Abstract
Arteriolar vasoreactivity tightly regulates tissue-specific blood flow and contributes to systemic blood pressure (BP) but becomes dysfunctional in the setting of cardiovascular disease. The mineralocorticoid receptor (MR) is known to regulate BP via the kidney and by vasoconstriction in smooth muscle cells. Although endothelial cells (EC) express MR, the contribution of EC-MR to BP and resistance vessel function remains unclear. To address this, we created a mouse with MR specifically deleted from EC (EC-MR-KO) but with intact leukocyte MR expression and normal renal MR function. Telemetric BP studies reveal no difference between male EC-MR-KO mice and MR-intact littermates in systolic, diastolic, circadian, or salt-sensitive BP or in the hypertensive responses to aldosterone +/− salt or angiotensin II (AngII) +/− L-NAME. Vessel myography demonstrated normal vasorelaxation in mesenteric and coronary arterioles from EC-MR-KO mice. After exposure to AngII-induced hypertension, impaired endothelial-dependent relaxation was prevented in EC-MR-KO mice in mesenteric vessels but not in coronary vessels. Mesenteric vessels from AngII-exposed EC-MR-KO mice showed increased maximum responsiveness to Ach compared to MR-intact vessels, a difference that is lost with indomethacin+L-NAME pretreatment. These data support that EC-MR plays a role in regulating endothelial function in hypertension. Although there was no effect of EC-MR deletion on mesenteric vasoconstriction, coronary arterioles from EC-MR-KO mice showed decreased constriction to endothelin-1 and thromboxane agonist at baseline and also after exposure to hypertension. These data support that EC-MR participates in regulation of vasomotor function in a vascular bed-specific manner that is also modulated by risk factors such as hypertension.
Keywords: endothelial cells, mineralocorticoid receptor, coronary function, blood pressure, endothelin-1
Introduction
Ample clinical trial data reveal that mineralocorticoid receptor (MR) antagonist (MRA) drugs decrease blood pressure (BP) and improve survival in systolic congestive heart failure (CHF)1;2;2;3. It is well established that MRAs prevent the hormone aldosterone from activating renal MRs thereby decreasing sodium and water retention4. More recently, it has become clear that MR is expressed in tissues outside of the kidney including in the cardiovascular system. In the vasculature, MR is expressed in medial smooth muscle cells (SMCs)5 and in intimal endothelial cells (ECs)6. SMCs and ECs function in concert to regulate arteriolar diameter, thereby globally controlling vascular resistance to contribute to systemic BP and locally modulating regional tissue blood flow7. Since the benefits of MRAs are disproportionately greater than their natriuretic properties, it has been postulated that some of their beneficial effects may be mediated by inhibition of vascular MR8 by mechanisms that are only beginning to be elucidated.
Recent studies in mice specifically deficient in MR in SMC have demonstrated that SMC-MR contributes directly to regulation of systemic BP and to vasoconstriction9–11. Despite substantial effort, our understanding of the specific role of EC-MR in BP control and vasoreactivity has been more elusive. In obese rats, MR inhibition improves coronary endothelial-dependent vasodilation and MR activation in healthy rats impairs coronary endothelial-dependent vasodilation12. In humans, MRA treatment improves brachial artery vasodilation in patients with CHF13 and improves coronary flow reserve in diabetics14 suggesting a role for endothelial MR in regulating vascular function in patients with cardiovascular disease or risk factors. Early studies exploring potential MR signaling mechanisms in cultured ECs revealed disparate effects of MR activation on endothelial nitric oxide synthase (eNOS) activity with reports of both MR-dependent inhibition and activation of eNOS activity or NO production (reviewed in 8;15). Likewise, extensive experimentation has been performed in isolated vessels with variable effects of MR activation on vasoconstriction and vasodilation that may depend on the species, vascular bed, or experimental strategy employed8. Overall, the data support a role for vascular MR in vasomotor control yet the specific role of EC-MR is unclear from such studies in which the MR is activated or inhibited in SMCs and ECs simultaneously.
To examine the role of EC-MR in BP regulation and vasoreactivity, recent studies have utilized transgenic animals in which MR expression was specifically modulated in the endothelium. Mice overexpressing human MR in ECs have elevated BP and increased mesenteric myogenic tone and constriction, with no change in mesenteric endothelial-dependent vasodilation16. However, two groups recently deleted MR from ECs using a Tie2 promoter strategy revealing no difference in basal BP or vasoconstriction17;18. Using this model, Rickard et. al. demonstrated decreased aortic and mesenteric endothelial-dependent relaxation17 while Schafer et. al. found no change in aortic endothelial function in healthy animals but protection from obesity-associated aortic endothelial dysfunction18. In addition to these conflicting results, these EC-MR knockout (EC-MR-KO) mouse models are complicated by MR deletion from leukocytes due to expression of the Tie2 promoter in bone marrow derived cells18. This has made interpretation of the specific role of EC-MR in this model difficult in light of the recent identification of a role for leukocytes in BP regulation19 and a role for MR in modulating leukocyte function20.
