Abstract
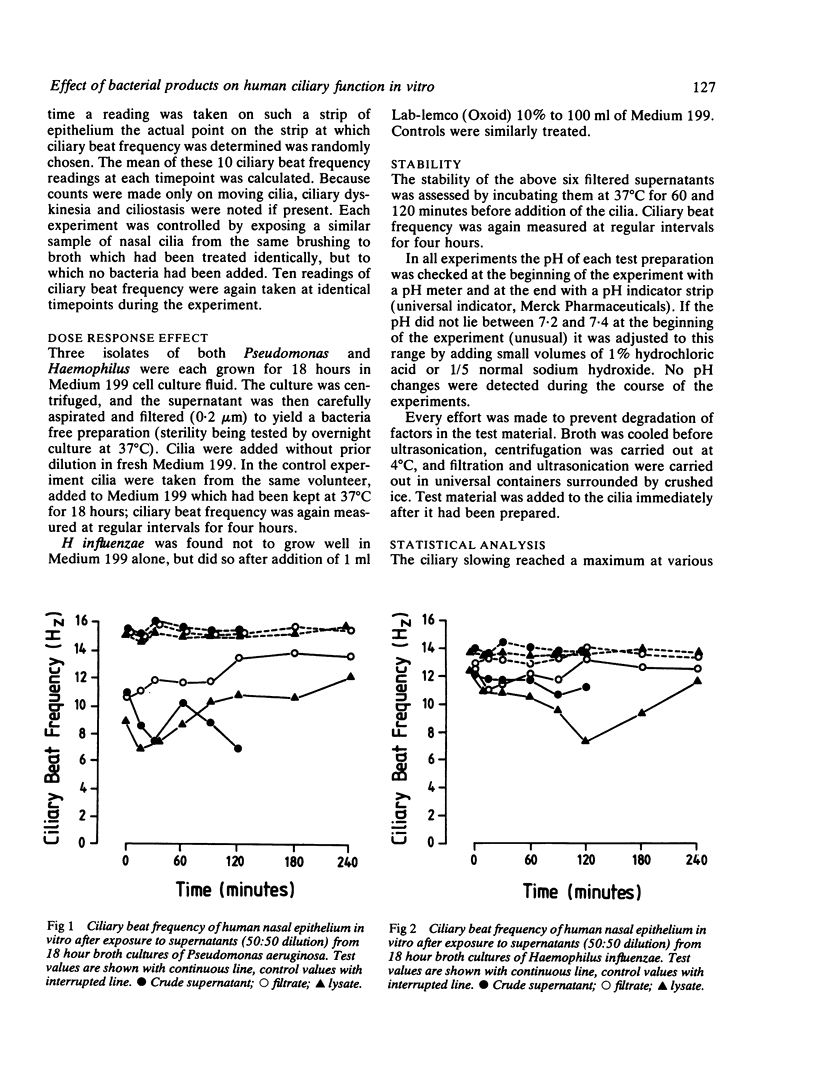
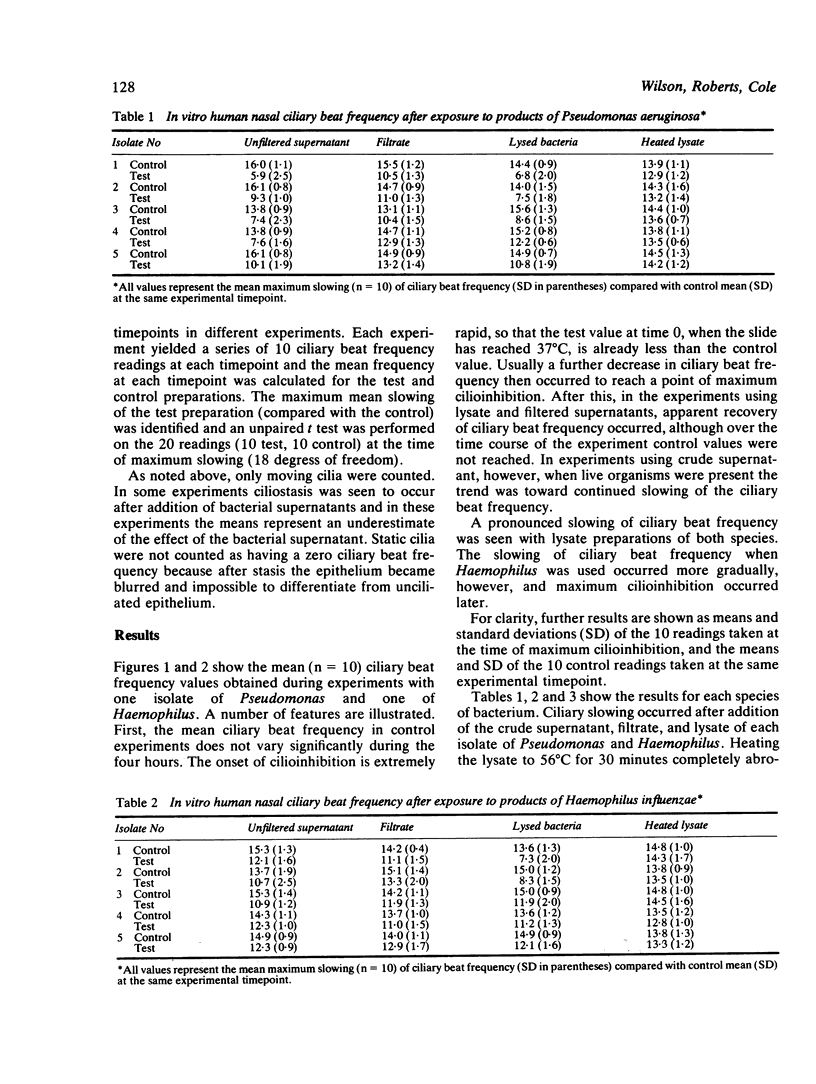
Ciliary activity protects the respiratory tract against inhaled particles, including bacteria, by transporting them trapped in mucus towards the pharynx. We have studied the effect of bacteria (Haemophilus influenzae, Staphylococcus aureus, and Pseudomonas aeruginosa) on human nasal cilia, measuring their in vitro ciliary beat frequency by a photometric technique. Supernatant fluids were obtained from 18 hour broth cultures by centrifugation alone, by filtration, and by lysis. Supernatants obtained from Ps aeruginosa and H influenzae caused a significantly lower ciliary beat frequency than controls (broth alone). Slowed cilia were dyskinetic and at times of maximal slowing ciliostasis occurred in some areas of the epithelium. A dose related effect was demonstrated. Abrogation of cilioinhibitory properties was achieved by heating the lysate to 56 degrees C for 30 minutes and by allowing the filtrate to stand at 37 degrees C for 120 minutes. Staphylococcal products were not cilioinhibitory. It is concluded that Ps aeruginosa and H influenzae release a factor (or factors) which causes slowing of human nasal cilia in vitro. The role of this factor in the pathogenesis of infection is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cash H. A., Straus D. C., Bass J. A. Pseudomonas aeruginosa exoproducts as pulmonary virulence factors. Can J Microbiol. 1983 Apr;29(4):448–456. doi: 10.1139/m83-072. [DOI] [PubMed] [Google Scholar]
