | 7 (6.36) | 4 (3.88) |
| Not available | 796 (11.84) | 15 (13.64) | 11 (10.68) |
| Cancer type | |||
| Nonadenocarcinoma | 385 (5.72) | 12 (10.91) | 10 (9.71) |
| Adenocarcinoma | 6340 (94.28) | 98 (89.09) | 93 (90.29) |
| Other complications | |||
| No | 5078 (75.51) | 21 (19.09) | 62 (60.19) |
| Yes | 1647 (24.49) | 89 (80.91) | 41 (39.81) |
| Surgery type | |||
| Open | 5434 (80.80) | 96 (87.27) | 89 (86.41) |
| Laparoscopic | 545 (8.10) | 5 (4.55) | 8 (7.77) |
| Other | 746 (11.09) | 9 (8.18) | 6 (5.83) |
| Primary site | |||
| Colon | 2135 (31.75) | 43 (39.09) | 31 (30.10) |
| Rectum | 955 (14.20) | 15 (13.64) | 24 (23.30) |
| Other gastrointestinal | 508 (7.55) | 9 (8.18) | 16 (15.53) |
| Female genital | 1200 (17.84) | 17 (15.45) | 13 (12.62) |
| Male genital | 1169 (17.38) | 10 (9.09) | 11 (10.68) |
| Urinary system | 758 (11.27) | 16 (14.55) | 8 (7.77) |
| Treatment before surgery | |||
| Chemotherapy and radiation | 140 (2.08) | 2 (1.82) | 5 (4.85) |
| Chemotherapy | 181 (2.69) | 2 (1.82) | 2 (1.94) |
| Radiation | 68 (1.01) | 1 (0.91) | 1 (0.97) |
| Neither | 6336 (94.22) | 105 (95.45) | 95 (92.23) |
| RUB | |||
| Very high | 2236 (33.25) | 94 (85.45) | 46 (44.66) |
| High | 2190 (32.57) | 14 (12.73) | 36 (34.95) |
| Low/moderate | 2299 (34.19) | 2 (1.82) | 21 (20.39) |
AJCC = American Joint Committee on Cancer; RUB = resource utilization band; SD = standard deviation; VTE = venous thromboembolism.
Accounts for multiple VTE diagnoses.
Denominators for tumour-specific variables are based on total number of tumours.
Among the 106 patients in whom VTE was diagnosed during the initial hospital visit, 50 patients (46.7%) had a DVT, 50 patients (46.7%) had a pulmonary embolus (PE), and 6 patients (6.5%) had both. Among the 96 patients diagnosed with VTE after discharge, 23 patients (24.0%) had DVT, 32 patients (33.3%) had PE, and 6 patients (6.3%) had both. For the remaining 35 patients (36.5%), the site could not be determined.
The median LOS for patients without VTE was 7 (range 4–11) days. For patients who had VTE in hospital, the median LOS was 19 (range 10–34) days. The median LOS for subsequent readmissions with VTE was 9 (range 6–15) days.
Predictors of postdischarge VTE on univariate analysis are shown in Table 2. Predictors were age, American Joint Committee on Cancer (AJCC) stage,17 the development of complications, noncolorectal gastrointestinal cancer, and high resource utilization band (RUB). Rectal cancer was associated with a higher risk of VTE than colon cancer. Other predictors, such as sex, cancer grade, cell type, surgery type and previous treatment, were not associated with VTE.
Table 2.