To clarify our understanding of the role of EC-MR in BP regulation and vasoreactivity, we generated an EC-MR-KO mouse with MR specifically deleted from ECs but with intact leukocyte MR using the VE-Cadherin (VE-Cad) promoter driving Cre-recombinase. Using this model, telemetry studies were performed to examine the role of EC-MR in BP regulation under normal ambulatory conditions and in response to sodium loading/restriction and to renin-angiotensin-aldosterone system (RAAS) activation. The role of EC-MR in endothelial-dependent relaxation and in vasoconstriction to multiple contractile agonists was explored in mesenteric and coronary arterioles to address the potential role of EC-MR in distinct vascular beds. The contribution of EC-MR to arteriolar vasoconstriction and to endothelial dysfunction after exposure to AngII hypertension was also examined in the model.
Methods
Mice were handled in accordance with US National Institutes of Health standards and all procedures were approved by the Institutions Animal Care and Use Committees. All experiments were conducted using male mice on the C57Bl/6 background and EC-MR-KO mice are compared to MR-intact littermate controls. Primers are listed in Table S1. Detailed Methods are available in the online-only Data Supplement.
Results
Creating a mouse model with MR specifically deleted from endothelial cells and intact in leukocytes
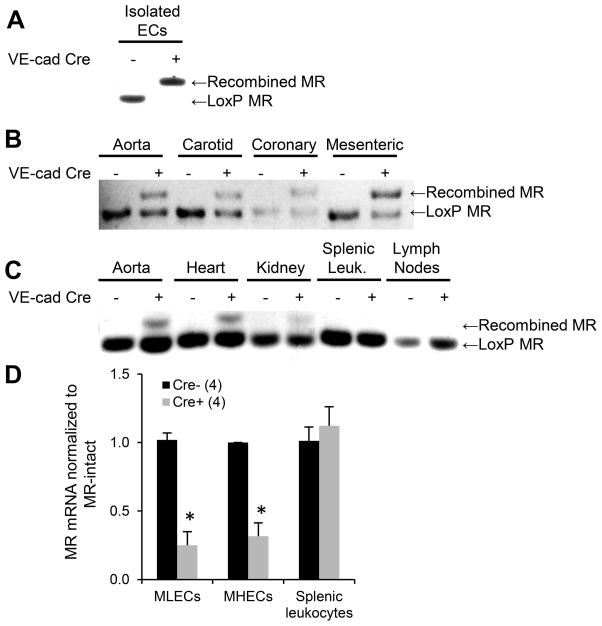
Mice with loxP sites flanking exons 5 and 6 of the MR gene (MRf/f)10 were bred with mice containing a Cre-recombinase transgene driven by the EC-specific VE-cadherin promoter21 (Cre+). EC-specific recombination of the MR gene was confirmed by PCR (Figure 1). Cultured ECs isolated from Cre+ mice showed complete MR DNA recombination while ECs from Cre− littermates showed no MR gene recombination (Figure 1A). DNA isolated from the aorta and from carotid, coronary and mesenteric arterioles shows MR recombination in all vessels but only from Cre+ mice (Figure 1B). DNA isolated from aorta, heart, and kidney of Cre+ mice revealed MR recombination consistent with the expected contribution of ECs in each tissue, while splenic leukocytes and lymph nodes contained only loxP MR, confirming lack of MR gene recombination in immune cells (Figure 1C). MR mRNA was also significantly decreased in primary cultured ECs from mouse lung and heart of Cre+ compared to Cre− mice. MR mRNA expression in splenic leukocytes was unchanged in Cre+ mice (Figure 1D). These data confirm that the MR gene is specifically recombined in ECs in this mouse model with depletion of MR mRNA in ECs but not in immune cells (protein was not measured due to lack of specific mouse MR antibodies). The mice were born in Mendelian frequencies with no gross developmental differences supporting the conclusion that EC-MR is not necessary during embryologic development (Table S2).
Figure 1. A mouse model with MR deleted specifically from EC and not leukocytes.
(A–C) MR genomic DNA was amplified with primers specific for the LoxP MR or recombined MR. (A) The MR gene is completely recombined in primary cultured mouse ECs only from Cre+ mice. (B) MR recombination occurs in Cre+ aorta, carotid, coronary, and mesenteric vessels. (C) MR recombination occurs only in EC-containing tissues in VE-cad-Cre+ mice and not in lymph nodes or splenic leukocytes (Leuk.) (D) MR mRNA is reduced in primary ECs cultured from mouse lungs (mouse lung endothelial cells, MLECs) and hearts (MHECs) but not in leukocytes from EC-MR-KO mice. Number of animals per group is indicated in parentheses. *p<0.05 versus MR-intact.
