Abstract
The prevalence of arterial stiffness increases with age while the level of the aging-suppressor protein klotho decreases with age. The objective of this study is to assess if haplodeficiency of klotho gene causes arterial stiffness and investigate the underlying mechanism. Pulse wave velocity, a direct measure of arterial stiffness, was increased significantly in klotho heterozygous (klotho+/−) mice vs. their age-matched wild-type (WT) littermates, suggesting that haplodeficiency of klotho causes arterial stiffening. Notably, plasma aldosterone levels were elevated significantly in klotho+/− mice. Treatment with eplerenone (6 mg/kg/day, IP), an aldosterone receptor blocker, abolished klotho deficiency-induced arterial stiffening in klotho+/− mice. Klotho deficiency was associated with increased collagen and decreased elastin contents in the media of aortas. In addition, arterial MMP2, MMP9 and TGFβ1 expression and myofibroblast differentiation were increased in klotho+/− mice. These klotho deficiency-related changes can be blocked by eplerenone. Protein expression of scleraxis, a transcription factor for collagen synthesis, and LC3-II/LC3-I, an index of autophagy, were upregulated in aortas of klotho+/− mice, which can be abolished by eplerenone. In cultured mouse aortic smooth muscle cells, aldosterone increased collagen-1 expression which can be completely eliminated by siRNA knockdown of scleraxis. Interestingly, alsosterone decreased elastin levels in smooth muscle cells which can be abolished by siRNA knockdown of Beclin-1, an autophagy-related gene.
Conclusion
This study demonstrated for the first time that klotho deficiency-induced arterial stiffening may involve aldosterone-mediated upregulation of scleraxis and induction of autophagy which led to increased collagen-1 expression and decreased elastin levels, respectively.
Keywords: arterial stiffness, scleraxis, autophagy, Beclin-1, myofibroblast, collagen, elastin, smooth muscle cell
Introduction
The klotho gene, which was originally identified as an ‘aging suppressor’ gene in mice, encodes a single-pass transmembrane protein that is predominantly expressed in the distal tubular epithelial cells of the kidneys and choroid plexus of the brain. 1–4 Insertional mutation of mouse klotho gene resulted in extensive premature aging phenotypes and shortens lifespan.1 However, overexpression of mouse klotho gene rescued aging phenotypes and extended lifespan by 20%–30%.5 Therefore, klotho is an anti-aging gene.3
Arterial stiffness is one of the earliest detectable manifestations of adverse structural and functional changes within the vessel wall. Increased stiffness of large conduit arteries is a major risk factor for hypertension.6–7 It has also been shown that arterial stiffness is an independent predictor of stroke and ischemic heart disease.8 The wall of large arteries, especially the aorta, thickens and loses elasticity over time which results in an increase in arterial stiffness.7 Elastin and collagen fibers are major determinants of mechanical properties of large arteries.9–10 Large conduit arteries become stiffer with age due to fragmentation and degradation of elastin and subsequent replacement by collagen which is 100–1000 times stiffer than elastin.11 Large artery stiffening may be influenced by aging, hemodynamic forces, and excessive hormones, such as aldosterone.12–14 It was reported that patients with hyperaldosteronism have increased arterial stiffness.15 Although excessive aldosterone may be involved in arterial stiffness,16 the underlying mechanism has yet to be determined.
The klotho level decreases with age17 while the prevalence of arterial stiffness increases with age.18 At age 70 years, the serum level of klotho is less than one half of what it was at age 40 years.17 Moreover, the serum klotho level is significantly decreased in patients with arterial stiffness and chronic kidney diseases.19 Therefore, we investigated if haplodeficiency of klotho causes arterial stiffening. We recently found that haplodeficiency of klotho upregulates adrenal CYP11B2 expression and aldosterone synthesis.20 Therefore, we plan to investigate if klotho deficiency causes arterial stiffness by upregulation of aldosterone levels. To study the potential involvement of aldosterone in the regulation of arterial stiffness, we assessed the effect of an aldosterone receptor blocker, eplerenone, on pulse wave velocity in klotho+/− mice. We further evaluated scleraxis (collagen transcription factor) and Beclin-1 (autopgagy-related gene) in aldosterone-induced collagen synthesis and elastin degradation in mouse vascular aortic smooth muscle cells (MOVAS).
Materials and Methods
See the Online Supplemental Methods.