- Denny F. W. Effect of a toxin produced by Haemophilus influenzae on ciliated respiratory epithelium. J Infect Dis. 1974 Feb;129(2):93–100. doi: 10.1093/infdis/129.2.93. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Lanyon H., Cole P. J. Use of lysostaphin to remove cell-adherent staphylococci during in vitro assays of phagocyte function. Br J Exp Pathol. 1978 Aug;59(4):381–385. [PMC free article] [PubMed] [Google Scholar]
- Lourenço R. V., Loddenkemper R., Carton R. W. Patterns of distribution and clearance of aerosols in patients with bronchiectasis. Am Rev Respir Dis. 1972 Dec;106(6):857–866. doi: 10.1164/arrd.1972.106.6.857. [DOI] [PubMed] [Google Scholar]
- Roberts D. E., Cole P. Use of selective media in bacteriological investigation of patients with chronic suppurative respiratory infection. Lancet. 1980 Apr 12;1(8172):796–797. doi: 10.1016/s0140-6736(80)91295-7. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Smallman L. A., Hill S. L., Stockley R. A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984 Sep;39(9):663–667. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley R. A., Burnett D. Serum derived protease inhibitors and leucocyte elastase in sputum and the effect of infection. Bull Eur Physiopathol Respir. 1980;16 (Suppl):261–272. doi: 10.1016/b978-0-08-027379-2.50027-2. [DOI] [PubMed] [Google Scholar]
- Tegner H., Ohlsson K., Toremalm N. G., von Mecklenburg C. Effect of human leukocyte enzymes on tracheal mucosa and its mucociliary activity. Rhinology. 1979 Sep;17(3):199–206. [PubMed] [Google Scholar]