EC-MR does not contribute to basal, diurnal, salt-sensitive, or RAAS-regulated blood pressure
To clarify the role of EC-MR in BP control, EC-MR-KO and MR-intact littermates were implanted with radiotelemetry devices for continuous ambulatory BP measurements. Specific deletion of EC-MR did not affect systolic, diastolic, or mean BP, heart rate, or activity level over the 24-hour cycle (Figure 2A and S1). Systolic BP was unchanged from 4 to 8 months of age in EC-MR-KO mice (Figure 2B). Since MR in the kidney contributes to BP control in response to varying sodium conditions22, salt sensitivity of BP was assessed. The modest BP decrease with low sodium intake and the increase with high sodium intake was equivalent in both genotypes (Figure 2C) with no change in serum and urine electrolytes and food and water consumption (Table S2). Fractional excretion of sodium (FENa+) and the expected decline in FENa+ on a low sodium diet were the same in both genotypes, thereby confirming intact renal MR function in EC-MR-KO mice (Figure 2D). We next investigated whether EC-MR participates in the BP response to RAAS activation, a common contributor to hypertension22. BP increased to a similar extent in EC-MR-KO and MR-intact mice in response to aldosterone infusion with a further increase with addition of 1% sodium in the drinking water as did the response to AngII infusion that is also further enhanced by addition of the eNOS inhibitor L-nitroarginine methyl ester (L-NAME) (Figures 2E and 2F).
Figure 2. EC-MR does not contribute to basal, salt sensitive, aldosterone/salt-enhanced, or angiotensin-enhanced BP.
(A) 24-hour ambulatory blood pressure in healthy 6-month old mice. (B) Average systolic blood pressure (SBP) at 4, 6 and 8 months of age in MR-intact and EC-MR-KO mice. (C) Average SBP in 5–6 month old mice on days 3–5 of normal, low, or high salt diets. *p<0.05 versus normal diet; #p<0.05 versus low salt diet. (D) Fractional excretion of sodium (FENa+) on normal and low-salt diet. *p<0.05 versus normal diet. (E–F) SBP assessment in hypertension models in 7–8 month old mice. (E) SBP on day 4 of aldosterone infusion; day 9 of aldosterone+1% NaCl in water.*p<0.05 versus baseline; #p<0.05 versus aldosterone alone. (F) BP on day 7 of AngII infusion; and on day 14 of AngII+L-NAME.*p<0.05 versus baseline; #p<0.05 versus AngII alone. Number of animals per group is indicated in parentheses.
EC-MR deletion does not affect basal mesenteric or coronary arteriolar vasodilation
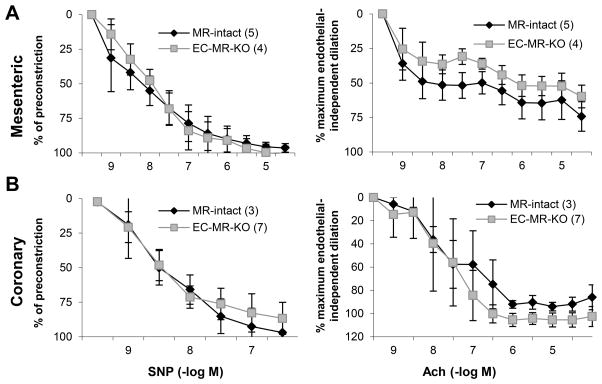
To address conflicting findings regarding the role of EC-MR in vasodilation, we examined endothelium-independent vasodilation to sodium nitroprusside (SNP) and endothelium-dependent relaxation to acetylcholine (Ach) in mesenteric and coronary arterioles. These vessels were chosen because mesenteric vasoreactivity contributes to regional blood flow and systemic vascular resistance and coronary vascular function is critical to coronary flow reserve, an important predictor of cardiovascular outcomes that was recently found to be modulated by MR antagonist treatment14. Wire myography revealed no difference in endothelium-independent or endothelium-dependent vasodilation in mesenteric or coronary arterioles from EC-MR-KO mice compared to MR-intact littermates (Figure 3).
Figure 3. EC-MR does not contribute to mesenteric or coronary vasodilation.
Sodium nitroprusside (SNP) and acetylcholine (Ach) dose-response curves were assessed by wire myography in (A) Mesenteric arterioles pre-constricted with PE (1 μM) and (B) Coronary arterioles pre-constricted with U46619 (0.1–0.3 μM). Number of animals per group is indicated in parentheses.
