Abstract
Primary aldosteronism is the most common form of secondary hypertension. Somatic mutations in KCNJ5, ATP1A1, ATP2B3 and CACNA1D are found in aldosterone producing adenoma. Additionally, adrenals with aldosterone producing adenomas show cortical remodeling and frequently multiple secondary nodules. Our aim was to investigate whether different aldosterone producing nodules from the same adrenal share the same mutational status. Aldosterone synthase expression was assessed in multinodular adrenals from 27 patients. DNA of 37 aldosterone producing secondary nodules was extracted from formalin fixed paraffin embedded tissues and genotyped for KCNJ5, ATP1A1, ATP2B3 and CACNA1D mutations. Among 17 adrenals with a somatic mutation in the principal nodule, four showed the same mutation in a secondary nodule, while ten had no mutation in any of the known genes. In one adrenal harboring the KCNJ5 p.Gly151Arg mutation in the principal nodule, the same mutation was present in two secondary nodules, but no mutation was found in a third nodule. Finally, in two adrenals with a CACNA1D mutation in the principal nodule, a KCNJ5 mutation was identified in the secondary nodule. Among ten adrenals without mutations in the principal nodule, one carried a KCNJ5 mutation in the secondary nodule. No mutations were detected in seven aldosterone producing cell clusters from six adrenals. No association was observed between the presence of mutations in secondary nodules and clinical parameters. In conclusion, different mutations are found in different aldosterone producing nodules from the same adrenal, suggesting that somatic mutations are independent events triggered by mechanisms that remain to be identified.
Keywords: Aldosterone, Mineralocorticoids, Endocrine Hypertension, Primary Aldosteronism, Aldosterone producing adenoma, Adrenal Cortex, Potassium Channels, Mutation
Introduction
Primary aldosteronism (PA), caused by the excessive production of aldosterone from the adrenal cortex, is the most common form of secondary hypertension with an estimated prevalence of 4% in primary care, ~10% in referred patients and as high as 20% in patients with resistant hypertension.1–5 PA is characterized by hypertension associated with high plasma aldosterone levels, low plasma renin activity, varying degrees of hypokalemia, and an increased risk of myocardial infarction, stroke, and atrial fibrillation at long term.6,7 Approximately 95% of all cases of PA are due to the subtypes aldosterone producing adenoma (APA) and bilateral adrenal hyperplasia.8
Somatic heterozygous mutations in the genes KCNJ5 coding for the potassium channel GIRK4, ATP1A1 coding for the α1 subunit of the Na+/K+-ATPase, ATP2B3 coding for the plasma membrane Ca2+-ATPase, type 3 PMCA3, and CACNA1D encoding the Cav1.3 voltage dependent calcium channel were identified in approximately 50% of APA.9-13 These mutations are responsible for chronic zona glomerulosa cell membrane depolarization (KCNJ5 and ATP1A1 mutations) leading to opening of voltage-dependent calcium channels, for impaired intracellular calcium recycling (ATP2B3 mutations), or voltage-dependent calcium channel activation and opening at lower voltages (CACNA1D mutations).9–12 All these genetic abnormalities converge towards an increase in intracellular calcium concentration and activation of calcium signaling, which plays a central role in the regulation of aldosterone production, in particular by increasing the expression of CYP11B2 encoding aldosterone synthase.
Although the direct link between the identified somatic mutations and aldosterone production is well characterized, the effects of these mutations on nodulation and cell proliferation remain to be clearly established. The first description of KCNJ5 mutations in APA, and in particular the discovery of similar mutations leading to familial hyperaldosteronism type 3 associated with massive bilateral hyperplasia, has indicated that this genetic abnormality was sufficient to drive both increased cell proliferation and autonomous aldosterone production.12 Recent work, however, has shown that cells transfected with mutant GIRK4 presented lower proliferation than cells transfected with wild-type GIRK4,14 or even apoptosis, which was due to the massive increase in intracellular Na+ concentration.15 This has led to the hypothesis that mutant K+ channels may cause sufficient Na+ permeability for tumor development but not as great as to cause cell death. We have shown that zona glomerulosa (ZG) hyperplasia, increased nodulation, and decreased vascularization are major features of adrenal cortex adjacent to APA,16 suggesting that KCNJ5 mutations could indeed occur within a proliferating cortex, leading to growth advantage, clonal expansion, and tumor formation. Alternatively, they may represent isolated events leading to APA formation, with adrenal cortex hyperplasia being secondary to reduced vascularization and/or tissue hypoxia. Indeed, no germline mutations are present in subjects carrying one of the different somatic mutations in APA and mutations are absent in somatic DNA from peritumoral cortices from adrenals carrying somatic KCNJ5 mutations in the corresponding APA.17 Recently, Deckers et al., have investigated the genotypes of multinodular adrenals and identified two aldosterone synthase positives nodules harboring two different recurrent KCNJ5 mutations in the same adrenal,18 supporting the hypothesis of independent events leading to nodulation and aldosterone overproduction.
