Abstract
Anaphylaxis is a severe allergic reaction that can be rapidly progressing and fatal; thus, establishing the etiology of anaphylaxis is pivotal to long-term risk management. Our recent work has identified a novel IgE antibody response to a mammalian oligosaccharide epitope, galactose-alpha-1,3-galactose (alpha-gal). IgE to alpha-gal has been associated with two distinct forms of anaphylaxis: i) immediate onset anaphylaxis during first exposure to intravenous cetuximab, and ii) delayed onset anaphylaxis 3–6 hours after ingestion of mammalian food products (e.g., beef and pork). Results of our studies and those of others strongly suggest that tick bites are a cause, if not the only significant cause, of IgE antibody responses to alpha-gal in the southern, eastern and central United States, Europe, Australia and parts of Asia. Typical immune responses to carbohydrates are considered to be T cell-independent, while IgE antibody production is thought to involve sequential class-switching that requires input from T cells. Therefore, establishing the mechanism of the specific IgE antibody response to alpha-gal will be an important aspect to address as this area of research continues.
Keywords: anaphylaxis, delayed reaction to red meat, galactose-alpha-1, 3-galactose
Introduction
Hypersensitivity in the allergic setting refers to immune reactions, stimulated by soluble antigens, that can be rapidly progressing and in the case of anaphylaxis are occasionally fatal. As the number of known exposures associated with anaphylaxis is limited, identification of novel causative agents is important in facilitating both education and other allergen-specific approaches that are crucial to long-term risk management. Within the last 10 years, several seemingly separate observations were recognized to be related, all of which resulted from the development of antibodies to a carbohydrate moiety on proteins where exposure differed from airborne allergens but which were nevertheless capable of producing anaphylactic and hypersensitivity reactions. Our recent work has identified these responses as being due to a novel IgE antibody (Ab) directed against a mammalian oligosaccharide epitope, galactose-alpha-1,3-galactose (alpha-gal) 1. This review will present the history and biology of alpha-gal and discuss the evidence that the IgE response to alpha-gal is different from typical IgE responses directed towards protein allergens.
Cetuximab-induced Hypersensitivity Reactions
In 2004, ImClone and Bristol Meyers Squibb were investigating a monoclonal antibody (cetuximab), specific for the epidermal growth factor receptor (EGFR), in clinical trials for the treatment of metastatic colorectal cancer. Early in those studies, it became clear that the antibody was causing hypersensitivity reactions: but they were occurring primarily in a group of southern US states. These reactions to cetuximab developed rapidly and symptoms often peaked within 20 minutes following or during the first infusion of the antibody and occasionally proved fatal 1–3. Due to delays in marketing, it was not until 2006 that the true severity of the reactions became obvious 2. At this time, our group began preliminary experiments examining the IgE response to this molecule. Dr. Hatley, who was working in Bentonville, AR, convinced our group to develop a new version of the IgE fluorometric enzyme immunoassay or CAP assay to cetuximab using the streptavidin technique. In this assay, streptavidin is coupled to the solid phase of the CAP to provide a matrix for the binding of biotinylated novel or purified allergens 4. We were subsequently asked to investigate the reactions to cetuximab, in part because we had already developed the IgE assay to cetuximab. In collaboration with Dr. Chung from Nashville, Dr. Mirakhur from Bristol Myers Squibb, and Dr. Hicklin from ImClone, we demonstrated that the patients who had reactions to cetuximab also had IgE antibodies specific for this molecule before they started treatment 1. The question remained as to what epitope the IgE antibody was recognizing on the cetuximab molecule.
Early work by Karl Landsteiner discovered that all humans had antibodies to a blood group “B-like” oligosaccharide found on non-primate red blood cells5. That antigen was subsequently identified as galactose-alpha-1,3-galactose (alpha-gal) and represents a major transplantation barrier between primates and other mammals 6–8. Antibodies against alpha-gal are present in all non-immunocompromised human subjects and some early studies suggested that the IgG antibodies against alpha-gal constituted about 1% of circulating immunoglobulins in human subjects, apes, and Old World monkeys 9. Recent work in our lab with specific assays for IgG antibodies suggests that the percentages are not this high. As discussed below, the fact that all non-primate mammals including mice can make oligosaccharides that are foreign to humans is an important component of our story.