EC-MR modulates mesenteric endothelial-dependent vasodilation in the setting of angiotensin II-induced hypertension
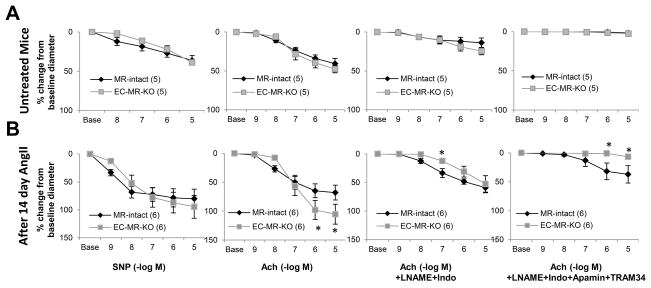
Pressure myography was also used to assess endothelial-dependent vasodilation in EC-MR-KO mice compared to controls at baseline and after exposure to two weeks of AngII hypertension (Figure 4). Differences in the COX1- and eNOS-dependent component (as measured after indomethacin and L-NAME inhibition) and in the endothelium-derived hyperpolarizing factor (EDHF)-dependent component (inhibited by SKca channel inhibitor Apamin and IKca channel inhibitor TRAM34) were also examined. Figure 4A confirms in pressurized vessels that in healthy animals, EC-MR does not contribute to vasodilation to SNP or Ach. Moreover, no difference was observed between the genotypes in the non-EDHF or EDHF contributions to vasodilation (Figure 4A) or in individual COX1- and eNOS-dependent components (Figure S2). However, after 14 day infusion with a pressor dose of AngII (same dose as in Figure 2F), EC-MR-KO vessels achieved a significantly greater maximal vasodilation compared to MR-intact controls in the absence of inhibitors (Figure 4B). There was equivalent preconstriction to phenylephrine (Figure S3) with no difference between genotypes in endothelial-independent vasodilation (Figure 4B). The improvement in endothelial function in EC-MR-KO mesenteric vessels was eliminated by pretreatment with L-NAME+indomethacin, supporting that EC-MR-KO maintains the Ach response after AngII-hypertension by enhancing eNOS or COX1 production of paracrine vasodilators. After L-NAME and indomethacin pretreatment, EC-MR-KO vessels displayed decreased dilation to 10−7M Ach suggesting a difference in EDHF. Addition of Apamin+TRAM34 eliminated vasodilation to Ach in EC-MR-KO vessels supporting that this is indeed due to an EDHF-mediated mechanism. Interestingly, in the MR-intact mice, there was residual vasodilation with all four inhibitors that may represent an additional EC-MR regulated mechanism of endothelial-dependent vasodilation that is activated in response to AngII hypertension.
Figure 4. EC-MR deletion modulates mesenteric endothelial-dependent relaxation after exposure to AngII-induced hypertension.
Mesenteric arterioles were isolated from untreated mice (A) or mice exposed to 14-day AngII infusion (B), and the change in vessel diameter after pre-constriction with PE (100 nM) was assessed by pressure myography. Relaxation-response curves are shown to sodium nitroprusside (SNP), Acetylcholine (Ach), Ach+L-NAME+indomethacin (Indo), or Ach+L-NAME+Indo+Apamin+TRAM34. Number of animals per group is indicated in parentheses. *p<0.05 versus MR-intact.
Contrary to the mesenteric vessels, after exposure to AngII hypertension, coronary endothelium-dependent vasodilation was not different between genotypes (Figure S4), supporting vascular bed-specific differences in the role of EC-MR in hypertension-induced alterations in endothelial function.
EC-MR differentially modulates vasoconstriction in a vascular bed-specific fashion
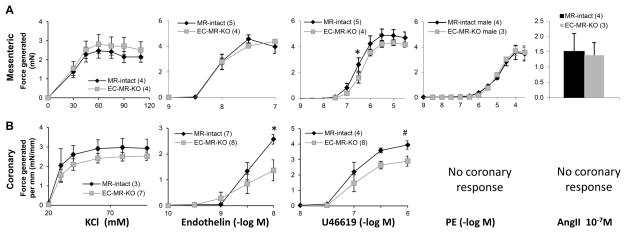
To clarify conflicting findings for the role of EC-MR in vasoconstriction8, we assessed the specific role of EC-MR in the responses of mesenteric and coronary arterioles to various contractile agonists. In mesenteric vessels, there was no difference in constriction between EC-MR-KO and MR-intact controls to potassium chloride (KCl), endothelin-1, PE, or AngII with a decrease in constriction only to the thromboxane agonist U46619 in EC-MR-KO at 3×10−7M (Figure 5A). Mesenteric myogenic constriction in response to increasing intraluminal pressure was also measured revealing no significant differences between EC-MR-KO and MR-intact mesenteric vessels in the level of myogenic tone (Figure S5), consistent with the lack of a difference in BP. Interestingly, coronary vessels from EC-MR-KO mice showed a decrease in constriction to U46619 (26% decrease at a dose of 10−6M) and a pronounced decrease in constriction to endothelin-1 (49% decrease at 10−8M) compared to vessels from MR-intact littermates (Figure 5B). These data support the concept that EC-MR differentially contributes to vasoconstriction depending on the vascular bed and on the contractile stimulus.
Figure 5. EC-MR differentially regulates vasoconstriction depending on the vascular bed and the contractile agonist.
Dose-response curves for contractile force were recorded by wire myography in (A) mesenteric arterioles in response to KCl, endothelin-1, the thromboxane agonist U46619, phenylephrine (PE), or a single dose of AngII; and in (B) coronary arterioles (normalized to length of the vessel segment) to KCl, endothelin-1, and U46619. No graph is included for PE and AngII as coronary arterioles did not respond to those agonists. Number of animals per group is indicated in parentheses. *p<0.05, #p<0.005 versus EC-MR-KO.
EC-MR also contributes to coronary vasoconstriction to endothelin-1 and thromboxane after hypertension and to expression of endothelin-B receptor in coronary ECs
Coronary vasoconstriction to endothelin-1 and thromboxane was also measured after 14 day exposure to AngII hypertension. The decreased coronary contractile response to endothelin-1 and thromboxane in EC-MR-KO mice persisted after exposure to hypertension and was significant over a greater range of agonist concentrations after exposure to AngII (Figure 6A and 6B).