The aim of the present study is to get further insight into the sequence of events leading to nodulation and aldosterone overproduction in adrenals with APA. To this purpose we have extensively characterized aldosterone production in secondary nodules from multinodular adrenals and investigated the presence of somatic KCNJ5, ATP1A1, ATP2B3 and CACNA1D mutations in these nodules. We have also investigated whether recurrent somatic mutations are present in aldosterone producing cell clusters (APCC), specific structures that have been suggested as the starting point for the development of APA.19
Subjects and Methods
An expanded Methods section is available in the online-only Data Supplement.
Patients
Patients with PA from the COMETE-HEGP cohort were recruited between 2002 and 2012 within the COMETE (COrtico- et MEdullo-surrénale, les Tumeurs Endocrines) network. Methods for screening and subtype identification of PA were performed according to institutional and Endocrine Society guidelines.20,21 In patients diagnosed with primary aldosteronism, a thin slice CT scan or MRI of the adrenal and/or an adrenal venous sampling (AVS) were performed to differentiate between unilateral and bilateral aldosterone hypersecretion. All patients gave written informed consent for genetic and clinical investigation. Procedures were in accordance with institutional guidelines. Further details are available in the online-only Data Supplement.
Pathological analysis
Details are available in the online-only Data Supplement.
DNA isolation and Sanger sequencing
Details are available in the online-only Data Supplement.
Droplet Digital PCR (ddPCR)
ddPCR was performed on a QX-100 system (Bio-Rad) by using Bio-Rad assays comprising primers and probes designed for the detection of the KCNJ5 mutations c.451G>A and c.451G>C, leading to the amino acid substitution p.Gly151Arg. Further details are available in the online-only Data Supplement.
Statistical analyses
Quantitative variables are reported as means ± standard deviation when Gaussian distribution or medians and interquartile range when no Gaussian distribution, and compared with unpaired t-test or Mann-Whitney test respectivelly. Categorical variables are reported as percentages and compared with Fisher’s exact test. A p value < 0.05 was considered significant for comparisons between 2 groups.
Results
Identification of functional secondary nodules in adrenals with APA
Based on the histopathological description of adrenals resected for lateralized PA performed in the pathological department of the Hospital Européen Georges Pompidou, adrenals from 27 patients were selected on whom we performed HES staining in order to identify secondary nodules in peripheral adrenal tissue (Figure 1A). All adrenals investigated in this study had functional APA as demonstrated by IHC of aldosterone synthase or ISH of CYP11B2. Clinical and biological characteristics of this group of patients were comparable to the other PA patients with APA of the Paris cohort, with no difference in the age at diagnosis of PA [44 years old (40;58) vs 40 years old (34;47); p=0.07)], gender (35% of females vs 54% of females; p=0.06), clinical and biochemical signs of PA, APA diameter, number of preoperative medications and follow up characteristics (Table 1). No differences were observed when comparing patients with multinodular adrenals to those with a single adenoma (table S2). Although median values of the lateralization index are lower and post-op SBP and number of medications are higher in patients with multinodular adrenals, this difference was not statistically significant. This might be indeed due to a lack of power of this study. After the identification of secondary nodules, CYP11B2 in situ hybridization or aldosterone synthase immunohistochemistry were performed to characterize the functionality of the secondary nodules (Figure 1B). Thirty-seven functional secondary nodules were identified in 27 adrenals (Table 1). In 18 adrenals we identified one secondary nodule, in eight adrenals two secondary nodules and in one adrenal three secondary nodules. In addition, in six adrenals we identified one APCC and in one adrenal two APCC. APCC are specific subcapsular cell clusters expressing high levels of CYP11B2,19 and are composed of morphological ZG cells in contact with the capsule and inner columnar zona fasciculate (ZF) like cells.16 These cells expression of CYP11B2 throughout the entire APCC suggested that these structures possess an intermediate phenotype between cells from ZG and from ZF.22
Figure 1. Immunohistochemical features of multinodular adrenal glands.