Carbohydrate Analysis of Cetuximab
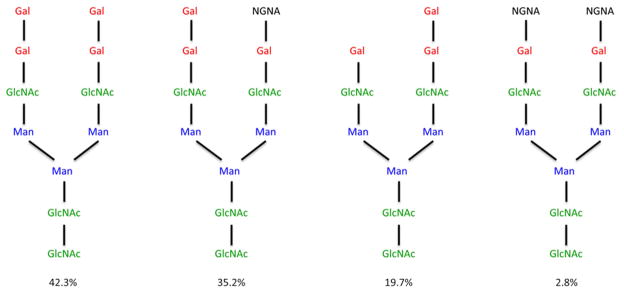
Glycosylation of proteins is a post-transcriptional modification that can play key roles in many processes including protein folding, protein stability, intracellular trafficking and cellular adhesion, reviewed by Huryado-Guerrero and Davies 10. Characterization of cetuximab glycosylation, as measured by peak area on TOF-MS spectra, revealed 21 distinct oligosaccharide structures, of which approximately 30% have one or more alpha-1,3 linked galactosyl residues 11. Analysis of the IgE antibodies to cetuximab demonstrated that these antibodies were specific for the oligosaccharide residues on the heavy chain of the Fab portion of the mAb. From the known glycosylation of the molecule at amino acids 88 and 299 (Figure 1), alpha-gal was identified as the relevant epitope 11. Of the total alpha-gal in cetuximab, most of it is located in the Fab domain (Fab 990 nmol alpha-gal/μmol IgG versus Fc 140 nmol alpha-gal/μmol IgG) 11. Recent mass spectrometry analysis indicates that glycosylation of cetuximab may be more complex than previously thought, containing both dianternary and trianternary structures 12. Synthesis of alpha-gal requires the gene encoding alpha-1,3-galactosyltransferase. In humans and higher primates this gene is not functional, so these species cannot produce alpha-gal – which in turn makes it possible for these animals to initially make IgG antibodies directed towards this oligosaccharide 7, 13. How IgE to alpha-gal gets made and the nature of the IgE response will be considered later. Of considerable importance to the development of biologics, in particular mAbs, is the observation that murine cell lines such as NS0 and Sp2/0 can synthesize galactose in an alpha-1,3 linkage such that alpha-gal is present on the molecules. Sp2/0 was the cell line used to produce cetuximab. In those individuals who have IgE to alpha-gal (≥0.35 IU/ml), reactions are likely to occur directed against this mAb 3.
Figure 1. Major carbohydrate structures on Fab portion of cetuximab.
Carbohydrate structure of N-linked glycans on the Fab portion of cetuximab as determined by mass spectrometry. Alpha-gal (red), manose (blue), N-acetylglucosamine (green), N-glycolyl neuraminic acid (black). The percentages represent the proportion of the indicated structures found on the Fab region of cetuximab. Adpated from Qian et al 11.
The Red Meat Connection
During this same time period (2006–2008), we evaluated a number of patients, most of whom spent a significant amount of time outdoors, who had presented with episodes of generalized urticaria, angioedema or recurrent anaphylaxis. The importance of the time spent outdoors was not clear at that time. There was no obvious immediate cause for the symptoms, but in several cases the patients reported that they felt the reactions might be due to consumption of meat 3–5 hours earlier. Prick tests were performed with commercial extracts of beef, pork or lamb, and produced small wheals only 2– 4 mm in diameter that often would be interpreted as negative. However, given the compelling history described by the patients, we extended our analysis to intradermal skin testing with commercial meat extracts or prick skin tests with fresh meat extracts both of which demonstrated strong positive results 14. These results were confirmed with blood tests for specific IgE Ab to red meats 14. Though not published, similar sensitivity to red meat had been previously noted in Georgia. Starting in 1989, Mrs. Sandra Latimer together with Dr. Antony Deutsch from Athens, Georgia collected ten cases of delayed reactions to mammalian meat and made a connection with the occurrence of tick bites several weeks or months prior to the first episode of hives or anaphylaxis. They presented these findings to the Georgia Allergy Society and to the CDC in 1991, but no additional reports or statements were issued by either of these organizations.
