Abstract
Pulmonary arterial hypertension (PAH), a rapidly fatal vascular disease, strikes women more often than men. Paradoxically, female PAH patients have better prognosis and survival rates than males. The female sex hormone 17β-estradiol has been linked to the better outcome of PAH in females; however, the mechanisms by which 17β-estradiol alters PAH progression and outcomes remain unclear. Since proximal PA stiffness, one hallmark of PAH, is a powerful predictor of mortality and morbidity, we hypothesized that 17β-estradiol attenuates PAH-induced changes in mechanical properties in conduit proximal PAs, which imparts hemodynamic and energetic benefits to RV function. To test this hypothesis, female mice were ovariectomized and treated with 17β-estradiol or placebo. PAH was induced in mice using SU5416 and chronic hypoxia (SuHx). Extra-lobar left PAs were isolated and mechanically tested ex vivo to study both static and frequency-dependent mechanical behaviors in the presence or absence of SMC activation. Our static mechanical test showed significant stiffening of large PAs with PAH (P < 0.05). 17β-estradiol restored PA compliance to control levels. The dynamic mechanical test demonstrated that 17β-estradiol protected the arterial wall from the PAH-induced frequency-dependent decline in dynamic stiffness and loss of viscosity with PAH (P<0.05). As demonstrated by the in vivo measurement of PA hemodynamics via RV catheterization, modulation by 17β-estradiol of mechanical proximal PAs reduced pulsatile loading, which contributed to improved ventricular-vascular coupling. This study provides a mechanical mechanism for delayed disease progression and better outcome in female PAH patients and underscores the therapeutic potential of 17β-estradiol in PAH.
Keywords: Pulmonary arterial hypertension, 17β-estradiol, sex difference, arterial stiffness, viscoelasticity
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a fast progressing vascular disease, characterized by proximal pulmonary arterial (PA) stiffening and distal PA occlusion, which increases right ventricular (RV) afterload, and ultimately leads to RV failure. PAH disproportionally affects women, with a female to male ratio of ~4:1 1; however, female PAH patients have better RV function 2 and a higher survival rate than male patients 3. Both animal and clinical studies suggest that 17β-estradiol plays an important role in the sex differences in PAH 4,5. However, the mechanistic role of 17β-estradiol in modifying PAH development and progression remains incompletely known.
17β-estradiol modifies age- and hypertension-related arterial stiffening in systemic arteries 6,7. 17β-estradiol deficiency (due to ovariectomy or menopause) increases arterial stiffness 8,9 and 17β-estradiol therapy restores stiffness (and its inverse, compliance) toward healthy values 6,7. Stiffening of large, proximal PAs, a common feature of PAH, occurs early in disease progression 10 and is a powerful predictor of mortality in PAH 11,12. PA compliance primarily affects the oscillatory component of RV afterload; loss of PA compliance (PA stiffening) increases RV afterload and thus affects RV adaption to PAH. In addition, the stiffening of large proximal PAs reduces their ability to dampen the pulsatility of pressure and flow to distal PAs, which may exacerbate distal PA remodeling and thus progression of PAH 13,14. To understand how and to what extent 17β-estradiol affects proximal PA stiffening and PAH progression, the first step is to comprehensively investigate the effects of 17β-estradiol on PAH-induced biomechanical changes in proximal PAs.
Most prior studies on the effects of 17β-estradiol on PA biomechanical properties have focused on its acute effects on PA vasoconstriction in healthy animals 15–17. The chronic effects of 17β-estradiol on biomechanical properties of remodeled PAs remain underexplored. Therefore, we tested the hypothesis that 17β-estradiol attenuates PAH-induced changes in proximal PA mechanical properties, which imparts hemodynamic and energetic benefits to RV adaption. We found that 17β-estradiol protected PAs from PAH-induced loss of arterial compliance and viscoelasticity by modulating vessel morphology and constituents. This finding provides a mechanical mechanism for delayed disease progression and better outcomes in female PAH patients.
METHODS
Animals
Female C57/BL6 mice (9–10 weeks old) were ovariectomized and treated with 17β-estradiol or placebo. PAH was induced using SU5416 and chronic hypoxia (SuHx). PA hemodynamics were measured in vivo via RV catheterization. Subsequently, left extra-lobar PAs were isolated and mechanically tested ex vivo to study both static and frequency-dependent behaviors in the presence or absence of SMC activation. All protocols and procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Detailed methods are provided in the online-only Data Supplement.