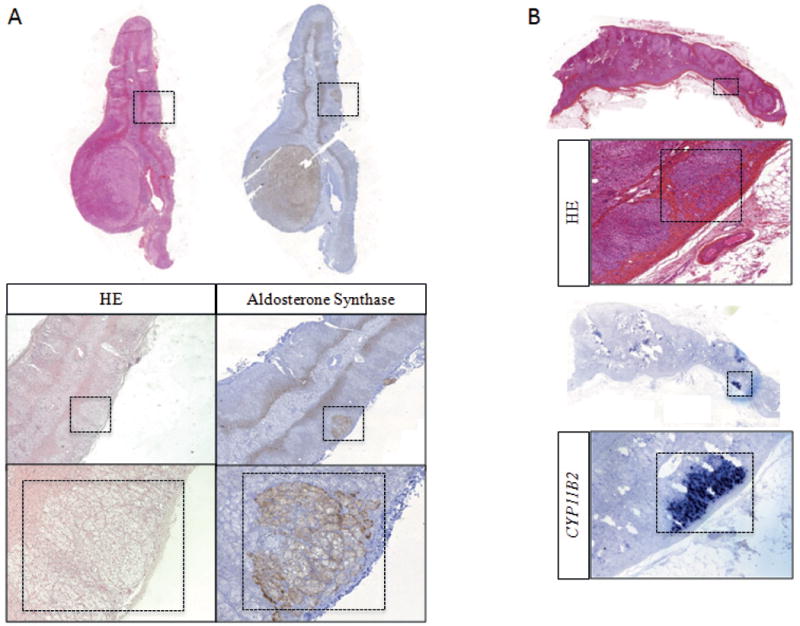
A. HES staining and aldosterone synthase IHC of an adrenal gland resected for lateralized PA showing an APA and secondary micronodules. Left. HES staining with the identification of secondary nodules in an adrenal carrying an APA. Right. Aldosterone synthase IHC positive in one secondary adrenal nodule. B. HES staining (upper panels) and CYP11B2 ISH (bottom panels) in an adrenal carrying an APA and one APCC region.
Table 1.
Phenotype of PA patients with secondary adrenal nodules investigated in this study and compared to 172 other PA patients from the COMETE-HEGP cohort.
| Parameters | Patients with multinodular adrenals | Other patients | p value |
|---|---|---|---|
| N | 27 | 172 | |
| gender F (%) | 35 | 54 | 0.06 |
| Age of diagnosis (years) | 44 (40;58) | 40 (34;47) | 0.07 |
| Duration of hypertension (years) | 3 (1.0;13.5) | 4.5 (1.0;8.7) | 0.8 |
| Preoperative SBP (mmHG) | 147 (138;160) | 147 (136;158) | 0.5 |
| Preoperative DBP (mmHg) | 91 (81;96) | 92 (86;102) | 0.07 |
| Minimal plasma K (mmol/L) | 3.1 (2.6;3.4) | 3.1 (2.9;3.4) | 0.6 |
| Plasma Aldosterone (pmol/L) | 823 (570;1230) | 820 (550;1172) | 0.9 |
| ARR (pmol/mU) | 160 (109;246) | 152 (102;217) | 0.7 |
| Anti-hypertensive drugs (n) | 3 (2;4) | 2 (1;3) | 0.06 |
| Lateralization index | 10 (6.5;28) | 19 (10;38) | 0.08 |
| APA size (mm) | 13 (10;18) | 15 (10;20) | 0.42 |
| Time of follow up (months) | 8.5 (6;13.5) | 7 (6;11) | 0.07 |
| Post op SBP (mmHG) | 131 (125;139) | 127 (120;135) | 0.06 |
| Post op DBP (mmHG) | 82 (77;90) | 82 (76;87) | 0.6 |
| Post op plasma K (mmol/L) | 4.1 (3.9;4.6) | 4.1 (3.9;4.4) | 0.6 |
| Post op plasma aldosterone (pmol/L) | 98 (46;246) | 115 (67;229) | 0.9 |
| Post op anti-hypertensive drugs (n) | 1 (0;2) | 0 (0;1) | 0.07 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; ARR: aldosterone to renin ratio
Presence of somatic mutations in secondary functional nodules
We genotyped hot spot regions of the KCNJ5, CACNA1D, ATP1A1 and ATP2B3 genes in 37 functional secondary nodules obtained from the 27 adrenals with an APA with known mutation status.13 Eight APA presented a KCNJ5 mutation (p.Gly151Arg), seven presented a CACNA1D mutations (p.Phe747Leu, p.Val259Asp, p.Pro1336Arg, p.Ala998Val, p.Ile750Met, p.Gly403Arg and p.Val1151Phe), two had an ATP1A1 mutation (p.Val332Gly and p.Leu104Arg), and in ten APA no mutation in any of the four known genes was identified (Table 2).
Table 2.
Adrenals with secondary nodules genotyped for somatic mutations.