Figure 6. Decreased coronary vasoconstriction to endothelin-1 and U46619 after hypertension and decreased EC endothelin-B receptor mRNA in EC-MR-KO mice.
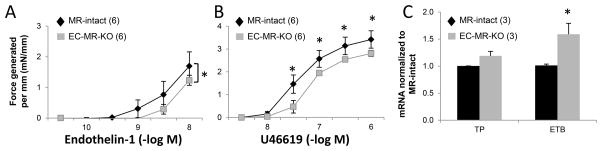
Coronary vasoconstriction was assessed by wire myography after 14 day AngII infusion to a dose escalation of (A) endothelin-1 and (B) thromboxane agonist U46619. *p<0.05 versus MR-intact. (C) Expression of the thromboxane receptor (TP) and the endothelin-B receptor (ETB) were measured in mRNA isolated from mouse cardiac endothelial cells. Number of animals or experiments is indicated in parentheses. *p<0.01 versus MR-intact.
Several potential mechanisms for decreased coronary vasoconstriction in EC-MR-KO mice were considered. The contractile differences were not due to alterations in coronary vasodilation at baseline (Figure 3) or after AngII exposure (Figure S4). SMC L-type calcium channels (LTCC) are required for vascular constriction however, single cell patch-clamp recordings in freshly dispersed coronary (and mesenteric) SMC revealed no difference in LTCC function in SMCs from EC-MR-KO mice (Figure S6). Expression levels of the thromboxane receptor and of the vasodilatory endothelin-B receptor (ETB; known to be expressed on ECs) were measured mRNA from primary mouse heart endothelial cells. ETB expression was significantly increased (59%) in coronary ECs from EC-MR-KO compared to MR-intact mice (Figure 6C), supporting the possibility that EC-MR contributes to coronary vasoconstriction in response to endothelin-1 by regulating coronary endothelial ETB expression. Thromboxane receptor (TP) mRNA was unchanged in coronary ECs from EC-MR-KO mice suggesting a different mechanism for the enhanced coronary constriction to U46619 in mice deficient in EC-MR.
Discussion
In summary, we developed a truly EC-specific MR-KO mouse model with intact immune cell MR to clarify the specific role of EC-MR in BP regulation and vascular function. Using this model, we demonstrated that: 1) EC-MR deletion does not alter renal sodium handling, basal BP, or BP after diverse hypertensive stimuli; 2) EC-MR does not contribute to basal endothelium-dependent relaxation in mesenteric or coronary arterioles; 3) EC-MR deletion improves mesenteric (but not coronary) endothelial function after exposure to AngII-hypertension and; 4) EC-MR differentially regulates vasoconstriction in distinct vascular beds, enhancing the responsiveness of coronary arterioles to endothelin-1 and thromboxane both at baseline and after AngII hypertension. Overall, these studies provide substantial new data supporting that EC-MR does not contribute to BP regulation in mice under basal and most experimental conditions. Rather, these data uncover a role for EC-MR in regulation of vasoconstriction and in modulation of endothelial function after hypertension that is vascular bed-specific and may explain some of the seemingly conflicting findings in prior studies. These results also support new therapeutic opportunities for targeting of pathways critical to maintenance of tissue-specific perfusion, particularly to the heart, in the setting of cardiovascular disease.
This study provides substantial clarification of the role of EC-MR in BP control in the context of the existing literature. It is quite clear that MR in the kidney contributes to BP regulation and it has recently been demonstrated by two groups that MR in SMC also contributes to BP regulation by modulating vascular tone9;10. This study provides the first data using telemetry as the gold standard approach to BP measurement in a model in which MR is deleted from ECs. From these data we conclude that EC-MR does not contribute to systolic, diastolic, mean, diurnal, or salt-sensitive hypertension. Furthermore, EC-MR deletion does not alter the response to typical hypertensive stimuli including mineralocorticoid excess+/−salt or AngII+/−L-NAME. This is consistent with the previously published Tie2-Cre EC-MR-KO model in which BP was measured by tail cuff and was not different in Cre+ animals at baseline or after uninephrectomy/DOCA/salt treatment17. The finding that EC-MR deletion does not modulate BP contrasts with the substantially elevated BP in the transgenic mouse overexpressing human MR in the endothelium16. While it is possible that the hypertension in the overexpression model is due to supraphysiologic levels of MR expression or a functional difference between human and mouse MR, it is also possible that there are conditions in which MR is substantially upregulated in the endothelium (such as aging or heart failure) in which EC-MR might play an enhanced role in BP control and further studies are needed to explore those possibilities.