Results
Haplodeficiency of Klotho gene (klotho+/−) increased arterial pulse wave velocity (PWV) and serum levels of aldosterone
Arterial pulse wave velocity (PWV) is a direct measure of arterial stiffness. Interestingly, PWV of klotho+/− mice was increased significantly compared to that of age-matched WT littermates (p<0.01) (Fig. 1A, 15 mo), indicating that klotho deficiency causes arterial stiffness. Following measurement of PWV, mice were euthanized for measuring serum aldosterone levels. Notably, serum aldosterone levels were higher in klotho+/− mice than in WT mice (Fig. 1B). Therefore, klotho deficiency increases circulating aldosterone levels.
Figure 1.
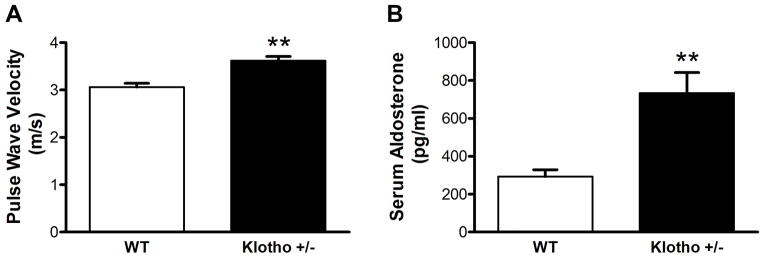
Haplodeficiency of Klotho gene (klotho+/−) increased arterial pulse wave velocity (PWV) and serum aldosterone levels. (A) PWV was measured in klotho+/− and age-mated WT mice by 10-MHz Doppler probes (n=14). (B) Serum aldosterone levels were measured by ELISA (n=6). Data are expressed as mean±SE and analyzed by a one-way ANOVA. **p<0.01 vs. WT group.
In another experiment, we monitored PWV and systolic blood pressure in mice from 14–16 weeks of age. PWV started to increase in klotho+/− mice around 14 weeks while blood pressure did not increase significantly by 16 weeks of age (Fig. S1A&B in the online-only Data Supplement). This result indicated that arterial stiffening preceded the development of elevation of blood pressure. Western blot analysis indicated that klotho protein expression was decreased significantly in kidneys and adrenal glands (Fig. S2), which is consistent with our recent studies.21
Blockade of aldosterone receptors abolished the increase in PWV in klotho+/− mice
To investigate if upregulation of aldosterone levels is involved in arterial stiffness, we treated klotho+/− mice and their WT littermates with eplerenone (6 mg/kg/day, IP), a specific aldosterone receptor antagonist. Blockade of aldosterone receptors decreased PWV of klotho+/− mice to the control level following a 3-week treatment (Fig. 2). Eplerenone did not affect PWV in WT mice (Fig. 2). The results suggest that upregulation of aldosterone levels may be involved in arterial stiffening due to klotho deficiency. Eplerenone did not affect body weight in either WT or klotho+/− mice (Fig. S3).
Figure 2.
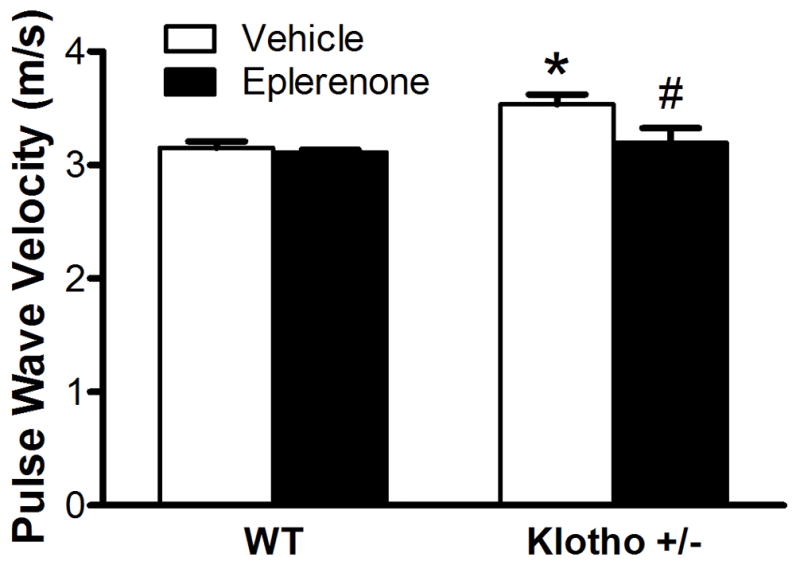
Blockade of aldosterone receptors abolished the increase of PWV in klotho+/− mice. PWV was measured after treatment with eplerenone for 3 weeks. Data are expressed as mean±SE and analyzed by two-way ANOVA. n=7. *p<0.05 vs. WT group; #p<0.05 vs. klotho+/−-vehicle group.