RESULTS
In vivo hemodynamics
SuHx caused hypertension as expected (Table). 17β-estradiol did not affect PAH severity, which is important because quantitative comparison of PA stiffness and elastic modulus is often confounded by differences in mean transmural pressure 18. Since cardiac output was preserved in both SuHx groups, total pulmonary vascular resistance (tPVR) significantly increased (P<0.05) in both SuHx groups and 17β-estradiol did not affect this increase (Table). In contrast, 17β-estradiol limited the increase in pulse pressure (PP) and decrease in global compliance index (SV/PP) induced by SuHx (Table), which suggests that 17β-estradiol modulates conduit PA stiffness.
Table.
Effects of 17β-estradiol on pulmonary hemodynamics in PAH
| Parameters | CTL_P | CTL_E | SuHx_P | SuHx_E |
|---|---|---|---|---|
| mPAP (mmHg) | 16.7±0.8 | 16.7±1.2 | 27.2±0.8* | 24.1±1.5* |
| sPAP (mmHg) | 21.5±1.4 | 22.4±1.3 | 37.5±1.2* | 30.8±1.8* |
| dPAP (mmHg) | 11.2±0.7 | 11.7±1.0 | 19.3±0.7* | 19.2±1.4* |
| PP (mmHg) | 12.0±0.5 | 12.0±0.5 | 18.2±1.0* | 11.6±1.1† |
| SV (μl) | 19.7±1.6 | 21.7±1.7 | 17.3±1.3 | 19.1±0.2 |
| CO (ml/min) | 10.6±0.8 | 10.0±0.8 | 9.8±0.7 | 9.5±0.9 |
| CI (ml/min/g) | 0.48±0.04 | 0.47±0.03 | 0.48±0.04 | 0.43±0.04 |
| SV/PP (μl/mmHg) | 1.76±0.13 | 1.89±0.12 | 0.98±0.12* | 1.68±0.23† |
| tPVR (mmHg min/ml) | 1.6±0.1 | 1.5±0.1 | 2.9±0.3* | 2.8±0.5* |
Mean ± SE values (n = 6–10 per group). mPAP, sPAP, and dPAP indicate mean, systolic and diastolic PA pressure, respectively; PP, pulse pressure, SV, stroke volume; CO, cardiac output; CI, cardiac index; tPVR (= mPAP/CO), total vascular resistance; CTL, control; P, placebo-treated; E, estradiol-treated.
P<0.05 vs Control;
P<0.05 vs Placebo.
Ex vivo mechanics
Vasoreactivity
The receptor-independent vasoconstrictor KCl caused a dose-dependent reduction in PA diameter measured ex vivo (Figure 1A). In control groups, 17β-estradiol treatment tended to reduce the magnitude change, suggesting that 17β-estradiol attenuated vasoreactivity in healthy arteries. In contrast, in the SuHx groups, 17β-estradiol treatment increased the magnitude change (P =0.05 vs. placebo-treated SuHx group at 50 mM KCl), suggesting that 17β-estradiol sensitizes diseased PAs to vasoconstriction by KCl.
Figure 1.
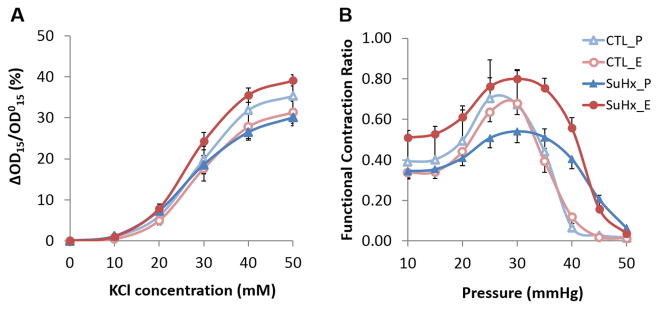
17β-estradiol enhances conduit PA vasoreactivity in PAH. (A) Dose-dependent response to receptor-independent vasoconstrictor KCl and (B) pressure-dependence of functional contraction ratio at 50 mM KCl.