Univariable and multivariable analysis between predictors and VTE developing postdischarge
| Predictor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age, yr | 0.028 | 0.047 | ||
|
| ||||
| ≥ 70 | 0.454 (0.228 – 0.906) | 0.325 (0.158–0.666) | ||
|
| ||||
| 60–69 | 0.617 (0.311–1.226) | 0.539 (0.269–1.083) | ||
|
| ||||
| 46–59 | 0.501 (0.243–1.031) | 0.455 (0.219–0.945) | ||
|
| ||||
| 18–45 | 1.000 | 1.000 | ||
|
| ||||
| AJCC stage | < 0.001 | < 0.001 | ||
|
| ||||
| Missing | 2.963 (1.046–8.390) | 2.100 (0.708–6.228) | ||
|
| ||||
| IV | 3.838 (1.912–7.708) | 3.248 (1.583–6.663) | ||
|
| ||||
| III | 3.352 (1.802–6.236) | 3.033 (1.600–5.751) | ||
|
| ||||
| II | 1.098 (0.541–2.229) | 1.070 (0.521–2.195) | ||
|
| ||||
| I | 1.000 | 1.000 | ||
|
| ||||
| Other complications | < 0.001 | < 0.001 | ||
|
| ||||
| Yes | 2.039 (1.369–3.037) | 1.748 (1.110–2.752) | ||
|
| ||||
| No | 1.000 | 1.000 | ||
|
| ||||
| Primary ste | 0.002 | 0.004 | ||
|
| ||||
| Other | 0.705 (0.429–1.158) | 0.843 (0.494–1.438) | ||
|
| ||||
| Other gastrointestinal | 2.171 (1.179–3.999) | 1.895 (0.994–3.613) | ||
|
| ||||
| Rectum | 1.731 (1.010–2.965) | 1.586 (0.92–2.736) | ||
|
| ||||
| Colon | 1.000 | 1.000 | ||
|
| ||||
| RUB | < 0.001 | < 0.001 | ||
|
| ||||
| Very high | 1.620 (1.095–2.397) | 1.204 (0.762–1.901) | ||
|
| ||||
| Low/moderate/high | 1.000 | 1.000 | ||
AJCC = American Joint Committee on Cancer; CI = confidence interval; OR = odds ratio; RUB = resource utilization band; VTE = venous thromboembolism.
Predictors of postdischarge VTE on multivariate analysis are also shown in Table 2. Independent predictors were stage, RUB, development of complications, primary site of cancer and age.
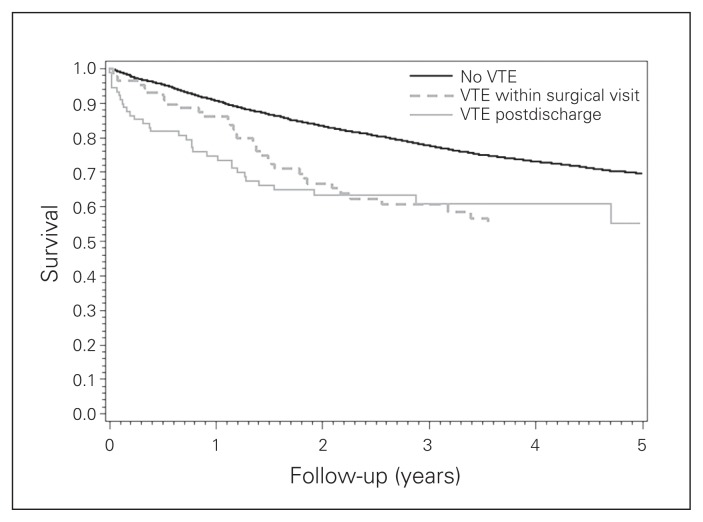
The development of VTE was associated with reduced 5-year survival (Fig. 1). The 5-year survival for those without VTE was 69.41%, whereas that for patients with VTE was 53.88% (p < 0.001).
Fig. 1.
Kaplan–Meier survival curve 90 days postsurgery, 5-year survival. The development of venous thromboembolism (VTE) was associated with reduced 5-year survival. The 5-year survival for those without VTE was 69.41%, while the 5-year survival for those in whom VTE developed was 53.88% (p < 0.001).