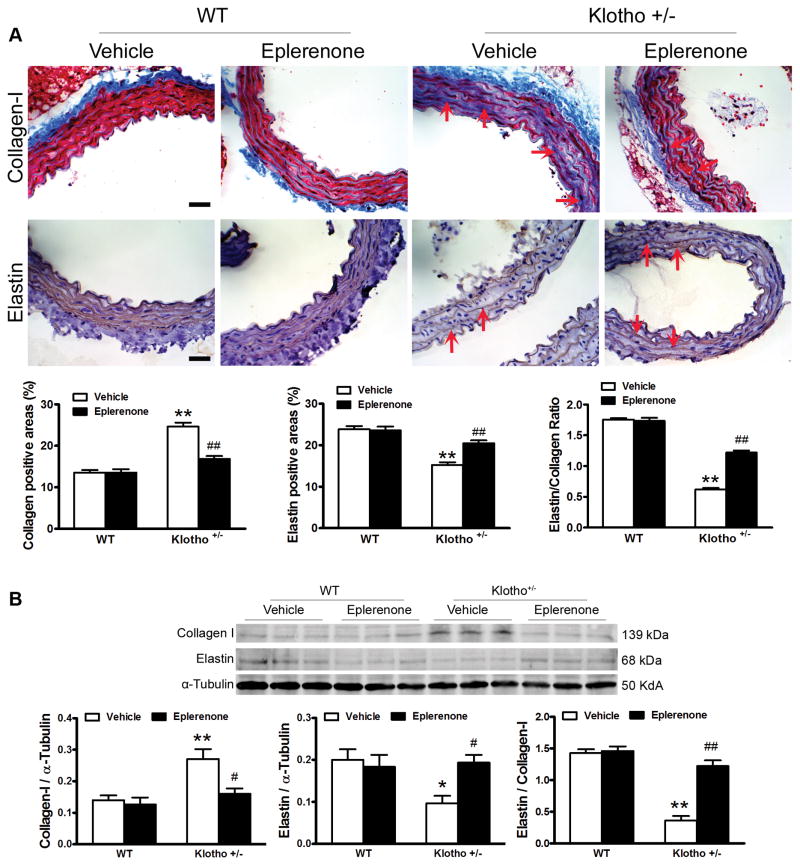
Haplodeficiency of klotho gene increased collagen expression but decreased elastin levels in aortas which can be abolished by blockade of aldosterone receptors
To investigate the molecular basis of arterial stiffening, we measured arterial collagen and elastin levels by immunostaining and western blot assays.22–24 The Immunostaining assay showed that aortic collagen expression levels were increased significantly in klotho+/− mice (Fig. 3A). Collagen deposition (blue) in klotho+/− mice was mainly found in the medial layer of the aorta. On the hand, aortic elastin levels (brown) were decreased significantly in klotho+/− mice (Fig. 3A). Collagen staining was increased in aortas but not in small arteries (carotid and femoral arteries) in klotho+/− mice (Fig. S4), suggesting that klotho deficiency causes remodeling primarily in large conduit arteries. Western blot analysis confirmed that klotho deficiency upregulated collagen I expression but downregulated elastin levels in aortas (Fig. 3B). The ratio of elastin to collagen in aortas was markedly decreased in klotho+/− mice (Fig. 3A&B), indicating that klotho deficiency causes arterial remodeling. Blockade of aldosterone receptors by eplerenone abolished the upregulation of collagen and downregulation of elastin in aortas leading to attenuation of arterial remodeling in klotho+/− mice. Eplerenone did not affect the ratio of elastin/collagen in WT mice (Fig. 3).
Figure 3.
Haplodeficiency of klotho gene increased collagen expression but decreased elastin levels in aortas which can be abolished by blockade of aldosterone receptors. (A) Immunohistochemical analysis of collagen-1 (blue) and elastin (brown). (B) Western blot analysis of collagen-1 and elastin. Data are expressed as mean±SE and analyzed by a two-way ANOVA. n=5. *p<0.05, **p<0.01 vs. WT group; #p<0.05, ##p<0.01 vs. klotho+/−-vehicle group. Scale bar = 20 μm.