These results also add to a growing body of literature supporting the idea that EC-MR may have a minimal (or even vasodilatory) role in vessels under basal conditions (reviewed in 8) but that EC-MR participates in the development of endothelial dysfunction in response to cardiac risk factors including obesity18 and now also after exposure to hypertension23. We demonstrate no difference in mesenteric or coronary vasodilation in healthy EC-MR-KO mice as did Schaefer et. al. in the aorta of Tie2-MR-KO mice18. This contrasts with the Rickard et. al. study showing decreased aortic and mesenteric endothelial-dependent relaxation in Tie2-MR-KO mice17. The difference in mesenteric vasodilation in the Rickard study could be due to the Tie2-Cre model if immune cell MR contributes to mesenteric vascular function, or to technical variations in how the vessel studies were performed. However, after exposure to risk factors, emerging data support a model in which EC-MR contributes to changes in endothelial function induced by obesity18 and hypertension. The enhanced vasodilation in EC-MR-KO mesenteric vessels after AngII-induced hypertension was eliminated by pretreatment with L-NAME+indomethacin supporting that EC-MR activation in the setting of AngII-induced hypertension may inhibit the production of NO or prostacyclin. The potential decrease in the EDHF contribution to relaxation in EC-MR-KO vessels is consistent with studies showing that EC-MR regulates the vasodilatory potassium channel component of EDHF, SKCa24. The presence of residual vasodilation after treatment with L-NAME+indomethacin+apamin+TRAM34 only in MR-intact mice suggests the possibility of an unidentified EC-MR dependent vasodilator component that is activated by AngII hypertension. Additional studies are needed to determine the detailed role and mechanism by which EC-MR regulates each component of vasodilation not only in this AngII hypertension model but also with other hypertensive stimuli including high sodium or other doses of AngII or aldosterone, or with other cardiovascular stresses such as obesity, diabetes, or heart failure.
In this study, EC-MR contributed to endothelial dysfunction in response to AngII without a difference in BP. Although endothelial dysfunction is not always associated with changes in BP, it is an independent risk factor for cardiovascular disease and therapies that improve endothelial function, improve outcomes. Although the specific role of EC-MR is difficult to determine in humans, clinical data support that MR activation may play a greater role in endothelial function in patients with high cardiovascular risk including those with hypertension, diabetes, and heart failure13;14;25;26 rather than in those with a healthy vasculature27. Thus, MR antagonism may provide clinical benefit in the setting of cardiovascular risk factors such as hypertension or obesity by interfering with the development of endothelial dysfunction, an early step in the development of atherosclerosis that also has negative prognostic implications28;29.
This study further demonstrates that EC-MR contributes to vasoconstriction in a manner that depends on the vascular bed examined and on the contractile agonist tested. This finding might explain what appear to be conflicting results in the literature. For example, the EC-MR-overexpressing mouse showed enhanced mesenteric vasoconstriction16 while the Tie2-MR-KO mouse showed no difference in vasoconstriction in the aorta18, perhaps due to vascular bed differences. However, our study is consistent with Rickard et. al. as EC-MR-deletion had no effect on mesenteric constriction in both studies. In addition, this is the first study to explore the specific role of EC-MR in coronary function and our data revealing a role for EC-MR in coronary vasoconstriction to endothelin-1 and thromboxane might explain the recent finding that MR antagonists reverse coronary vascular dysfunction in a rat model of obesity12.
The mechanism by which EC-MR exerts site-specific effects on vascular function remains to be explored. Endothelial cells from different locations in the vasculature have distinct biochemical and biomechanical forces to which they are exposed resulting in unique mRNA transcriptomes and epigenetic profiles30. Thus, potential mechanisms may include vascular bed-specific differences in expression of MR itself, of other components of the RAAS, of other transcription factors and cofactors that interact with the MR, and of downstream mediators of contractile signaling pathways in distinct EC populations. Intact endothelial-independent vasodilation and coronary SMC calcium channel function, suggests that SMC function is intact in EC-MR-KO mice. The finding that decreased endothelin-induced coronary vasoconstriction is associated with increased ETB receptor mRNA in mouse cardiac ECs from EC-MR-KO mice may suggest one potential mechanism. Activation of endothelial ETB receptors by endothelin-1 induces vasorelaxation that is known to counter the constrictive response in the SMCs in an NO-dependent manner31. Previous work has also shown a role for MR in post-translational inactivation of ETB in cultured ECs32. However, the lack of a change in thromboxane receptor mRNA despite a change in thromboxane-induced constriction demonstrates that additional mechanisms remain to be elucidated.
The finding of decreased constriction to endothelin-1and thromboxane in coronary arteries from EC-MR-KO mice has important clinical implications. Thromboxane is released by activated platelets in the coronary arteries during acute myocardial infarction (MI) thereby contributing to vasoconstriction at the site of plaque rupture that exacerbates myocardial necrosis. Thromboxane-mediated vasoconstriction has been implicated in the morbidity of acute myocardial infarction, heart failure, diabetes, and disorders of primary vasospasm such as migraine headaches33. In patients presenting with acute MI, higher circulating thromboxane A2 or endothelin-1 levels correlate with worse outcomes, including increased risk of poor myocardial perfusion following percutaneous coronary intervention (no reflow)34;35. Endothelin-1 levels are also associated with decreased coronary flow reserve and decreased ejection fraction following MI36. MR antagonists reduce mortality following MI complicated by left ventricular dysfunction2 and improve coronary flow reserve in patients with diabetes14. Thus, clinical studies suggest that exaggerated thromboxane and endothelin-1 signaling worsens outcomes after MI and that MR antagonism is protective. Data from this study supports a novel explanation for the benefits of MR antagonism in coronary artery disease by demonstrating that EC-MR deletion attenuates thromboxane and endothelin-1-mediated coronary vasoconstriction.