The characteristics of red meat allergy are different from typical allergic reactions. Common complaints include both gastrointestinal symptoms and urticaria, but unlike most allergic reactions, patients do not develop any symptoms for until after at least 2 hours after eating red meat, while many reactions are delayed for 3–5 hours or even longer. Nonetheless, symptoms can be severe or even life threatening. Many of the patients described nausea, diarrhea or indigestion before a reaction, however the most common symptom reported was itching. The presence of symptoms before a severe reaction is common, but not a requirement. Many patients do not have any symptoms and even those who have had them previously, the symptoms do not occur with every exposure to red meat. All of the patients had consumed red meat without complications for many years prior to the onset of the syndrome. While some individuals had a prior history of allergy, most of the cases had no previous allergic symptoms, thus an atopic disposition does not appear to predispose patients to this kind of IgE response.
Three observations led us to investigate whether IgE antibodies to alpha-gal were present in the sera of adult patients reporting reactions to beef. Alpha-gal is known to be present on both tissues and meat from non-primate mammals 15, the antibodies causing reactions to cetuximab were directed against alpha-gal, and the geographical distribution of the reactions to cetuximab overlapped the same geographical area where the red meat reactions were occurring. Not surprisingly, the patients’ sera tested positive for IgE to beef, pork, lamb, cat and dog, but not to non-mammalian meat such as turkey, fish or chicken 14, 16. The presence of alpha-gal was confirmed using two different absorption assays, one with alpha-gal on human serum albumin and the other using thyroglobulin, which is heavily decorated with alpha-gal. The glycosylated antigens were bound to sepharose beads 14. In each case, the specific IgE binding to beef, pork, lamb, cat and dog was reduced by >75%. More recently, evidence has come from a study examining beef extracts using 2D gel electrophoresis. The authors demonstrated seven alpha-gal containing IgE binding proteins, four of which survived heating the beef extract 17. How alpha- gal is structurally expressed on red meat remains unclear. Also unclear is whether differences in the structure do exist and if these differences impact IgE binding? The terminal carbohydrate residue on red meat is likely alpha-gal based on the binding of IgE from sera of subjects with red meat allergy to cetuximab, which as discussed also has terminal alpha-gal residues. However, one can envision a difference in carbohydrate structure such that only a single exposed alpha-gal binding site is present in the oligosaccharide chain on meat, contrasting the two found predominately on cetuximab. The majority of alpha-gal found on cetuximab has a dianternary structure (Figure 1). The structure on meat has not been determined. Whether or not having two alpha-gal residues on the terminus of the carbohydrate structure has an impact on the strength of IgE binding is unknown?
Are Tick Bites Responsible for the Induction of IgE Antibody to Alpha-gal
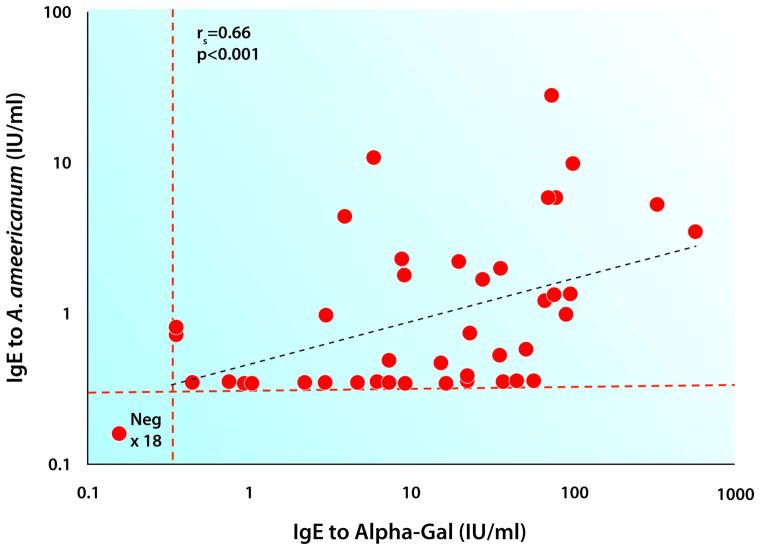
In 2008, as the specificity of the IgE antibodies to alpha gal that caused reactions to cetuximab became clearer, the number of reports describing delayed reactions to red meat was also increasing. A relationship between mammalian meat allergy and tick bites had already been suggested in Australia 18, however the role of alpha-gal was not known and the tick connection was not yet obvious in the United States. What caught our attention was that both cetuximab reactions and delayed reactions to red meat were being reported from the same region of the country: a group of southeastern states. However, it was not clear why these cases were geographically localized and the only area that was comparable was the maximum incidence of Rocky Mountain Spotted Fever (RMSF). At this time, three members