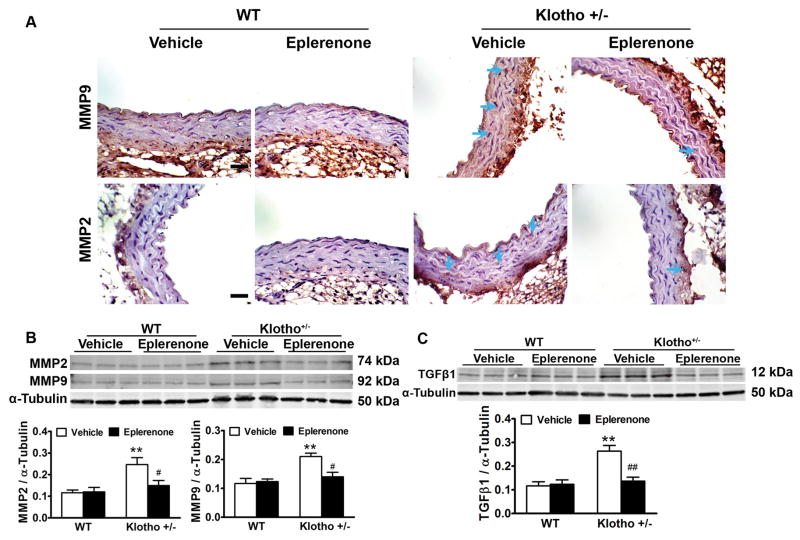
Haplodeficiency of klotho gene increased arterial MMP2, MMP9 and TGFβ1 expression which can be eliminated by blockade of aldosterone receptors
MMPs are a family of proteases that play important roles in extracellular matrix (ECM) remodeling and degradation. Increased MMP activity could contribute to ECM remodeling and fibrosis. The immunostaining assay showed that MMP2 and MMP9 expression were localized in smooth muscle layers (Fig. 4A). We further measured MMPs protein expression levels by western blots. MMP2 and MMP9 protein expressions were increased significantly in aortas of klotho+/− mice (Fig 4B). Blockade of aldosterone receptors by eplerenone decreased MMP2 and MMP9 expressions to the control levels (Fig. 4B). Eplerenone did not affect MMP2 and MMP9 expressions in WT mice (Fig. 4B).
Figure 4.
Haplodeficiency of klotho gene increased arterial MMP2, MMP9 and TGFβ1 expression which can be eliminated by blockade of aldosterone receptors. (A) Immunohistochemical staining results of MMP2 and MMP9. (B) Western blot analysis of MMP2 and MMP9 expression. (C) Western blot analysis of TGFβ1 expression. Data are expressed as mean±SE and analyzed by two-way ANOVA. n=5, *p<0.05, **p<0.01 vs WT group; #p<0.05, ##p<0.01 vs klotho+/−-vehicle group. Scale bar = 20 μm
TGFβ increases matrix protein synthesis and decreases matrix protein degradation, catalyzed by several enzyme families, including matrix metalloproteinases (MMP), resulting in tissue fibrosis.25 Western blot analysis showed that TGFβ1 expression was increased significantly in klotho+/− mice (Fig. 4C), indicating that klotho deficiency upregulated TGFβ1 expression. Blockade of aldosterone receptors by eplerenone decreased TGFβ1 expression to the control level in klotho+/− mice (Fig. 4C), suggesting that klotho deficiency-induced upregulation of TGFβ1 was mediated by the upregulation of aldosterone. Eplerenone did not affect TGFβ1 expressions in WT mice (Fig. 4C).
Klotho+/− increased aortic myofibroblast differentiation which can be abolished by blockade of aldosterone receptors
Myofibroblasts are primarily involved in collagen synthesis and fibrotic formation. The differentiated myofibroblasts lie in between fibroblasts and smooth muscle cells in differentiation. Myofibroblasts are characterized by the intermediate filament vimentin like alpha smooth muscle actin (α-SMA). α-SMA-positive cells were significantly increased in klotho+/− mice (Fig. 5), indicating that klotho deficiency promoted myofibroblast differentiation. Blockade of aldosterone receptors by eplerenone abolished klotho deficiency-induced myofibroblast differentiation (Fig. 5), suggesting that klotho deficiency-induced myofibroblast differentiation was mediated by upregulation of aldosterone levels. Eplerenone did not affect myofibroblasts differentiation in WT mice (Fig. 5).