Functional contraction ratio, a measure of arterial contractility, exhibited a downward parabolic relationship with transmural pressures ranging from 10 to 50 mmHg (Figure 1B). 17β-estradiol enhanced the maximal functional contraction ratio in the SuHx groups but had no effect in the control groups, further indicating that 17β-estradiol sensitizes diseased conduit PAs to vasoconstriction.
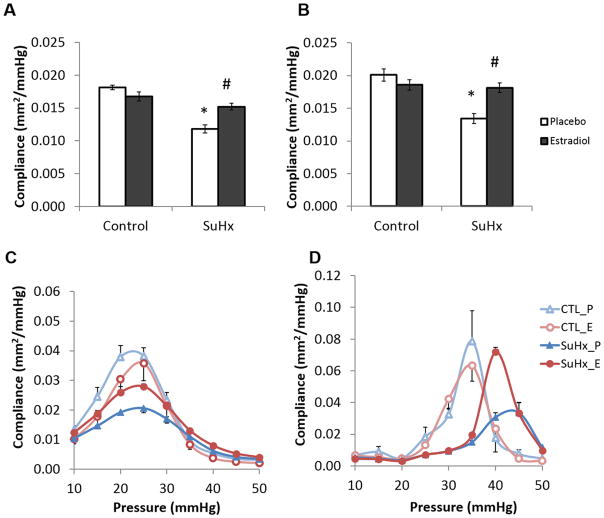
Compliance
SuHx reduced conduit PA compliance, and 17β-estradiol treatment restored it to control levels (Figure 2A). SMC activation increased total PA compliance in all groups (Figure 2B). A sigmoid relationship between PA compliance and pressure was observed. Without SMC tone, PA compliance peaked at a pressure of approximately 25 mmHg (Figure 2C). 17β-estradiol treatment attenuated the loss of maximal compliance seen in the placebo-treated SuHx group, but did not restore maximal compliance to control levels. SMC activation increased the maximal compliance and shifted it to higher pressures in all groups (Figure 2D). 17β-estradiol further enhanced the effect of SMC activation on compliance and restored the maximal compliance to control levels.
Figure 2.
17β-estradiol preserves conduit PA compliance in PAH (mean ± SE). Total PA compliance in the pressure range of 5 to 50 mmHg (A) in the absence of SMC tone and (B) with SMC activation at 50 mM KCl; pressure-compliance curves (C) in the absence of SMC tone and (D) with SMC activation at 50 mM KCl. *, P<0.05 vs. Control; #, P<0.05 vs. Placebo.*, P<0.05 vs. Control; #, P<0.05 vs. Placebo.
Static mechanical properties
SuHx shifted the stress-strain relationships upwards and to the left (Figure 3A). 17β-estradiol treatment attenuated the shift of the stress-strain curves, although the 17β-estradiol-induced change was small compared to the SuHx-induced change. Compared to the stress-strain curve without SMC tone, isobaric SMC contraction resulted in a more linear stress-strain relationship in the low strain regime (Figure 3B). SMC activation had no effect in the high strain regime, indicating that collagen remains the primary load-bearing wall component at high pressures and strains.
Figure 3.
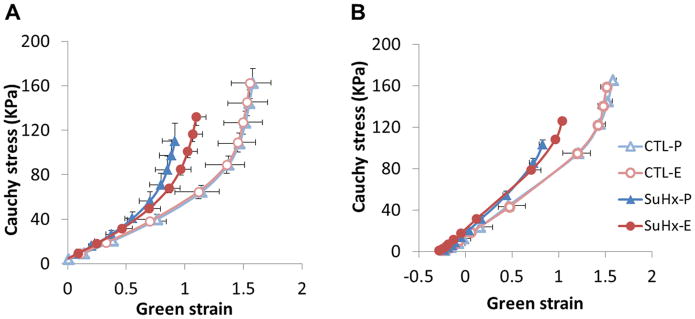
17β-estradiol attenuates changes in conduit PA static mechanical properties in PAH. Stress-strain curves (A) in the absence of SMC tone and (B) with SMC activation at 50 mM KCl.
SuHx decreased the transition strain, a threshold above which collagen engages and bears load, (Figure S2A). 17β-estradiol treatment attenuated this decrease. A similar trend was observed with SMC activation (Figure S2B).