of our group developed red meat allergy and each one distinctly remembered being bitten by ticks weeks or months prior to the development of symptoms. Sera from these individuals that had been obtained prior to the tick bite was compared to sera collected after the bite and it was found that serum levels of IgE to alpha-gal had increased dramatically (4 to 10-fold). Following up on this connection, we started to ask patients about tick bites and rapidly became aware that most of those with delayed anaphylaxis had experienced recent bites from adult or larval ticks. Examination of CDC maps of the distribution of the tick Amblyomma americanum (Lone Star tick) revealed an overlap with the region of both cetuximab sensitivity and red meat allergy. Additional indications that tick bites are involved in the development of specific IgE to alpha-gal include: histories of bites that have itched for two or more weeks, a significant correlation between IgE Ab to alpha-gal and IgE to Lone Star tick (Figure 2) as well as the prospective data on the increase in IgE to alpha-gal following known Lone Star tick bites 19. Allergy to red meat is now being reported in other countries, but the ticks giving rise to this response are not the same species as in the United States. In Europe, Ixodes ricinus has been implicated while in Australia the relevant tick is Ixodes holocyclus 18, 20, 21. It appears that Ixodes scapularis, the main vector of Lyme disease (Borrelia burgdorferi infection) in the United States, does not induce IgE to alpha-gal and unlike bites of the Lone Star Tick, bites of Ixodes scapularis that transmit Lyme disease are not associated with itching 22.
Figure 2. Relationship of IgE to alpha-gal with IgE to Amblyomma americanum (Lone Star tick).
Specific IgE to alpha-gal and Amblyomma americanum was measured using ImmunoCAP and the correlation between the two was determined using Spearman’s correlation rs=0.66: p<0.001.
Given that tick bites represent the most important cause of alpha-gal sensitization in USA, Sydney, and Stockholm, why has our recognition of this problem increased so dramatically over the past 10 years? The increase in Lone Star ticks parallels the increase in the deer population, a major carrier of these ticks, throughout the United States over the last 30–40 yrs 23, 24 making it more likely that people who walk in the woods or in long grass will be bitten at some point. The increasing deer population can also be linked to the enactment of leash laws for dogs, a decrease in the number of hunters and movement of the deer into suburban areas. This last point is important as the deer provide a means for the ticks to be transported over large geographic areas quickly. Clearly, the increase in tick exposure is one plausible explanation for the increase in the number of cases. However, the data from different countries demonstrate that not all tick bite per se or a tick bite from one particular species result in the problem (Table II). The epidemiological evidence in the USA would suggest that the rise in the deer population has played an important role. However, it is important to remember that there are at least three theories about how tick bites give rise to an IgE response:
Table II.
Ticks that commonly bite humans in countries where IgE to alpha-gal has been reported
| USA | Tick | Outcome |
|---|---|---|
| Ixodes scapularis (deer tick) | Lyme disease | |
| Dermancentor variabillis (dog tick) | Rocky mountain spotted fever (RMSF) | |
| Amblyomma americanum (Lone Star tick) | IgE to alpha-gal, Ehrlichiosis and RMSF | |
| Australia | ||
| Ixodes holocyclus | IgE to alpha-gal and/or tick bite anaphylaxis | |
| Europe | ||
| Ixodes ricinus | IgE to alpha-gal and Lyme disease | |
| Argas reflexus (pigeon tick) | Anaphylactic reactions to tick bite |
That the response is induced by the normal (i.e. tick derived) constituents of their saliva.
That residual mammalian glycoproteins or glycolipids are present in the tick from a previous blood meal, and that they are responsible for inducing the response to alpha-gal.