There are several important limitations to this study. First, the lack of a specific mouse MR antibody restricts our ability to compare EC MR protein levels in different beds or to show loss of MR protein from ECs. Instead we confirmed MR DNA recombination in multiple vascular beds and MR DNA recombination and loss of MR mRNA in primary ECs cultured from EC-MR-KO mice. MR mRNA expression is reduced 75% in cultured EC-MR-KO ECs, consistent with reductions in mRNA seen in other endothelial-targeted MR KO mice18 and in other KO mice using the VE-Cadherin promoter37. Whether this is due to contaminating SMC in the EC cultures or from incomplete MR deletion from ECs is difficult to discern but incomplete recombination would only underestimate the role of EC-MR in vascular function. Since we identified a vascular-bed specific role for EC-MR in the mesentery and coronary microcirculation, another important limitation is that the results cannot be generalized to other vascular beds. Additional studies are needed to understand the role of EC-MR in regulating blood flow to other critical tissues, by examining cerebral, carotid, renal and skeletal muscle microvessels in this model. Throughout the study, EC-MR-KO mice and MR-intact littermates were compared under identical conditions however, direct comparisons cannot be made between healthy mice and those treated with AngII since the studies were not performed simultaneously (not littermates) and the untreated mice did not undergo osmotic minipump implantation surgery. Finally, for practical reasons, all experiments were performed only in male mice. There is substantial evidence that male and female mice and humans differ in their vascular function and BP responses38;39 and MR transcriptional activity was recently found to be modulated by estrogen40. Indeed, the coronary response to endothelin-1 is modified by aging in a sex-dependent manner 41. Thus, additional studies are needed to characterize the role of EC-MR in female mice.
Supplementary Material
Perspectives.
MR antagonists decrease BP and mortality in cardiovascular disease. Over the past decade it has become clear that MR contributes to cardiovascular function and disease in multiple tissues in addition to the kidney. This study used a unique mouse with EC-specific MR deletion with intact leukocyte MR to demonstrate that EC-MR does not contribute to BP regulation. EC-MR does contribute to vasoconstriction, specifically in the coronary arteries in response to endothelin-1 and thromboxane. This study suggests that inhibition of EC-MR may contribute to the mechanism by which MR antagonists improve coronary vascular function in diabetic patients and in animal models of obesity. Furthermore, although EC-MR does not play a role in endothelial function in healthy vessels, it contributes to mesenteric endothelial dysfunction in the setting of AngII-hypertension. Thus although EC-MR does not appear to contribute to BP regulation, EC-MR differentially regulates vasoconstriction depending on the vascular bed and modulates vasodilation in the context of cardiovascular risk factors with important clinical implications.
Novelty and significance.
What is new?
Using a novel mouse with the MR specifically deleted from endothelial cells (and not leukocytes), this study demonstrates that EC-MR does not play a role in basal blood pressure control or in salt-sensitive or RAAS driven hypertension. This study reveals that EC-MR contributes to mesenteric vasodilation after exposure to AngII hypertension and to regulation of coronary but not mesenteric vasoconstriction.
What is relevant?
These results reveal vascular bed- and disease-specific roles for EC-MR that may explain conflicting findings in the literature. Moreover, the results suggest that one mechanism by which MR antagonists improve cardiovascular outcomes is by inhibiting EC-MR, thereby preventing coronary vasoconstriction in patients with cardiovascular disease.
Summary
Endothelial MR does not contribute to blood pressure regulation but does contribute to coronary vasoconstriction and to hypertension-induced mesenteric endothelial dysfunction.
Acknowledgments
Sources of Funding: This work was funded by grants from the National Institutes of Health (HL095590 and HL119290 to IZJ), the American Heart Association (EIA18290005 to IZJ and PRE16920014 to KBM), and the Department of Veterans Affairs BLR&D (CDA-2 BX002030 to SBB). This work was also supported by resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Footnotes
Disclosures: The authors declare no conflicts of interest.