Dynamic mechanical properties
SuHx increased dynamic elastic modulus at all frequencies (Figure 4A) and 17β-estradiol tended to attenuate this increase in elastic modulus. SuHx caused the dynamic modulus to decrease progressively with frequency in a range of 1 to 10 Hz, such that the dynamic modulus at 10Hz was significantly lower than that at 0.1Hz. 17β-estradiol tended to attenuate the rate of frequency-dependent decrease.
Figure 4.
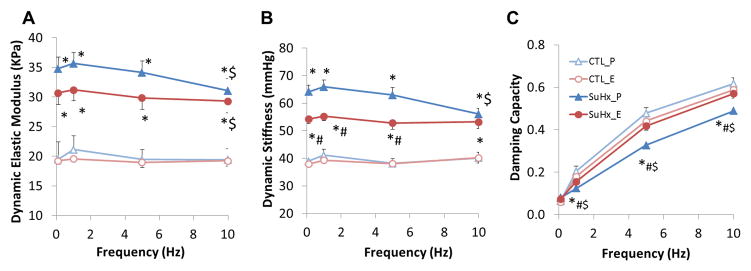
17β-estradiol attenuates changes in conduit PA dynamic mechanical properties in PAH. (A) Dynamic elastic modulus, (B) dynamic stiffness, and (C) damping capacity. *, P<0.05 vs. Control; #, P<0.05 vs. Placebo; $, P<0.05 vs. 0.1 Hz.
Similarly, SuHx significantly increased dynamic stiffness and 17β-estradiol significantly reduced the increase up to 5 Hz (Figure 4B). Since the frequency-dependent reduction in dynamic stiffness only occurred in the placebo-treated SuHx group, no difference was detected between the 17β-estradiol- and placebo-treated SuHx groups at 10 Hz. Damping capacity increased with frequency in all groups (Figure 4C). SuHx significantly reduced the damping capacity and 17β-estradiol restored damping capacity to control levels, indicating that 17β-estradiol protects the PAH-induced reduction in wall viscosity. We did not find any significant differences in dynamic elasticity, dynamic stiffness, or damping capacity at 10 Hz between with and without SMC activation (data not shown).
Morphological and histological data
SuHx increased RV weight normalized by body weight (Table S1), which is evidence of RV hypertrophy. 17β-estradiol had a limited effect on the normalized RV weight. PA outer diameter measured optically at 15 mmHg was significantly larger in the 17β-estradiol-treated SuHx group compared to both the control and placebo-treated SuHx groups (Table S1). The wall thickness measured by histology increased significantly in SuHx groups and this increase was attenuated by 17β-estradiol (Table S1). The same trend was observed in the medial wall thickness.
SuHx increased the area fraction of collagen measured histologically, and 17β-estradiol attenuated this increase (Figure S1M and N). Elastin did not significantly change with PAH or with 17β-estradiol treatment (data not shown). SuHx increased proteoglycans in both 17β-estradiol- and placebo-treated groups (Figure S1O and P). Changes in nitrotyrosine, a biomarker of oxidative stress, were not detected (8–10% of the vessel wall). Smoothelin, a biomarker for the fully differentiated, contractile phenotype of SMCs 19, increased significantly in the medial layer of the 17β-estradiol-treated SuHx group compared to the control groups (Figure S1Q and R); no significant difference was detected between the 17β-estradiol- and placebo-treated SuHx groups.
Discussion
Here we investigated the effects of 17β-estradiol on changes in conduit PA mechanical properties in mice with PAH via ex vivo mechanical testing. The novel findings include (1) 17β-estradiol enhanced vasoreactivity with PAH; (2) 17β-estradiol attenuated stiffening with PAH; and (3) 17β-estradiol preserved wall viscosity. Our in vivo pulmonary hemodynamics data indicate that 17β-estradiol reduced RV pulsatile load and improved ventricular-vascular coupling efficiency. Since conduit PA mechanical properties play an important role in distal PA remodeling and RV adaption to PAH, the protective effects of 17β-estradiol likely contribute to improved outcomes for female PAH patients compared to male patients.