That the response is induced by another organism that is present in the tick. The best recognized organisms present as commensals on ticks are Rickettsia, such as those that cause RMSF or bacteria such as B. burgdorpheri which is found in the Lone Star tick (Amblyomma americanum). Other organisms are possible, but none have been recognized.
It might be thought that the IgE response that has been seen with seed ticks would argue against either residual mammalian proteins or other organisms, however, transovarial transmission of RMSF is well recognized. Sorting out these possibilities is the subject of ongoing investigation.
A Broader Understanding of Alpha-gal
The early reports of alpha-gal sensitivity were mostly from adults with very few reports of affected children. However, children often have urticaria, angioedema or recurrent anaphylaxis for which the cause is unknown. We identified 51 children ages 4–17 with symptoms consistent with possible delayed allergic reactions to mammalian foods and measured IgE to alpha-gal in their sera. Serum IgE to alpha-gal was high in 45 of the subjects and there was a strong correlation with beef IgE as previously observed in the adults 16. When questioned, these children gave a history of symptoms 3–6 hrs after ingestion of meat and many could recall recent tick bites. The geographic distribution of affected children matches that of adults, namely the southeastern United States.
For protein allergens there is a strong correlation between atopic sensitivity and asthma 25–27. It is unknown whether or not this same relationship exists when an oligosaccharide is the target of the IgE response. Three populations were examined: one with high levels of IgE to alpha-gal that had anaphylaxis, angioedema or acute urticarial after ingestion of red meat, people admitted to the emergency room for an acute asthma exacerbation and a cohort of children in Kenya that showed sensitization to cat despite limited cat exposure. These studies involved extensive investigation of lung function, exhaled nitric oxide measurements, histories of asthma symptoms as well as serum assays for IgE Ab to alpha-gal. Taken together, those studies showed no association between sensitization to alpha-gal and asthma 28. One caveat is that people develop this syndrome differently than the typical areoallergen sensitivity, as alpha-gal does not appear to be airborne, and it may be that if given enough time following the onset of symptoms, these subjects would develop asthma. This would either require a prospective study following patients and seeing how their lung function changes over time when they have a high alpha-gal IgE titer or a retrospective study many years from now after individuals have had the disease for years and comparing the new lung function to their lung function prior to development of disease. Previously, we had studied an asthmatic cohort from Sweden and found a strong correlation between atopic sensitization to cat allergens and asthma 29. However, when we examined a population from rural Kenya, we saw high titer IgE to cat allergens but no association with asthma or atopic disease. What we did not understand was why the level of sensitization to cat was so high in Kenya, while exposure to cats was low. A clue to that riddle was provided when we found that the subjects in the USA with delayed anaphylaxis to red meat had positive skin prick tests and serum assays to cat. Further investigation revealed that the alpha-gal positive subjects were also positive to cat epithelium extracts, but not to Fel d 1 28. Upon reexamination of the Swedish and Kenyan cohorts, it was discovered that for Sweden there was a strong correlation between IgE to Fel d 1 and IgE to cat dander, whereas in the Kenyan population there was no correlation 28. Instead, there was a strong correlation between IgE to cat dander and IgE to alpha-gal, thus explaining the apparent high levels of serum IgE to cat we had observed in the Kenyan cohort. Care must be taken when interpreting skin test or serum results in subjects who present with symptoms of urticaria, angioedema and idiopathic anaphylaxis.
In Zimbabwe, Dr Elopi Sibanda, working with colleagues in Austria and Sweden, identified a group of patients who had IgE to cat allergens, which was explained by IgE to alpha-gal 30. In that report, they focused on the potential for IgE to alpha-gal to give “false positive” or confusing results for cat allergy. However, equally interesting was the observation that these patients did not report allergic reactions to red meat. In fact, given the evidence that many children and adults in Africa have IgE to alpha-gal there are remarkably few reports of delayed or other allergic reactions to meat on that continent. Whether this reflects i) a difference in the IgE response; ii) some aspect of the fat content of meat or the digestion of meat or iii) a difference in the response of mast cells is not clear.