Discussion
In the present study the cumulative incidence of VTE was 3.01% (1.60% during the initial hospital stay and 1.45% after discharge), which is similar to that in other reports.18,19 Another finding of our study was the decreased 5-year overall survival associated with the development of VTE. This result has been found by others,18 but does not prove causation as it is possible that more biologically aggressive tumours that would be expected to have a poorer prognosis anyway were also associated with VTE formation.
The existing literature has some limitations that may be partly responsible for the practice of extended thromboprophylaxis not having been uniformly adopted. The ENOXACAN II study12 found a relative risk reduction of 60% (p = 0.02); however, the benefit of extended treatment was predominantly in the reduction of asymptomatic distal DVT, which is of questionable significance. Another major limitation was that only about half of the patients recruited for the study were accounted for in the outcome assessment. The presence of a few additional events among the lost patients in either group could have had dramatic effects on the results either way. The FAME study8 found a relative risk reduction of 55%, but again the number of patients who dropped out was significant. In a third study, the number of patients who were included in the outcome assessment was only one-third of the total.13 The study did not demonstrate a statistically significant benefit.
Recently, the Cochrane Collaboration published a meta-analysis of trials evaluating prolonged thromboprophylaxis for abdominal or pelvic surgery.14 This meta-analysis included the 3 trials listed above8,12,13 plus a fourth study that was published only as an abstract.20 By combining the studies, the meta-analysis demonstrated a significant reduction in overall episodes of VTE (14.3% in the control group v. 6.1% in the treatment group, OR 0.41, 95% confidence interval [CI] 0.26–0.63). There was also a significant decrease in proximal DVT, with an incidence of 5.1% in the control group compared with 1.1% in the treatment group (OR 0.27, 95% CI 0.13–0.57). Importantly, the Cochrane review did find a significant decrease in symptomatic VTE. The incidence of symptomatic VTE in the control group was 1.8% (8 of 455 patients) compared with 0.22% (1 of 446 patients) in the treatment group (OR 0.22, 95% CI 0.06–0.80). Still, this must be interpreted cautiously because owing to the large number of patients lost to follow-up in the studies and the low number of symptomatic VTEs, only a few additional events in either group could have resulted in quite different conclusions.
Our study found that 1.45% of patients undergoing major abdominal or pelvic oncologic surgery had a VTE diagnosed after discharge and could have potentially benefitted from extended VTE prophylaxis. Assuming the same risk reduction as the Cochrane review,14 a number needed to treat of 117 can be calculated.
One of the biggest unresolved dilemmas is how much of a reduction would justify introducing thromboprophylaxis for up to 28 days and whether the risks outweigh the benefits. However, the existing literature has not reported an increased bleeding risk,8,12–14,21 and the consequences of VTE can be lethal. In the Cochrane meta-analysis,14 the rate of bleeding events in the treatment group was 4.1% (25 of 614 patients) compared with 3.7% (23 of 628 patients) in the control group. This risk was not significant (p = 0.73), although it is possible that there is a very small risk of bleeding for which the existing trials and meta-analyses were underpowered to detect.
Another dilemma is determining which patients might derive the greatest benefit from extended thromboprophylaxis in order to guide treatment decisions. All patients undergoing major abdominal or pelvic surgery for cancer are considered to be at high risk for VTE.22 We found that patients with noncolorectal gastrointestinal cancers, advanced stage of disease and postoperative complications and those requiring a higher intensity of nursing care were at even higher risk for VTE. Other studies have identified additional risk factors, such as advanced age, higher Charlson comorbidity score, prior VTE, sepsis and longer LOS.23–25 Unlike others, we did not find an association between older age and VTE; in fact, younger age was associated with increased odds of presenting with VTE after discharge. The reason for this result is not clear. It may be that younger patients underwent more extensive surgical procedures, which was not fully controlled for in the multivariate analysis. Also, we assumed that all patients received VTE prophylaxis with heparin in the postoperative period, but perhaps some younger patients were deemed to be at lower risk and did not receive heparin.