17β-estradiol enhances conduit PA vasoreactivity in PAH
Vasoconstriction is an important contributor to PAH 20. Both endogenous and exogenously administered estrogen attenuated vasoconstriction of healthy PA induced by vasoactive agents or hypoxia16,17. We hypothesized that these effects of 17β-estradiol are altered by PAH. Our results show that in the PAH-remodeled PA, 17β-estradiol heightened drug-induced vasocontractility. Without 17β-estradiol, the ability of conduit PAs to respond to vasoconstrictors was impaired, as previously observed in both hypoxic- and MCT-treated rats 21. This suppressed vasoreactivity can result from de-differentiation of pulmonary SMCs to a more proliferative/synthetic and less contractile phenotype in PH 21. This view is supported by the evidence of altered growth responses of pulmonary SMCs from PAH patients to antiproliferative BMP and TGF-β 21. Here we observed that 17β-estradiol upregulates smoothelin in the PA medial layer of diseased animals (Figure S1R), suggesting estradiol prevents SMCs de-differentiation in PAH.
17β-estradiol attenuates PA stiffening in PAH
Premenopausal women have greater arterial distensibility than age-matched men and postmenopausal women 22. 17β-estradiol protects systemic arteries from age- and hypertensive-related stiffening 6–8. Here we show that 17β-estradiol attenuates loss of PA compliance in PAH, consistent with our previous study 4. Furthermore, we demonstrate that SMC activation significantly altered the pressure-compliance relationship and estrogen enhanced the effects of SMC activation on PA compliance. The increase in PA compliance with SMC activation is consistent with previous studies 23 and can be attributed to disengagement of collagen at a given pressure. The rightward shift of the pressure-compliance curves enables the PAs to maintain compliance at higher pressures. These findings suggest that increased conduit PA SMC reactivity is a protective adaptation to hypertension and that 17β-estradiol enhances the benefits of SMC activation. However, as increased SMC reactivity in distal pulmonary vasculature is detrimental in PAH 20, whether 17β-estradiol also sensitizes distal PA SMC reactivity warrants further exploration.
Arterial compliance is primarily influenced by material properties and morphology 24. Our results show that rather than significantly reducing wall elasticity, chronic administration of 17β-estradiol increased diameter and attenuated wall thickening, which compensated for the increase in elastic modulus in PAH. 17β-estradiol also significantly reduced the PAH-induced collagen accumulation and postponed early engagement of collagen, both contributing to attenuating arterial stiffening with PAH. In addition, as suggested by the pressure-compliance curves, differences in compliance can result from the pressure ranges in which the conduit PA operates in vivo, which would engage different wall components in normal and hypertensive groups. For the 17β-estradiol-treated hypertensive group, the conduit PA operates around the maximal region of compliance, which further reduces the difference in compliance between the control and the 17β-estradiol-treated PAH groups in vivo.
17β-estradiol protects PA viscoelasticity in PAH
Viscoelastic properties of the arterial wall determine the relationship between pulsatile pressure and flow in cardiovascular system. Arterial viscoelasticity deteriorates with aging and hypertension 25,26. A recent study showed that carotid artery viscoelastic deterioration is a significant risk factor for coronary artery disease 27. Women after menopause had increased arterial viscoelastic deterioration compared to age-matched men, suggesting that 17β-estradiol protects against viscoelastic deterioration. Our group recently reported that PAH modified frequency-dependent viscoelastic behaviors in conduit PAs of male mice 28. Here, we are the first to address the interaction between viscoelasticity and 17β-estradiol in PAs.
We found that 17β-estradiol tended to attenuate the PAH-induced increase in dynamic elasticity and stiffness for all frequencies studied. 17β-estradiol also prevented the frequency-dependent decline in the dynamic modulus and dynamic stiffness in the hypertensive groups. The biomechanical mechanisms for frequency-dependent changes in elasticity and stiffness remain unknown. Since 17β-estradiol attenuated collagen deposition and limited the increase in SMC content in PAs with PAH, we speculate that interactions between collagen, vascular cell types and intracellular SMC proteins are important contributors to frequency-dependent arterial mechanics.