Mechanisms of Anaphylaxis
Currently, it is our belief that the initial step in sensitization to the oligosaccharide alpha-gal is through bites that occur from ticks. The patients develop IgE antibodies to this hapten that is present on all non-primate mammalian food products. This is comparable to the sensitization that occurs to inhaled plant oligosaccharides such as MUXF3-- a hapten on the glycoproteins of many plant species 31–33. Unlike alpha-gal, IgE antibodies to these plant-derived cross-reactive carbohydrate determinants (CCD) have not been shown to contribute to symptoms related to pollen exposure 31, 34. Patients with IgE to alpha-gal typically report symptoms beginning 3–5 hours after eating meat. Despite detailed and aggressive questioning, the patients do not recognize any oral or gastrointestinal symptoms less than 2 hours after eating a meal. Similarly, in challenge studies using pork, hives and other symptoms are delayed at least two hours after meat ingestion 35. This is different than the reactions to cetuximab that develop rapidly, where symptoms often peak within 20 minutes of initial administration of the drug 1,2, 3. This rapid time frame is similar to the in vitro responses of basophils following activation with glycoproteins, such as beef thyroglobulin or cetuximab, which can be detected within 25 minutes. Skin test responses to cetuximab, beef extract, pork sausage or beef thyroglobulin are also rapid. Thus, the delay in response after eating meat does not reflect a delayed response or inability of basophils or mast cells to be activated by these glycoproteins. The obvious explanation is that the oligosaccharide is absorbed from the gut in a form that enters the circulation slowly. Given that alpha-gal is present on both glycoproteins and glycolipids (including chylomicrons), it is our belief that the most likely explanation for the delay in symptoms is due to a delay in the appearance of the antigen in the circulation. Since chylomicrons enter the circulation via the thoracic duct after a several hour process of absorption, re-packaging and transit, mediator release triggered by the accumulated metabolic products (e.g., VLDL or LDL) may account for the now documented delay. Our studies have shown that during a challenge, circulating basophils assessed ex vivo upregulate the expression of CD63 in a similar time frame as the patients develop symptoms 35. Surprisingly, a proportion of non-allergic controls also demonstrated upregulation of CD63, although they do not experience any symptoms. Evidence that basophils and mast cells have receptors for LDL was reported many years ago 36, 37. We postulate that the likely explanation for this enigmatic finding is that, although VLDL or LDL can cause basophils to upregulate CD63, the quantity of histamine released in non-allergic controls is not sufficient to cause symptoms. The implication is that LDL particles with alpha-gal on the surface can cause mast cell mediator release, but only in individuals with IgE Ab to alpha-gal. In keeping with this model, three of the challenge patients, but none of the controls, had tryptase in their circulation following the challenge 35. In the United States, delayed allergic reactions are almost uniformly related to eating beef, pork or lamb with a minority of cases reporting reactions to milk or cheese. However, in most cases the reactions have followed consumption of over 100 grams of mammalian meat. By contrast, in Europe, it is normal to eat meat and organs from a much wider range of mammals. This includes not only horse, goat and rabbit, but also liver, heart, tripe (intestine) or kidney. Two separate groups have reported that reactions to pork kidney can be both severe and more rapid, i.e. 2 hours rather than 4 hours 38, 39. In addition, there are increasing anecdotal reports from both the USA and Europe that drinking alcohol at the same time as eating red meat or kidney can increase the probability of a reaction.
Nature of alpha-gal IgE development
While our data and that of others support the theory that bites from ecto-parasitic ticks initiate the development of an IgE response to alpha-gal in humans, the mechanistic aspects of this response have not yet been elucidated. There is already extensive evidence that i) IgE Ab responses can occur outside mature germinal centers 40, 41, ii) that the switch to IgE can occur in B cells locally in the nose 42, and iii) that antibody responses to oligosaccharides can be – or normally are – relatively T-independent 43, 44. Thus, there is a real possibility that the IgE response to alpha-gal involves switching that occurs outside germinal centers and it is possible that the skin is the site of such a switch. It will be important to establish the extent of rearrangement in the complementarity-determining region (CDR) which could provide additional clues regarding the antigen(s) 45. Previously, it has been documented that individuals bitten by the pigeon tick (Argas reflexus) can develop specific IgE to extracts made from the whole tick body 46. From preliminary experiments, we have noted that in subjects with IgE to alpha-gal there appears to be proliferation of a subset of plasmablasts in response to tick extract that was not present in control subjects. In keeping with the observed decreases in IgE and reactivity to alpha-gal over time in patients who avoid further tick bites 14, the formation of plasmablasts could occur in this setting without the development of long-lived plasma cells. Overall, the alpha-gal IgE response has some features resembling an IgM response to an oligosaccharide. Certainly, understanding why exposure to one antigen leads to a long-lived IgE response when another exposure does not would be an important and potentially therapeutically- manipulateable insight.