Limitations
This study has several important limitations. First, we assumed that patients received appropriate VTE prophylaxis during their initial postoperative stay. This would have consisted of a single preoperative dose of unfractionated heparin (UFH) or LMWH continuing with subcutaneous doses postoperatively.26 Others have shown that VTE prophylaxis is underutilized in patients undergoing oncologic surgery.27 Second, we assumed that new outpatient prescriptions for LMWH were for newly diagnosed VTEs that did not require hospitalization. It is possible that some of these prescriptions were for other indications and the true number of postdischarge VTEs was overestimated. Third, the proportion of DVTs related to central venous catheters could not be determined from the available data. A fourth limitation of the study is the inability to determine whether the surgical procedures were done with a curative or palliative goal, or if they were done in an elective or emergency setting. These factors may have important implications in the decision whether or not to offer extended thromboprophylaxis, but we could not determine their influence on the incidence of VTE.
Perhaps the biggest limitation of this study is that the true proportion of how many of these VTEs diagnosed within 90 days of hospital discharge could have been prevented is not known. It is not known whether the same risk reduction seen in the Cochrane review14 would have been found in these patients. Some of the VTEs diagnosed after discharge may have formed before discharge while patients were still receiving standard prophylaxis and may not have been preventable with longer prophylaxis. In addition, we used a cut off of 90 days from discharge to determine VTEs that might have been preventable with extended prophylaxis. This arbitrary cut-off was chosen to capture patients in whom a VTE might have developed within 28 days of surgery, but were not diagnosed until later and may have resulted in an overestimate.
This study was designed to determine the number of patients who might have had a preventable VTE. It makes the assumption that most of the 1.45% of patients with postdischarge VTE could have benefited. Although the percentage who might benefit is small, if the majority of these patients might benefit, extended prophylaxis should be considered, especially in high-risk patients. This remains an area where further research is needed and is ongoing.28,29
Conclusion
The cumulative incidence of VTE within 90 days of major abdominopelvic oncologic surgery was 3.01%; of those patients 1.45% had VTE diagnosed within 90 days after hospital discharge. Predictors of VTE after discharge were advanced AJCC stage, the development of postoperative complications, a high RUB, noncolorectal gastrointestinal cancer, and age 45 years or younger. The development of VTE was associated with longer LOS and reduced overall survival. Presumably, the patients (1.45%) in whom VTE was diagnosed after discharge may have benefited most from extended thromboprophylaxis for 28 days after hospital discharge. Although the benefit is small, extended prophylaxis should be considered, especially in high-risk patients.
Footnotes
Competing interests: None declared.
Contributors: H. Alsubaie, D. Hochman and A. McKay designed the study. H. Alsubaie, C. Leggett, P. Lambert and A. McKay acquired the data, which all authors analyzed. H. Alsubaie and A. McKay wrote the article, which all authors reviewed and approved for publication.