| Adrenal | Genotype APA | Secondary nodule | Size of sec nodule (macro/micro)* | Genotype secondary nodule | APCC (n) | Genotype APCC |
|---|---|---|---|---|---|---|
| 1 | KCNJ5 p.Gly151Arg | A | micro | KCNJ5 p.Gly151Arg | 1 | neg |
| 2 | KCNJ5 p.Gly151Arg | A | micro | KCNJ5 p.Gly151Arg | 1 | neg |
| 3 | KCNJ5 p.Gly151Arg | A | micro | KCNJ5 p.Gly151Arg | ||
| B | micro | KCNJ5 p.Gly151Arg | ||||
| C | micro | neg | ||||
| 4 | neg | A | micro | KCNJ5 p.Gly151Arg | 2 | neg |
| 5 | CACNA1D p.Phe747Leu | A | micro | neg | ||
| 6 | CACNA1D p.Val259Asp | A | micro | neg | ||
| 7 | neg | A | micro | neg | ||
| 8 | neg | A | micro | neg | ||
| 9 | KCNJ5 p.Gly151Arg | A | micro | KCNJ5 p.Gly151Arg | ||
| 10 | neg | A | micro | neg | 1 | neg |
| B | micro | neg | ||||
| 11 | neg | A | micro | neg | ||
| B | micro | neg | ||||
| 12 | neg | A | micro | neg | ||
| B | micro | neg | ||||
| 13 | ATP1A1 p.Val332Gly | A | macro (6 mm) | neg | ||
| 14 | CACNA1D p.Pro1336Arg | A | micro | neg | ||
| 15 | CACNA1D p.Ala998Val | A | micro | KCNJ5 p.Gly151Arg | ||
| 16 | ATP1A1 p.Leu104Arg | A | micro | neg | 1 | neg |
| B | micro | neg | ||||
| 17 | KCNJ5 p.Gly151Arg | A | micro | KCNJ5 p.Gly151Arg | 1 | neg |
| 18 | KCNJ5 p.Gly151Arg | A | micro | neg | ||
| 19 | neg | A | micro | neg | ||
| 20 | CACNA1D p.Ile750Met | A | micro | neg | ||
| B | micro | neg | ||||
| 21 | KCNJ5 p.Gly151Arg | A | micro | neg | ||
| 22 | neg | A | micro | neg | ||
| 23 | neg | A | micro | neg | ||
| B | micro | neg | ||||
| 24 | CACNA1D p.Gly403Arg | A | micro | neg | ||
| B | micro | KCNJ5 p.Gly151Arg | ||||
| 25 | neg | A | macro (5 mm) | neg | ||
| 26 | KCNJ5 p.Gly151Arg | A | micro | neg | ||
| B | micro | neg | ||||
| 27 | CACNA1D p.Val1151Phe | A | micro | neg |
APCC: aldosterone producing cell cluster.
Secondary nodules were defined as macroscopic nodules if larger than 5 mm of diameter; microscopic nodules were defined as nodules smaller than 5 mm located in a hyperplastic adrenal cortex.
Genotyping results of secondary nodules are described in Table 2. Among 17 adrenals with a somatic mutation identified in the APA, four showed the same mutation in a secondary nodule (Figure 2A and figure S1A to S1D). All these APA were carrying the KCNJ5 mutation p.Gly151Arg (adrenals 1, 2, 9, and 17). In ten adrenals with a somatic mutation in the APA (adrenals 5, 6, 13, 14, 16, 18, 20, 21, 26 and 27), among which five carrying CACNA1D mutations, three with KCNJ5 mutations and two with ATP1A1 mutations, no mutation in any of the known genes was detected in 13 functional secondary nodules analyzed (Figure 2B and Table 2). In one adrenal harboring the KCNJ5 p.Gly151Arg mutation in the APA (adrenal 3), we identified the same mutation in two functional secondary nodules, but no mutation in a third functional secondary nodule (Figure S1E). In two adrenals harboring a CACNA1D mutation in the APA (p.Ala998Val and p.Gly403Arg in adrenals 15 and 24 respectively) a KCNJ5 p.Gly151Arg was identified in the secondary nodules (Figure 2C, 2D, S1F and S1G). We also analyzed functional secondary nodules from ten adrenals without mutations in the APA. In one adrenal we identified a KCNJ5 mutation (c.451G>A; p.Gly151Arg) in a secondary nodule (adrenal 4, Figure 2E and S1H); no mutations were identified in the remaining 9 adrenals (Table 2). We did not observe differences in the cellular composition between APA and secondary nodules positive for KCNJ5 mutations (Table S3). Finally, no mutation was detected in seven APCC regions from six adrenals (data not shown).
Figure 2. Genotyping of secondary nodules identified in adrenals with APA. A. Adrenal 1.