Figure 5.
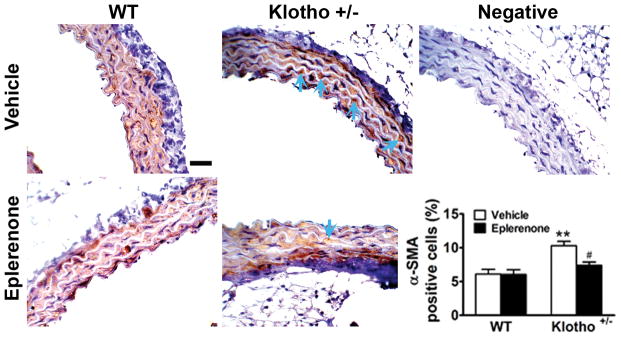
Klotho+/− increased aortic myofibroblasts differentiation which can be abolished by blockade of aldosterone receptors. Myofibroblast differentiation was evaluated by α-SMA positive cells using immunohistochemical staining. The semi-quantitative data are expressed as mean±SE and analyzed by two-way ANOVA. n=5, **p<0.01 vs WT group; #p<0.05 vs klotho+/−-vehicle group. Scale bar = 20 μm.
Aldosterone increased collagen-1 expression in smooth muscle cells by upregulation of transcriptional factor scleraxis
One of the interesting findings is that scleraxis was up-regulated in aortas of klotho+/− mice (Fig. S5A). Blockade of aldosterone receptors by eplerenone decreased scleraxis expression in klotho+/− mice to the control level (Fig. S5A), suggesting that klotho deficieny-induced upregulation of scleraxis was mediated by aldosterone. Scleraxis is a transcription factor that is implicated in regulating the development of collagen-rich tissues such as tendons.26–27 However, whether it is involved in the collagen synthesis in arterial cells is not clear.
To answer this question, mouse aortic smooth muscle cells (MOVAS) were treated with aldosterone for 16 h and then harvested for western blot analysis. Aldosterone increased scleraxis expression and collagen-1 expression in MOVAS (Fig. S5B). To investigate if scleraxis plays a role in aldosterone-induced up-regulation of collagen-1, we silenced scleraxis in MOVAS. Scleraxis siRNA was effective in knocking down scleraxis (Fig. S5C). Briefly, MOVAS were transfected with scleraxis siRNA (SiSCX) for 48 h before treatment with aldosterone for another 16 h. Aldosterone increased collagen-1 and scleraxis expression levels in MOVAS. siRNA-mediated knockdown of scleraxis completely eliminated upregulation of collagen-1 induced by aldosterone (Fig. S5D). Scleraxis siRNA did not affect the inhibitory effect of aldosterone on elastin expression in MOVAS (Fig. 6D). These results suggest that upregulation of scleraxis may mediate the aldosterone-induced increase in collagen-1 expression in MOVAS.
Aldosterone decreased elastin levels in smooth muscle cells through induction of autophagy
The ratio of LC3-II to LC3-I, a reliable marker of autophagy, was upregulated in aortas of klotho+/− mice (Fig. S6A). Blockade of aldosterone receptors by eplerenone abolished klotho deficiency-induced upregulation of the ratio of LC3-II/LC3-I. In cultured MOVAS, aldosterone increased the ratio of LC3-II/LC3-I ratio and decreased elastin expression (Fig. S6B).
To assess if autophagy plays a role in aldosterone-induced downregulation of elastin, we silenced Beclin-1, an autophagy-related gene. Beclin-1 siRNA effectively knocked down Beclin-1 and inhibited autophagy in MOVAS (Fig. S6C). Briefly, MOVAS were transfected with Beclin-1 siRNA (siBCN1) for 48 h before treatment with aldosterone for another 16 h. Aldosterone upregulated Beclin-1, elastase, MMP2, and MMP9 expression but decreased elastin levels in MOVAS (Fig. S6D). These changes were abolished by siRNA knockdown of Beclin-1 (Fig. S6D), suggesting that autophay may be involved in elastin degradation induced by aldosterone. Beclin-1 siRNA did not affect the promoting effect of aldosterone on collagen-1 expression in MOVAS (Fig. S6D).