References
- 1.Huse DM, Cummins G, Taylor DC, et al. Outpatient treatment of venous thromboembolism with low-molecular-weight heparin: an economic evaluation. Am J Manag Care. 2002;8(Suppl):S10–6. [PubMed] [Google Scholar]
- 2.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 3.Negus JJ, Gardner JJ, Tann O, et al. Thromboprophylaxis in major abdominal surgery for cancer. Eur J Surg Oncol. 2006;32:911–6. doi: 10.1016/j.ejso.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Galster H, Kolb G, Kohsytorz A, et al. The pre-, peri-, and postsurgical activation of coagulation and the thromboembolic risk for different risk groups. Thromb Res. 2000;100:381–8. doi: 10.1016/s0049-3848(00)00342-x. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen MS. Prolonged thromboprophylaxis with low molecular weight heparin after major abdominal surgery. Curr Opin Pulm Med. 2007;13:389–92. doi: 10.1097/MCP.0b013e3282058ba6. [DOI] [PubMed] [Google Scholar]
- 6.Scurr JH, Coleridge-Smith PD, Hasty JH. Deep venous thrombosis: a continuing problem. BMJ. 1988;297:28. doi: 10.1136/bmj.297.6640.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sørensen C, Andersen M, Kristiansen VB, et al. The occurrence of late thromboembolic complications after elective abdominal surgery. Ugeskr Laeger. 1990;152:1586–7. [PubMed] [Google Scholar]
- 8.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–90. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol. 2007;95:167–74. doi: 10.1002/jso.20625. [DOI] [PubMed] [Google Scholar]
- 10.Negus JJ, Gardner JJ, Tann O, et al. Thromboprophylaxis in major abdominal surgery for cancer. Eur J Surg Oncol. 2006;32:911–6. doi: 10.1016/j.ejso.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen MS, Wille-Jorgensen P, Jorgensen LN. Postoperative fatal pulmonary embolism in a general surgical department. Am J Surg. 1995;169:214–6. doi: 10.1016/S0002-9610(99)80139-1. [DOI] [PubMed] [Google Scholar]
- 12.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–80. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 13.Lausen I, Jensen R, Jorgensen LN, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: randomised controlled study of prolonged thromboprophylaxis. Eur J Surg. 1998;164:657–63. doi: 10.1080/110241598750005534. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;(1):CD004318. doi: 10.1002/14651858.CD004318.pub2. (1) [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.The Johns Hopkins University ACG case-mix Adjustment System [computer program] Johns hopkins university school of hygiene and public health.version 9.0. baltimore, MD: Johns hopkins university; 2012. [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 18.Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg. 2011;254:131–7. doi: 10.1097/SLA.0b013e31821b98da. [DOI] [PubMed] [Google Scholar]
- 19.Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the national surgical quality improvement program database. Dis Colon Rectum. 2010;53:1355–60. doi: 10.1007/DCR.0b013e3181eb9b0e. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen LN, Lausen I, Rasmussen MS, et al. Prolonged thromboprophylaxis with low-molecular weight heparin following major general surgery: an individual patient data meta-analysis. Blood. 2002;100 [Google Scholar]
- 21.Raftopoulos I, Martindale C, Cronin A, et al. The effect of extended post-discharge chemical thromboprophylaxis on venous thromboembolism rates after bariatric surgery: a prospective comparison trial. Surg Endosc. 2008;22:2384–91. doi: 10.1007/s00464-008-0031-9. [DOI] [PubMed] [Google Scholar]
- 22.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e227S–77S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahl V, Jacob P, III, Havel C, et al. Thirdhand cigarette smoke: factors affecting exposure and remediation. PLoS ONE. 2014;9:e108258. doi: 10.1371/journal.pone.0108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spyropoulos AC, Hussein M, Lin J, et al. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951–7. doi: 10.1160/TH09-02-0073. [DOI] [PubMed] [Google Scholar]
- 25.Bahl V, Hu HM, Henke PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251:344–50. doi: 10.1097/SLA.0b013e3181b7fca6. [DOI] [PubMed] [Google Scholar]
- 26.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 27.Wright JD, Lewin SN, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Ann Surg. 2011;253:1140–6. doi: 10.1097/SLA.0b013e31821287ac. [DOI] [PubMed] [Google Scholar]
- 28.Auer R, Scheer A, Wells PS, et al. The use of extended perioperative low molecular weight heparin (tinzaparin) to improve disease-free survival following surgical resection of colon cancer: a pilot randomized controlled trial. Blood Coagul Fibrinolysis. 2011;22:760–2. doi: 10.1097/MBC.0b013e328349f1a8. [DOI] [PubMed] [Google Scholar]
- 29.Carrier M, et al. ClinicalTrials.gov, NCT01455831. Oct, 2011. Extended peri-operative tinzaparin to improve disease-free survival in patients with resectable colorectal cancer (PERIOP-01) [Google Scholar]