While previous studies have shown that damping capacity, a measure of arterial wall viscosity, decreases in PAH28, we show that 17β-estradiol preserved damping capacity. Changes in ECM components such as collagen, proteoglycans and SMC content have been linked with changes in arterial viscosity 28–30. Here, we found a positive correlation between collagen content and damping capacity at 0.1 Hz (Figure S3A). The relationship became negative at higher frequencies (Figure S3B), suggesting the contribution of collagen content to arterial viscosity is frequency-dependent. Furthermore, we found a negative correlation between the medial wall thickness and wall viscosity in conduit PAs at a physiological frequency (Figure S4). Although this finding is not consistent with Bia’s study on healthy carotid and aortic arterial segments30, we speculate that this inconsistency is due to inherent differences between systemic and pulmonary arteries or differences between healthy and diseased states. It is difficult to attribute the change in wall viscosity to changes in any single PA constituent since arterial viscoelasticity is likely affected by interactions between cells and matrix proteins 31, organization of matrix 29, and hemodynamic loading 32.
17β-estradiol is an important contributor to sex disparities in PAH outcomes
The pulsatile pulmonary pressure-flow relationship provides insight into the impact of changes in conduit PA mechanical properties on RV afterload, RV-PA coupling efficiency, and distal arterial remodeling, which play major roles in the progression of PAH. Our study shows that 17β-estradiol restored PP without significantly affecting the elevation of mean pressure and tPVR, indicating that 17β-estradiol had limited effects on distal occlusive remodeling in early PAH. This is expected as considerable changes in arterial stiffness occur in early PAH with a small change in pulmonary resistance 10. The ability of 17β-estradiol to preserve PA compliance likely contributed to attenuated RV pulsatile load in PAH (Figure S5), which may explain the improved RV function and ventricular-vascular coupling in the 17β-estradiol-treated SuHx group observed in our previous study 4.
Loss of large PA compliance with PAH also has consequences for distal PAs. In particular, loss of compliance can result in significantly higher PP (by 2–3 fold), which has been linked to endothelial cell dysfunction and SMC hypertrophy 13,14. Here, we report that 17β-estradiol restores large artery compliance and thus the PP (Figure S5), which should lessen remodeling of the distal PAs, a key feature in PAH disease progression.
The conduit PA mechanical property changes we found in the placebo-treated SuHx group were also found in the male SuHx group in our previous study 28. We also found similar cardiac response to SuHx exposure in the current placebo-treated female and prior male groups 4. Since the similar mean PA pressure excluded the impact of pressure on mechanical and structural remodeling of conduit PA between the 17β-estradiol and placebo-treated SuHx groups, we conclude that the estrogenic modulation of arterial wall mechanical properties is, at least partially, responsible for sex disparities in PAH outcomes.
Study limitations
First, this study focuses on the effects of 17β-estradiol on the mechanical properties of conduit PAs. The conclusions derived from this study cannot directly apply to other forms of estrogens such as conjugated equine estrogens and synthetic estrogens without further confirmation. In addition, whether the protection of estradiol on PAs is mediated through its metabolites such as 2-methoxyestradiol 33 or estrogen receptors 34 is not clear and warrants further study. Second, for better comparison, we tested material properties of isolated conduit PAs under the same condition (same pressure- and frequency-range) rather than under their respective conditions in vivo. However, changes in material properties may be an adaptation to the differences in pressures and flow. For example, the reduction in arterial wall viscosity in the placebo-treated SuHx group may be a compensatory mechanism for the increased PP 32. Lastly, the effects of 17β-estradiol were studied using relatively young adult mice with mild PAH, so the results may not be applicable to aged animals or more severe disease. Investigations into the interaction of 17β-estradiol, age and PAH severity are warranted.
Perspective
Our study demonstrated that 17β-estradiol attenuated stiffening and limited viscoelastic deterioration of conduit PAs in PAH. The modulation in arterial mechanical properties by 17β-estradiol improved pulmonary hemodynamics and RV-PA coupling efficiency. As large PA stiffening is a powerful predictor of morbidity and mortality 11,12, a key implication is that exogenous 17β-estradiol may act as a novel therapy to prevent PA stiffening and thus delay the progression of PAH. This finding is profound because commonly used antihypertensive medications in PAH are based largely on their vasodilating effects on smaller resistance vessels and have little effect on large arterial stiffness 35. In addition, this study suggests that the effects of 17β-estradiol on PA stiffness and viscoelasticity are important contributors to sex differences in PAH progression and outcomes.
Supplementary Material
Novelty and Significance.
What is new?