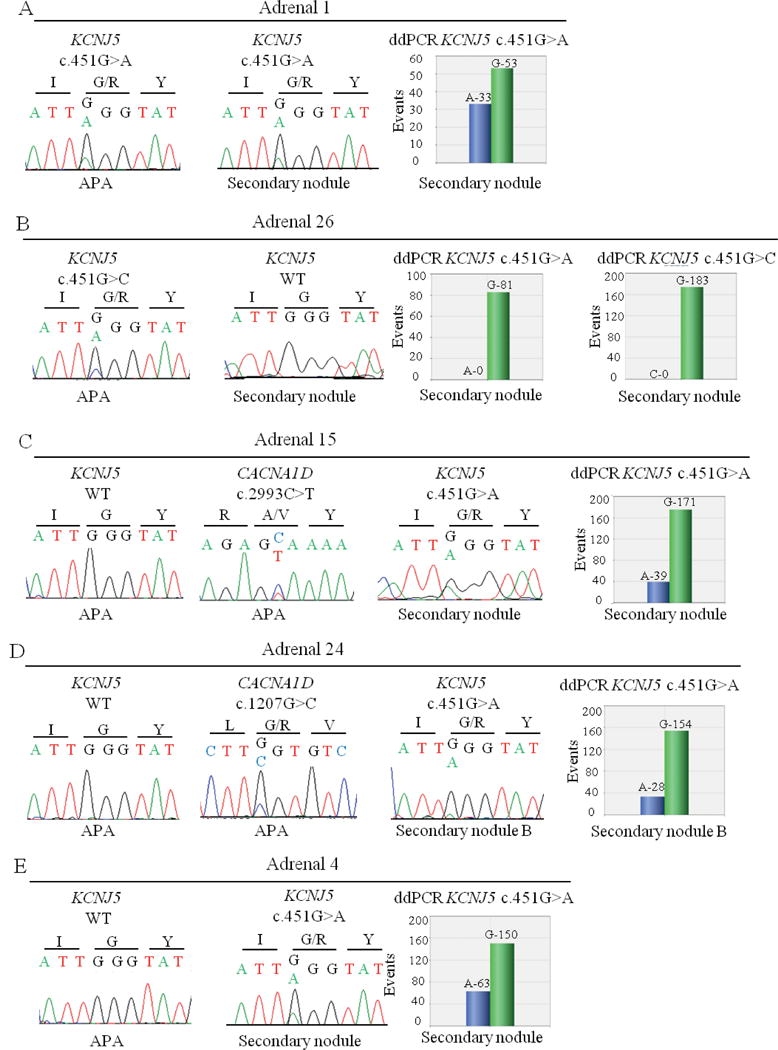
Left and middle panels: Sanger sequencing chromatograms showing the KCNJ5 mutation c.451G>A in APA and the wild type KCNJ5 sequence in the secondary nodule. Right panel: ddPCR analysis showing the amount of wild type G allele and mutant A allele of the KCNJ5 mutation c.451G>A in the secondary nodule. B. Adrenal 26. Left panels: Sanger sequencing chromatograms showing the KCNJ5 mutation c.451G>C in APA and the wild type KCNJ5 sequence in the secondary nodule. Right panels: ddPCR analysis showing the amount of wild type G allele and mutant A allele (c.451G>A) and the amount of wild type G allele and mutant C allele (c.451G>C) in the secondary nodule. C. Adrenal 15. Left and middle panels: Sanger sequencing chromatograms showing the wild type KCNJ5 sequence in APA, the CACNA1D mutation c.2293C>T in APA, and the KCNJ5 mutation c.451G>A in the secondary nodule. Right panel: ddPCR analysis showing the amount of wild type G allele and mutant A allele of the KCNJ5 mutation c.451G>A in the secondary nodule. D. Adrenal 24. Left and middle panels: Sanger sequencing chromatograms showing the wild type KCNJ5 sequence in APA, the CACNA1D mutation c.2293C>T in APA, and the KCNJ5 mutation c.1207G>C in the secondary nodule. Right panel: ddPCR analysis showing the amount of wild type G allele and mutant A allele of the KCNJ5 mutation c.451G>A in the secondary nodule. E. Adrenal 4. Left and middle panels: Sanger sequencing chromatograms showing the wild type KCNJ5 sequence in APA and the KCNJ5 mutation c.451G>A in the secondary nodule. Right panel: ddPCR analysis showing the amount of wild type G allele and mutant A allele of the KCNJ5 mutation c.451G>A in the secondary nodule.
To verify the absence of small proportions of KCNJ5 mutations in secondary nodules negative for KCNJ5 mutations that could have been missed by Sanger sequencing, we performed ddPCR analysis of the KCNJ5 mutations c.451G>A and c.451G>C, leading to the amino acid substitution p.Gly151Arg in secondary nodules negative for KCNJ5 mutations. In adrenals 26 and 21, with KCNJ5 mutation c.451G>C identified in APA, no KCNJ5 mutations were identified in secondary nodules by ddPCR (Figure 2B and Figure S2A). ddPCR analysis of adrenals 7 and 8, without KCNJ5 mutations identified in APA, did not reveal KCNJ5 mutations in the secondary nodules (Figure S2B and S2C), confirming the results of Sanger sequencing.