References
- 1.Pitt B, Zannad F, Remme, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Remme W, Zannad F, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 3.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 4.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65:61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 6.Caprio M, Newfell BG, LaSala A, Baur WE, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 8.McCurley A, Jaffe IZ. Mineralocorticoid Receptors in Vascular Function and Disease. Molecular and Cellular Endocrinology. 2011;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarjus A, Belozertseva E, Louis H, El Moghrabi S, Labat C, Lacolley P, Jaisser F, Galmiche G. Role of smooth muscle cell mineralocorticoid receptor in vascular tone. Pflugers Arch. 2015;467:1643–1650. doi: 10.1007/s00424-014-1616-x. [DOI] [PubMed] [Google Scholar]
- 12.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR. Mineralocorticoid Receptor Antagonism Treats Obesity-Associated Cardiac Diastolic Dysfunction. Hypertension. 2015;65:1082–1088. doi: 10.1161/HYPERTENSIONAHA.114.04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90:765–770. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64:236–242. doi: 10.2337/db14-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCurley A, McGraw AP, Pruthi D, Jaffe IZ. Smooth muscle cell mineralocorticoid receptors: role in vascular function and contribution to cardiovascular disease. Pflugers Archiv. 2013;465:1661–1670. doi: 10.1007/s00424-013-1282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen Dinh CA, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 17.Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension. 2014;63:1033–1040. doi: 10.1161/HYPERTENSIONAHA.113.01803. [DOI] [PubMed] [Google Scholar]
- 18.Schafer N, Lohmann C, Winnik S, Van Tits L, Miranda M, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Luscher TF, Matter C. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J. 2013;34:3515–3524. doi: 10.1093/eurheartj/eht095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10:203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids. 2014;91:38–45. doi: 10.1016/j.steroids.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 22.Mentz RJ, Bakris GL, Waeber B, McMurray JJV, Gheorghiade M, Ruilope LM, Maggioni AP, Swedberg K, Pina IL, Fiuzat M, O’Connor CM, Zannad F, Pitt B. The past, present and future of renin-angiotensin aldosterone system inhibition. Int J Cardiol. 2013;167:1677–1687. doi: 10.1016/j.ijcard.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffe IZ, Jaisser F. Endothelial cell mineralocorticoid receptors: turning cardiovascular risk factors into cardiovascular dysfunction. Hypertension. 2014;63:915–917. doi: 10.1161/HYPERTENSIONAHA.114.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Celerier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli Ml, Offret O, Curan A, Farman N, Jaisser Fdr, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672–2679. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 26.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 27.Nietlispach F, Julius B, Schindler R, Bernheim A, Binkert C, Kiowski W, Brunner-La Rocca HP. Influence of acute and chronic mineralocorticoid excess on endothelial function in healthy men. Hypertension. 2007;50:82–88. doi: 10.1161/HYPERTENSIONAHA.107.088955. [DOI] [PubMed] [Google Scholar]
- 28.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 29.Sunbul M, Ozben B, Durmus E, Kepez A, Pehlivan A, Midi I, Mutlu B. Endothelial dysfunction is an independent risk factor for stroke patients irrespective of the presence of patent foramen ovale. Herz. 2013;38:671–676. doi: 10.1007/s00059-013-3759-5. [DOI] [PubMed] [Google Scholar]
- 30.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 31.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 32.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellers MM, Stallone JN. Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. Am J Physiol Heart Circ Physiol. 2008;294:H1978–H1986. doi: 10.1152/ajpheart.01318.2007. [DOI] [PubMed] [Google Scholar]
- 34.Niccoli G, Giubilato S, Russo E, Spaziani C, Leo A, Porto I, Leone AM, Burzotta F, Riondino S, Pulcinelli F, Biasucci LM, Crea F. Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur Heart J. 2008;29:1843–1850. doi: 10.1093/eurheartj/ehn325. [DOI] [PubMed] [Google Scholar]
- 35.Niccoli G, Lanza GA, Shaw S, Romagnoli E, Gioia D, Burzotta F, Trani C, Mazzari MA, Mongiardo R, De Vita M, Rebuzzi AG, Luscher TF, Crea F. Endothelin-1 and acute myocardial infarction: a no-reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J. 2006;27:1793–1798. doi: 10.1093/eurheartj/ehl119. [DOI] [PubMed] [Google Scholar]
- 36.Cuculi F, Dall’Armellina E, Manlhiot C, De Caterina AR, Colyer S, Ferreira V, Morovat A, Prendergast BD, Forfar JC, Alp NJ, Choudhury RP, Neubauer S, Channon KM, Banning AP, Kharbanda RK. Early change in invasive measures of microvascular function can predict myocardial recovery following PCI for ST-elevation myocardial infarction. Eur Heart J. 2014;35:1971–1980. doi: 10.1093/eurheartj/eht434. [DOI] [PubMed] [Google Scholar]
- 37.Torisu T, Torisu K, Lee IH, Liu J, Malide D, Combs CA, Wu XS, Rovira II, Fergusson MM, Weigert R, Connelly PS, Daniels MP, Komatsu M, Cao L, Finkel T. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat Med. 2013;19:1281–1287. doi: 10.1038/nm.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan MV, Bubb KJ, Noyce A, Villar IC, Duchene J, Hobbs AJ, Scotland RS, Ahluwalia A. Distinct endothelial pathways underlie sexual dimorphism in vascular auto-regulation. Br J Pharmacol. 2012;167:805–817. doi: 10.1111/j.1476-5381.2012.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, Thor D, Han X, Anderson L, Rahimian R. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: a shift in the relative importance of EDRFs. Am J Physiol Heart Circ Physiol. 2012;303:H1183–H1198. doi: 10.1152/ajpheart.00327.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett MK, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155:4461–4472. doi: 10.1210/en.2014-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leblanc AJ, Chen B, Dougherty PJ, Reyes RA, Shipley RD, Korzick DH, Muller-Delp JM. Divergent effects of aging and sex on vasoconstriction to endothelin in coronary arterioles. Microcirculation. 2013;20:365–376. doi: 10.1111/micc.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.