Pulmonary arterial wall stiffening, a hallmark of PAH, occurs early in PAH and has been associated with increased mortality and mobility in advanced PAH. Our study demonstrated that estrogen attenuates PAH-induced arterial stiffening.
Frequency-dependent behaviors of the pulmonary arterial wall including dynamic elasticity, stiffness and viscosity are altered in PAH. 17β-estradiol protects conduit PAs from PAH-induced deterioration in viscoelasticity.
What is relevant?
The modulation by 17β-estradiol of conduit PA stiffness and viscoelasticity provides a mechanical mechanism by which 17β-estradiol contributes to sex differences in PAH progression and outcomes.
Our data suggest that 17β-estradiol may be an effective therapy for preventing or reducing PA stiffening in PAH.
Summary.
Our study demonstrates that 17β-estradiol attenuates arterial stiffening and limits viscoelastic deterioration of conduit PAs in PAH. The attenuation of arterial mechanical property changes by 17β-estradiol also preserves pulmonary hemodynamics and RV-PA coupling efficiency.
Acknowledgments
Sources of Funding
This work was supported by NIH R01HL-086939 (to N.C. Chesler) and AHA 13POST16910091 (to A. Liu).
Footnotes
Conflict of interest/Disclosure
None.
References
- 1.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M. Pulmonary Arterial Hypertension Baseline Characteristics From the REVEAL Registry. CHEST J. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 2.Kawut SM, Lima JAC, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, Kronmal RA, Bluemke DA. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, Chaouat A, Chabot F, Cordier J-F, Habib G, Gressin V, Jing Z-C, Souza R, Simonneau G. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 4.Liu A, Schreier D, Tian L, Eickhoff JC, Wang Z, Hacker TA, Chesler NC. Direct and indirect protection of right ventricular function by estrogen in an experimental model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2014;307:H273–283. doi: 10.1152/ajpheart.00758.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JAC, Kawut SM. Sex Hormones Are Associated with Right Ventricular Structure and Function The MESA-Right Ventricle Study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawecka-Jaszcz K, Czarnecka D, Olszanecka A, Rajzer M, Jankowski P. The effect of hormone replacement therapy on arterial blood pressure and vascular compliance in postmenopausal women with arterial hypertension. Journal of Hum Hypertens. 2002;16:506–516. doi: 10.1038/sj.jhh.1001431. [DOI] [PubMed] [Google Scholar]
- 7.Miura S, Tanaka E, Mori A, Toya M, Takahashi K, Nakahara K, Ohmichi M, Kurachi H. Hormone replacement therapy improves arterial stiffness in normotensive postmenopausal women. Maturitas. 2003;45:293–298. doi: 10.1016/s0378-5122(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 8.Tatchum-Talom R, Martel C, Marette A. Influence of estrogen on aortic stiffness and endothelial function in female rats. Am J Physiol Heart Circ Physiol. 2002;282:H491–498. doi: 10.1152/ajpheart.00589.2001. [DOI] [PubMed] [Google Scholar]
- 9.Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, Motobe K, Hori S, Yamashina A. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis. 2006;184:137–142. doi: 10.1016/j.atherosclerosis.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev. 2010;19:197–203. doi: 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of Pulmonary Arterial Capacitance and Mortality in Idiopathic Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 12.Gan CT-J, Lankhaar J-W, Westerhof N, Marcus JT, Becker A, Twisk JWR, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively Assessed Pulmonary Artery Stiffness Predicts Mortality in Pulmonary Arterial Hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High Pulsatility Flow Induces Adhesion Molecule and Cytokine mRNA Expression in Distal Pulmonary Artery Endothelial Cells. Ann Biomed Eng. 2009;37:1082–1092. doi: 10.1007/s10439-009-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott D, Tan Y, Shandas R, Stenmark KR, Tan W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol - Lung Cell Mol Physiol. 2013;304:L70–L81. doi: 10.1152/ajplung.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender Differences in the Vasomotor Effects of Different Steroid Hormones in Rat Pulmonary and Coronary Arteries. Horm Metab Res. 2001;33:645–652. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 16.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock. 2008;30:660–667. doi: 10.1097/SHK.0b013e31816f239f. [DOI] [PubMed] [Google Scholar]