We also performed ddPCR analysis of the KCNJ5 mutation p.Gly151Arg, in the nine secondary nodules positive for a KCNJ5 mutation in order to confirm the genotype and determine the proportion of mutational events (Table S3). We identified 38% (adrenal 1), 12% (adrenal 2), 33% and 13% (adrenal 3, nodules A and B), 15% (adrenal 9) and 13% (adrenal 17) of the mutant allele in the secondary nodules (Figure 2A, S2D and S2E, Table S3). In the corresponding principal nodules, 39% (adrenal 1), 40% (adrenal 2), 36% (adrenal 3), 38% (adrenal 9) and 33% (adrenal 17) of the mutant allele were detected (Figure S2F and S2G, Table S3). In adrenals 15 and 24, carrying a CACNA1D mutation in APA and the KCNJ5 mutation c.451G>A in the secondary nodules, the mutant A allele was present in a proportion of 18% (adrenal 15) and 15% (adrenal 24) in the secondary nodule (Figure 2C and 2D) and the absence of mutated KCNJ5 alleles was confirmed in the APA (Table S3). In adrenal 4, no mutated KCNJ5 alleles were identified in the APA (Table S3) and 29% of the mutated allele A was identified in the secondary nodule (Figure 2E). Additionally, in the principal nodule of adrenal 13, positive for an ATP1A1 mutation by Sanger sequencing, no mutated KCNJ5 alleles were detected in the ddPCR analysis.
Clinical correlates of the presence of mutations in secondary nodules
We compared clinical and biochemical findings between patients with and without identified mutations in secondary nodules (Table S4). Remarkably, all patients with a mutation in the secondary nodule harbored the KCNJ5 p.Gly151Arg mutation. There was no differences between the two groups in the age at PA diagnosis [44 years old (39;53) vs 48 years old (42;52); p=0.3) and gender distribution (37.5% of females vs 31.5% of females; p=0.5). There was no association between the presence of mutations in the secondary nodules and preoperative plasma aldosterone or renin levels, the aldosterone to renin ratio or the number of medications taken before surgery. There was also no association with post-operative blood pressure outcome as measured by blood pressure and treatment score at follow up, cure or improvement of hypertension.
Discussion
Although the functional link between somatic mutations and aldosterone production is clearly established, it is still not clear whether and how mutations lead to increased proliferation and nodulation. Here we report the results of the genotyping of somatic mutations in KCNJ5, ATP1A1, ATP2B3 and CACNA1D in 37 aldosterone producing secondary nodules from 27 adrenals carrying an APA with known mutation status. In 13 adrenals we identified the same mutation status between the APA and the secondary nodule. In eight adrenals we identified the somatic mutation KCNJ5 p.Gly151Arg in at least one secondary nodule. In 14 adrenals the mutation status was different between the APA and the secondary nodule. In ten adrenals with an APA harboring a known mutation, no mutations were identified in the secondary nodule. Remarkably, in one adrenal without mutation in APA, we identified a KCNJ5 mutation in the secondary nodule, while in two adrenals harboring a CACNA1D mutation in the APA, a KCNJ5 mutation was identified in the secondary nodule.
We have observed in many adrenals carrying an APA the presence of macro- or micronodulations of the peripheral adjacent cortex. We have previously shown that adrenal cortex remodeling, decreased vascularization, and ZG hyperplasia are major features of adrenals with APA.16 Although a certain degree of nodularity can be observed in normal adrenals associated to older age and severity of hypertension,23 we observed an increase of nodulation of the cortex adjacent to an APA when compared to control adrenals.16 This raises the possibility that remodeling of the adrenal cortex precedes the development of an APA, and thus, that somatic mutations could be a secondary event in APA development. Alternatively, they may represent isolated events leading to APA formation, with increased nodulation being secondary to reduced vascularization and/or tissue hypoxia 24 or to the secretion of factors promoting nodularity by the APA itself. On the other hand, genetic background could also play a role in the nodulation of adrenals with APA. A recent study performed on 56 patients with PA showed the presence of germline heterozygous ARMC5 variants in subjects of African origin.25 ARMC5 mutations were described in primary macronodular adrenal hyperplasia with Cushing’s syndrome, with patients showing a germline mutation on one allele and a second somatic abnormality on the other allele.26 ARMC5 codes for armadillo repeat containing 5, which is likely to be a tumor suppressor gene. Tumors carrying ARMC5 mutations are supposed to be polyclonal, as different nodules carry different type of mutations. In vitro studies of the mutations observed in patients with PA did not identify a link between these mutations and increased aldosterone production,25 suggesting that they could represent a genetic predisposition for nodule formation preceding the occurrence of somatic mutations responsible for inappropriate aldosterone production.
A recent study has assessed the genotypic characteristics of nodules from adrenals with a solitary nodule or multinodular adrenals of 53 patents with PA.18 Adrenals were classified as harboring an adenoma characterized by one well demarcated or encapsulated nodule without nodulation in the adjacent adrenal cortex, or as a nodular hyperplasia if the presence of multiple nodules with an increase in cortex thickness or a distortion of the surrounding adrenal cortex was observed. Most adrenals contained only one nodule positive for aldosterone synthase harboring or not a mutation in one of the known genes. Only in one case a KCNJ5 mutation was observed in a secondary nodule expressing aldosterone synthase.18 In the present study, all adrenals carried a well-defined larger nodule defined as the APA and expressing aldosterone synthase and a different number of macro- or micronodulations in the peripheral adjacent adrenal cortex. Only nodules expressing CYP11B2 or aldosterone synthase were included in our analysis, in order to discriminate nodules with the capacity of aldosterone production and, therefore, candidates to somatic mutations associated to inappropriate aldosterone secretion.