Discussion
Klotho was originally identified as an aging-suppressor gene.3 Mutation of klotho gene causes multiple premature aging phenotypes and shortens life span.1 This study demonstrates, for the first time, that haplodeficiency of klotho gene caused arterial stiffening. Arterial stiffening is one of the earliest detectable manifestations of adverse structural and functional changes within the vessel wall.8 An increase in arterial stiffness is an independent risk factor for cardiovascular morbidity and mortality.7, 28–31 In humans, a decrease in serum level of soluble klotho is an independent biomarker of pronounced arterial stiffness in patients with chronic kidney disease.19 Conversely, an increase in plasma klotho levels is associated with reduced arterial stiffness in postmenopausal women.32
This study demonstrated that arterial stiffening occurred prior to the elevation of blood pressure, suggesting that arterial stiffening was not attributed to hypertension. The recent Framingham study showed that large artery stiffness precedes the development of hypertension.33 This report indicated that arterial stiffening may be the cause of hypertension.33 Two longitudinal studies have demonstrated that arterial stiffness predicts an increase in systolic blood pressure and incident hypertension.34–35 High fat diet-induced arterial stiffening also preceded the development of hypertension.36 In this study, we showed that hyperaldosteronism may mediate arterial stiffening due to klotho deficiency (Fig. 1–5). Indeed, a high level of aldosterone itself is sufficient to cause elastin degradation and increase collagen synthesis in smooth muscle cells (Fig. S5 & S6). Although hypertension could also contribute to vascular remodeling and stiffening, it is, however, a slow process. Nevertheless, this study does not exclude the possibility that persistent elevation of BP may also contribute to the progression of arterial stiffening in this model in its later stage. The limitation of this study is that it does not elucidate the relationship of arterial stiffening and hypertension (causality).
Serum levels of klotho decrease with age after age 4017 while the prevalence of arterial stiffening and hypertension increases with age.7 Our study provides the first experimental evidence that klotho deficiency may be a pathological factor for arterial stiffness. Klotho+/− mice were used which mimics a half klotho reduction in the aged population 17. The development of arterial stiffening in klotho+/− mice is a slow and gradual structural remodeling process starting at low level of stiffening (Fig. 1, S1). Arterial stiffening in klotho+/− mice is a natural model which may be moderate vs other models, e.g., the high fat/high sucrose-induced model.36 Klotho homozygous (−/−) mice demonstrate early and extensive aging phenotypes and die before the age of 8 weeks (body weight = 8 grams).1 Klotho homozygous mice also develop severe hyperphosphatemia and soft tissue calcification.3–4 As a result, klotho homozygous mice were not used.
Interestingly, haplodeficiency of klotho gene increased the level of circulating aldosterone (Fig. 1), which was supported by an observation by Fischer et al who showed that plasma levels of aldosterone were elevated significantly in klotho−/− mice.37 Our recent study showed that haplodeficiency of klotho gene upregulates adrenal CYP11B2 expression leading to increased aldosterone synthesis.20 To investigate if upregulation of aldosterone levels is involved in klotho deficiency-induced arterial stiffness, we treated klotho+/− mice with an aldosterone receptor blocker, eplerenone. Notably, blockade of the aldosterone action by eplerenone: (1) abolished the increase of PWV, (2) largely rescued arterial collagen deposition and elastin degradation, (3) eliminated the increases in arterial MMP2, MMP9 and TGFβ1 expression, and (4) attenuated myofibroblasts differentiation in aortas in klotho+/− mice. Together, this study provides the first evidence that klotho deficiency-induced arterial stiffening is mediated by upregulation of aldosterone levels.
Using mouse vascular aortic smooth muscle cells (MOVAS), we further investigated the molecular mechanism of klotho deficiency-induced upregulation of collagen expression and downregulation of elastin levels, the critical basis of arterial stiffness. Scleraxis, a member of the basic helix-loop-helix (bHLH) family of transcription factors, is specifically expressed in tendons and ligaments, where it can be detected from early progenitor cells to mature fibroblasts.38–40 Scleraxis is sufficient to upregulate expression of the collagen 1α2 gene in primary cardiac fibroblasts.41–42 Indeed, scleraxis regulates expression of the collagen 1α1 gene in tenocytes, and scleraxis gene deletion results in defects in the development of intermuscular and force-transmitting tendons concomitant with type I collagen loss.26–27 We showed that scleraxis was upregulated in the aorta of klotho+/− mice (Fig. S5). Interestingly, blockade of aldosterone receptors by eplerenone abolished upregulation of scleraxis expression, suggesting that aldosterone may be involved in klotho deficiency-induced upregulation of scleraxis. Although aldosterone is known to increase arterial stiffening,16 the underlying mechanism is unclear. In cultured MOVAS, aldosterone increased scleraxis expression and collagen-1 expression. Scleraxis may mediate aldosterone-induced upregulation of collagen-1 expression which can be eliminated by siRNA knockdown of scleraxis. This is the first study demonstrating that scleraxis is expressed in aortic SMCs and may be involved in collagen synthesis. In addition, the findings reveal a previously unidentified role of aldosterone in regulating expression of scleraxis, a key transcription factor for collagen synthesis.