- 17.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol - Endocrinol Metab. 2007;293:E865–E871. doi: 10.1152/ajpendo.00201.2007. [DOI] [PubMed] [Google Scholar]
- 18.Tian L, Kellihan HB, Henningsen J, Bellofiore A, Forouzan O, Roldán-Alzate A, Consigny DW, Gunderson M, Dailey SH, Francois CJ, Chesler NC. Pulmonary artery relative area change is inversely related to ex vivo measured arterial elastic modulus in the canine model of acute pulmonary embolization. J Biomech. 2014;47:2904–2910. doi: 10.1016/j.jbiomech.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Loop FTL, Gabbiani G, Kohnen G, Ramaekers FCS, van Eys GJJM. Differentiation of Smooth Muscle Cells in Human Blood Vessels as Defined by Smoothelin, a Novel Marker for the Contractile Phenotype. Arterioscler Thromb Vasc Biol. 1997;17:665–671. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- 20.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho Kinase–Mediated Vasoconstriction Is Important in Severe Occlusive Pulmonary Arterial Hypertension in Rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 21.Mam V, Tanbe AF, Vitali SH, Arons E, Christou HA, Khalil RA. Impaired Vasoconstriction and Nitric Oxide-Mediated Relaxation in Pulmonary Arteries of Hypoxia- and Monocrotaline-Induced Pulmonary Hypertensive Rats. J Pharmacol Exp Ther. 2010;332:455–462. doi: 10.1124/jpet.109.160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London GM, Guerin AP, Pannier B, Marchais SJ, Stimpel M. Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension. 1995;26:514–519. doi: 10.1161/01.hyp.26.3.514. [DOI] [PubMed] [Google Scholar]
- 23.Santana DB, Barra JG, Grignola JC, Ginés FF, Armentano RL. Pulmonary artery smooth muscle activation attenuates arterial dysfunction during acute pulmonary hypertension. J Appl Physiol. 2005;98:605–613. doi: 10.1152/japplphysiol.00361.2004. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ. 2011;1:212–223. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Learoyd BM, Taylor MG. Alterations with Age in the Viscoelastic Properties of Human Arterial Walls. Circ Res. 1966;18:278–292. doi: 10.1161/01.res.18.3.278. [DOI] [PubMed] [Google Scholar]
- 26.Simon A, Levenson J. Effect of hypertension on viscoelasticity of large arteries in humans. Curr Hypertens Rep. 2001;3:74–79. doi: 10.1007/s11906-001-0084-9. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi R, Hosaka A, Miyahara T, Hoshina K, Okamoto H, Shigematsu K, Miyata T, Sugiura R, Yokobori T, Watanabe T. Viscoelastic Deterioration of the Carotid Artery Vascular Wall is a Possible Predictor of Coronary Artery Disease. J Atheroscler Thromb. 2014;21:415–423. doi: 10.5551/jat.24513. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Lakes RS, Golob M, Eickhoff JC, Chesler NC. Changes in Large Pulmonary Arterial Viscoelasticity in Chronic Pulmonary Hypertension. PLoS ONE. 2013;8:e78569. doi: 10.1371/journal.pone.0078569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Chesler NC. Role of collagen content and cross-linking in large pulmonary arterial stiffening after chronic hypoxia. Biomech Model Mechanobiol. 2012;11:279–289. doi: 10.1007/s10237-011-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bia D, Zócalo Y, Cabrera-Fischer EI, Wray S, Armentano RL. Quantitative Analysis of the Relationship between Blood Vessel Wall Constituents and Viscoelastic Properties: Dynamic Biomechanical and Structural In Vitro Studies in Aorta and Carotid Arteries. Physiol J. 2014;2014:e142421. [Google Scholar]
- 31.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 32.Boutouyrie P, Boumaza S, Challande P, Lacolley P, Laurent S. Smooth Muscle Tone and Arterial Wall Viscosity An In Vivo/In Vitro Study. Hypertension. 1998;32:360–364. doi: 10.1161/01.hyp.32.2.360. [DOI] [PubMed] [Google Scholar]
- 33.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul Pharmacol. 2006;45:358–367. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Demark MV, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol Attenuates Hypoxic Pulmonary Hypertension via Estrogen Receptor–mediated Effects. Am J Respir Crit Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? Pulm Circ. 2014;4:560–580. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.