Among the 37 secondary nodules investigated in this study, we have identified somatic mutations in nine secondary nodules from eight adrenals with APA. In all cases, we identified a KCNJ5 p.Gly151Arg mutation (c.451G>C or c.451G>A), the most frequent somatic mutation associated to APA 13. In 28 secondary nodules we did not observe somatic mutations in the four genes studied, including 10 adrenals harboring a somatic mutation in the APA. This could be explained by a low sensitivity of Sanger sequencing for the detection of rare events in somatic DNA due to small amount of cells carrying the mutation. To avoid this bias in our results, we performed sensitive droplet digital PCR experiments to deeply genotype KCNJ5 mutations in negative secondary nodules. Using this approach we are able to identify rare mutation events with high sensitivity and we confirmed the absence of KCNJ5 mutations in the secondary nodules analyzed. In different studies, only 50% of APA carried a somatic mutation in the four investigated genes,13,17,27 thus somatic mutations in genes not yet described and associated with aldosterone production cannot be excluded. Interestingly, in two adrenals with APA positive for CACNA1D mutations we observed a KCNJ5 mutation in a secondary nodule. Additionally, in one adrenal without a mutation in APA, a KCNJ5 mutation was observed in the secondary nodule. In this adrenal, aldosterone synthase was highly expressed in the APA as well as in the secondary nodule (Fig. S3). Different somatic mutations in nodules from the same adrenal were previously described in primary macronodular adrenal hyperplasia, where the same germline ARMC5 mutation was associated with different, nodule-specific, somatic ARMC5 alterations.26 More recently, Dekkers et al. identified two different KCNJ5 mutations in two nodules from the same adrenal in a patient with APA.18 The discordance of mutation status between APA and secondary nodules in the same adrenal suggests that a specific genetic or epigenetic hit could be responsible for the remodeling in the adrenal cortex resulting in nodule formation. In this case, the somatic mutations described in KCNJ5, CACNA1D, ATP1A1 or ATP2B3 genes could represent second hits occurring in a previously altered adrenal cortex, being responsible only for the excessive aldosterone secretion. Alternatively, a somatic mutation may lead to the formation of an APA, followed by secondary mutations and nodule formation triggered by changes in the tumor microenvironment. Against this hypothesis is however the fact that in multinodular adrenals Dekkers et al identified somatic mutations only in aldosterone expressing nodules.18
Some authors suggested that APCC, isolated subcapsular cell clusters strongly expressing CYP11B2 without an apparent fibrous capsule, share morphological characteristics with cells composing an APA and could eventually be the starting point for the development of an APA.19 In order to investigate a possible continuum between APCC and APA development we have genotyped seven APCC structures from six adrenals with APA; no somatic mutations in the four target genes were identified. These data do not support the possible origin of APA from APCC and corroborate previous findings showing different molecular characteristics between APA and APCC.16 However, we cannot exclude that APCC might represent very precocious structures in which an event inducing an abnormal proliferative state happened before the occurrence of one of the known somatic mutations.
We have compared clinical and biological characteristics of patients with PA enrolled in this study with patients of our entire cohort. We did not find differences in the clinical presentation of PA and the follow up between patients from the two groups. This lack of difference may be due to the fact that we did not analyze the adrenals of all patients of the Paris cohort for the presence of peripheral cortical micronodules. As the patients were randomly selected for the presence of multinodular adrenals, the frequency of somatic mutations in the APA is slightly different compared to that previously reported.13 We did not observe differences in the disease presentation in patients carrying adrenals with secondary nodules positives for somatic mutations and patients carrying adrenals with secondary nodules negatives for somatic mutations. However, given the small number of patients in each group, the comparison may lack statistical power to identify genotype-phenotype correlations.
Perspectives
Although the functional link between somatic mutations in KCNJ5, ATP1A1, ATP2B3 and CACNA1D and aldosterone production has clearly been established, it is less clear whether and how these mutations also lead to adrenal cortex cell proliferation and nodule formation. Adrenals with aldosterone producing adenoma show increased nodulation and cortical remodeling with zona glomerulosa hyperplasia and often multiple secondary aldosterone producing nodules. Our work shows the presence of different recurrent somatic mutations in different aldosterone producing nodules from a same adrenal, suggesting that somatic mutations are independent events triggered by mechanisms that remain to be identified. Our results also show that in some cases a genetic defect is present in the secondary nodule but absent in the APA, challenging our current genetic classification of APA and the correlations with clinical measures that have been previously established. It will be particularly relevant in the future to identify additional genetic, epigenetic or local environmental factors predisposing to somatic mutagenesis as well as to establish the sequence of events leading to APA formation.
Supplementary Material
Novelty and Significance.
What Is New?
Mutations in different genes were observed among APA and functional secondary nodules in the same adrenal.