Autophagy is a primary cellular pathway for lysosomal degradation and recycling of long-lived proteins and organelles. Autophagy plays an important role in maintaining cell and organ homeostasis under both basal and various stressful conditions.43–44 Accumulating evidence suggests that aldosterone may induce autophagy to remove protein aggregation.45–46 However, whether autophagy causes extracellular matrix protein changes and contributes to arterial stiffening has never been investigated. We demonstrate that klotho deficiency upregulated autophagy in aortas which was mediated by aldosterone (Fig. S6). In cultured SMCs, aldosterone induced autophagy and increased elastase, MMP2, and MMP9 expression leading to decreased elastin levels (Fig. S6). We showed that upregulation of autophagy may mediate aldosterone-induced degradation of elastin because inhibition of autophagy by siRNA knockdown of Beclin 1 abolished aldosterone-induced upregulation of elastase, MMP2 and MMP9 expression and downregulation of elastin. This is the first report showing that autophagy may induce elastin degradation. Further studies are warranted to assess the relationship of Beclin-1 and elastase/MMPs.
Supplementary Material
Perspective.
Our study provides the first experimental evidence that arterial stiffness due to klotho deficiency is mediated by upregulation of aldosterone levels which increase scleraxis expression and induce autophagy. It is new and interesting that klotho deficiency-induced upregulation of scleraxis expression and induction of autophagy induce collagen synthesis and elastin degradation, respectively. Accumulation of stiffer collagen and degeneration of compliant elastin fibers are considered the key structural remodeling contributing to arterial stiffening.
Novelty and Significance.
1. What is new?
It is new and interesting that haplodeficiency of klotho gene causes arterial stiffening via aldosterone-mediated upregulation of scleraxis and induction of autophagy.
This study demonstrates, for the first time, that upregulation of autophagy may increase MMPs and eastase and cause elastin degradation.
2. What is relevant?
It is significant that klotho deficiency causes arterial stiffening, an aging-related disorder.
This study reveals that inhibition of scleraxis expression and autophagy may be a new therapeutic strategy for arterial stiffening, an independent risk factor for cardiovascular mortality and morbidity.
3. Summary
Klotho deficiency causes arterial stiffening via upregulation of aldosterone levels which increases scleraxis expression and induces autophagy leading to increased collagen synthesis and elastin degradation, respectively.
Acknowledgments
Sources of Funding
This work was supported by NIH R01 HL118558, DK093403, HL105302, HL102074, HL116863, AG049780, and HL122166.
This publication was made possible by NIH Grant Number 9P20GM104934-06 from the COBRE Program of the National Institute of General Medical Sciences.
Footnotes
Disclosure
K. Chen, None
X Zhou: None
Z. Sun: None
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y, Sun Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safar ME. Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr Opin Nephrol Hyper. 2001;10:257–261. doi: 10.1097/00041552-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–1191. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 10.Pasterkamp G, de Kleijn DP, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc Res. 2000;45:843–852. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 11.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovas Translat Res. 2012;5:264–273. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier SR, Sandberg K, Moody AM, Frechette V, Curry CD, Ji H, Gowdar R, Chaudhuri D, Meucci M. Reduction of plasma aldosterone and arterial stiffness in obese pre- and stage1 hypertensive subjects after aerobic exercise. J Hum Hypertens. 2015;29:53–57. doi: 10.1038/jhh.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamide K. Role of Renin-Angiotensin-Aldosterone System in Metabolic Syndrome and Obesity-related Hypertension. Curr Hypertens Rev. 2014 Epub ahead of print. [PubMed] [Google Scholar]
- 14.Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, Sowers JR. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol. 2013;4:161. doi: 10.3389/fendo.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Kim JB, Shim CY, Ko YG, Choi D, Jang Y, Chung N. The influence of serum aldosterone and the aldosterone-renin ratio on pulse wave velocity in hypertensive patients. J Hypertens. 2007;25:1279–1283. doi: 10.1097/HJH.0b013e3280f31b6e. [DOI] [PubMed] [Google Scholar]
- 16.Leibovitz E, Ebrahimian T, Paradis P, Schiffrin EL. Aldosterone induces arterial stiffness in absence of oxidative stress and endothelial dysfunction. J Hypertens. 2009;27:2192–2200. doi: 10.1097/HJH.0b013e328330a963. [DOI] [PubMed] [Google Scholar]
- 17.Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117:742–747. [PubMed] [Google Scholar]
- 18.Kotsis V, Stabouli S. Arterial stiffness, vascular aging, and intracranial large artery disease. Am J Hypertens. 2011;24:252. doi: 10.1038/ajh.2010.251. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PloS One. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Wang Y, Lei H, Sun Z. Mutation of anti-aging gene klotho causes hypertension via upregulation of adrenal CYP11B2 expression and aldosterone synthesis. Circulation. 2014;130:A17793. (Abstract) [Google Scholar]
- 21.Lin Y, Sun Z. In Vivo Pancreatic beta-Cell-Specific Expression of Antiaging Gene Klotho: A Novel Approach for Preserving beta-Cells in Type 2 Diabetes. Diabetes. 2015;64:1444–1458. doi: 10.2337/db14-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crosswhite P, Chen K, Sun Z. AAV delivery of tumor necrosis factor-alpha short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension. 2014;64:1141–1150. doi: 10.1161/HYPERTENSIONAHA.114.03791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Sun Z. Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens. 2014;32:1629–1636. doi: 10.1097/HJH.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Skelley L, Wang B, Mejia A, Sapozhnikov V, Sun Z. AAV-Based RNAi Silencing of NADPH Oxidase gp91(phox) Attenuates Cold-Induced Cardiovascular Dysfunction. Hum Gene Ther. 2012;23:1016–1026. doi: 10.1089/hum.2012.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Kuro-o M, Sun Z. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology. 2013;154:3855–3863. doi: 10.1210/en.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 27.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 28.Georgianos PI, Sarafidis PA, Lasaridis AN. Arterial stiffness: a novel cardiovascular risk factor in kidney disease patients. Curr Vasc Pharmacol. 2015;13:229–238. doi: 10.2174/15701611113119990147. [DOI] [PubMed] [Google Scholar]
- 29.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 30.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23:1211–1216. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 31.Liao J, Farmer J. Arterial stiffness as a risk factor for coronary artery disease. Curr Atheroscler Rep. 2014;16:387. doi: 10.1007/s11883-013-0387-8. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra SG, Tanahashi K, Kumagai H, Oikawa S, Maeda S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart & Circ Physiol. 2014;306:H348–355. doi: 10.1152/ajpheart.00429.2013. [DOI] [PubMed] [Google Scholar]
- 33.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 35.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer SS, Kempe DS, Leibrock CB, Rexhepaj R, Siraskar B, Boini KM, Ackermann TF, Foller M, Hocher B, Rosenblatt KP, Kuro OM, Lang F. Hyperaldosteronism in Klotho-deficient mice. Am J Physiol Renal physiol. 2010;299:F1171–1177. doi: 10.1152/ajprenal.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 39.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 40.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 41.Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol. 2009;47:188–195. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Bagchi RA, Czubryt MP. Synergistic roles of scleraxis and Smads in the regulation of collagen 1alpha2 gene expression. Biochim Biophys Acta. 2012;1823:1936–1944. doi: 10.1016/j.bbamcr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Huett A, Goel G, Xavier RJ. A systems biology viewpoint on autophagy in health and disease. Curr OpinGgastroenterol. 2010;26:302–309. doi: 10.1097/MOG.0b013e32833ae2ed. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi S, Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta. 2015;1852:252–261. doi: 10.1016/j.bbadis.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Mao N, Cheng Y, Shi XL, Wang L, Wen J, Zhang Q, Hu QD, Fan JM. Ginsenoside Rg1 protects mouse podocytes from aldosterone-induced injury in vitro. Acta Pharmacol Sinica. 2014;35:513–522. doi: 10.1038/aps.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia AG, Wilson RM, Heo J, Murthy NR, Baid S, Ouchi N, Sam F. Interferon-gamma ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart &Circ Physiol. 2012;303:H587–596. doi: 10.1152/ajpheart.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.