One adrenal without somatic mutation identified in the APA carried a KCNJ5 mutation in a secondary aldosterone producing nodule.
What Is Relevant?
Recurrent somatic mutations in KCNJ5 were identified in secondary nodules of adrenals with APA.
A different mutation status between APA and secondary nodules suggests independent genetic events leading to nodulation and aldosterone overproduction.
All APCC are negatives for somatic mutations in KCNJ5, CACNA1D, ATP1A1 and ATP2B3.
Summary.
Different mutations in APA and functional secondary nodules were identified in the same adrenal cortex. Our data suggest that somatic mutations found in APA are independent events leading to increased aldosterone production. The sequence of events underlying formation of aldosterone producing nodules remains to be elucidated.
Acknowledgments
The authors wish to thank Pierre-François Plouin (Hypertension unit, HEGP) and the COMETE (COrtico and MEdullary adrenal Tumors) network for providing tissue samples from APA.
Source of Funding: This work was funded through institutional support from INSERM and by the Agence Nationale pour la Recherche (ANR Blanc 2011, No.: 11-BSV1 005 03, ANR-13-ISV1-0006-01), the Fondation pour la Recherche Médicale (DEQ20140329556), the Programme Hospitalier de Recherche Clinique (PHRC grant AOM 06179), and by grants from INSERM and Ministère Délégué à la Recherche et des Nouvelles Technologies.
Abbreviations
- PA
primary aldosteronism
- APA
aldosterone producing adenoma
- APCC
aldosterone producing cell cluster
Footnotes
Conflict of interest/Disclosure statement
NONE
References
- 1.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 2.Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167:7–15. doi: 10.1530/EJE-11-1013. [DOI] [PubMed] [Google Scholar]
- 3.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF., Jr Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. The New England journal of medicine. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E-HS. Eplerenone in patients with systolic heart failure and mild symptoms. The New England journal of medicine. 2011;364:11–21. [Google Scholar]
- 6.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 7.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 8.Amar L, Plouin PF, Steichen O. Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism. Orphanet J Rare Dis. 2010;5:9. doi: 10.1186/1750-1172-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 10.Scholl UI, Goh G, Stolting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 12.Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–361. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- 14.Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782. doi: 10.1210/en.2011-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, Wang WH, Lifton RP. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A. 2012;109:2533–2538. doi: 10.1073/pnas.1121407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A, Meatchi T, Zennaro MC. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56:885–892. doi: 10.1161/HYPERTENSIONAHA.110.158543. [DOI] [PubMed] [Google Scholar]
- 17.Boulkroun S, Beuschlein F, Rossi GP, et al. Prevalence, Clinical, and Molecular Correlates of KCNJ5 Mutations in Primary Aldosteronism. Hypertension. 2012;59:592–598. doi: 10.1161/HYPERTENSIONAHA.111.186478. [DOI] [PubMed] [Google Scholar]
- 18.Dekkers T, ter Meer M, Lenders JW, Hermus AR, Schultze Kool L, Langenhuijsen JF, Nishimoto K, Ogishima T, Mukai K, Azizan EA, Tops B, Deinum J, Kusters B. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99:E1341–1351. doi: 10.1210/jc.2013-4255. [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical Zonation in Humans under Normal and Pathological Conditions. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 20.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 21.Letavernier E, Peyrard S, Amar L, Zinzindohoue F, Fiquet B, Plouin PF. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens. 2008;26:1816–1823. doi: 10.1097/HJH.0b013e3283060f0c. [DOI] [PubMed] [Google Scholar]
- 22.Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, Lefebvre H, Louiset E, Jeunemaitre X, Meatchi T, Benecke A, Lalli E, Zennaro MC. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205. [DOI] [PubMed] [Google Scholar]
- 23.Wolkersdorfer GW, Bornstein SR. Tissue remodelling in the adrenal gland. Biochem Pharmacol. 1998;56:163–171. doi: 10.1016/s0006-2952(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 24.Sasano H, Suzuki T, Moriya T. Adrenal Cortex. In: Lloyd RV, editor. Endocrine Pathology: Differential Diagnosis and Molecular Advances. Totowa, NJ: Humana Press Inc; 2003. pp. 211–226. [Google Scholar]
- 25.Zilbermint M, Xekouki P, Faucz FR, et al. Primary Aldosteronism and ARMC5 variants. J Clin Endocrinol Metab. 2015 doi: 10.1210/jc.2014-4167. jc20144167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assie G, Libe R, Espiard S, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369:2105–2114. doi: 10.1056/NEJMoa1304603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azizan EA, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, Brown MJ, O’Shaughnessy KM. Somatic Mutations Affecting the Selectivity Filter of KCNJ5 Are Frequent in 2 Large Unselected Collections of Adrenal Aldosteronomas. Hypertension. 2012;59:587–591. doi: 10.1161/HYPERTENSIONAHA.111.186239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