Conclusion
The finding that IgE to alpha-gal explains two novel forms of anaphylaxis has not only changed several established rules about allergic disease, but has opened up at least two new areas of research. The results provide evidence that: IgE responses to an oligosaccharide can induce significant or severe allergic symptoms, demonstration of sensitization to this epitope by skin tests often requires intradermal as well as prick test, ticks can induce high titer food specific IgE responses in adult life, and also that eating mammalian products carrying this epitope does not give rise to any symptoms during the first hour or more. Like so many new findings, this area of research provides both challenges and opportunities. The delay in onset of symptoms following eating red meat is best explained by delayed arrival of the relevant form of antigen in the circulation, but the question remains as to what form of glycoprotein or more likely glycolipid takes 3 hours or more to appear in the circulation. Finally, the often-rapid production of IgE antibodies to alpha-gal after tick bites provides a striking model of a parasite induced IgE response (Figure 3). This parasite only enters through the skin and the tick saliva contains a wide variety of agents that could act as antigens and/or as adjuvants. However, it remains a striking challenge to identify why the response is so strong and why it is directed so consistently against the alpha-gal carbohydrate residue.
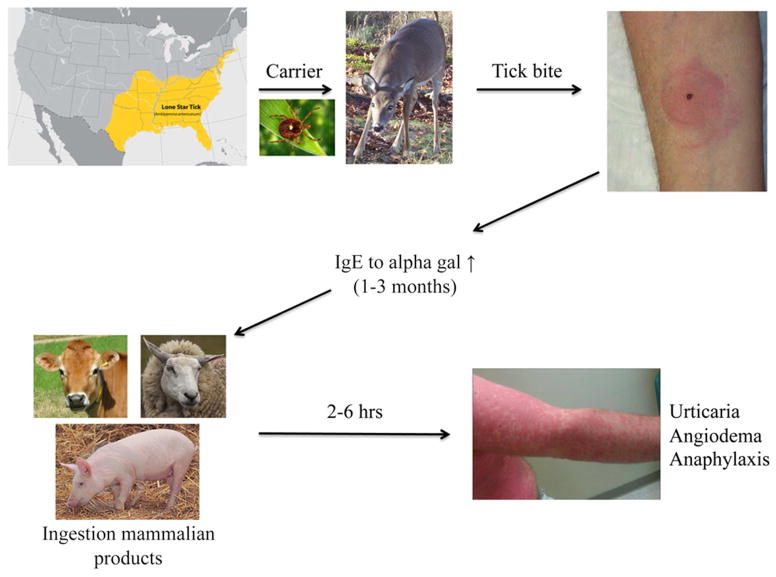
Figure 3. Summary of alpha-gal sensitization leading to clinical symptoms of red meat allergy.
The southeastern section of the US is where most of the reactions to red meat have been reported. This region overlaps with the distribution of the Lone Star tick. The current hypothesis is that people are bitten by Lone Star ticks carried by deer into rural and urban areas. Following a period of time, IgE to alpha-gal develops. Once IgE to alpha-gal reaches sufficient levels, ingestion of red meat can trigger reactions.
Table I.
Time course of the alpha-gal story
| Year | Events leading to our understanding of red meat allergy |
|---|---|
| ~2000 | At least two groups reported cases of meat allergy that started after tick bites. |
| 2003 | IgE to cat allergens common in an African village but not related to symptoms. |
| 2005 | Reports of hypersensitivity reactions to first infusion of Cetuximab in clinical trials. |
| 2007 | Severe reactions to Cetuximab common in TN, NC, AR, MS, and VA. |
| 2007 | Two cases in VA of adult-onset delayed anaphylaxis occurring 3–6 hrs after eating beef. |
| 2008 | Identification of galactose-alpha-1,3-galactose as the epitope on Cetuximab. |
| 2009 | 24 cases of delayed anaphylaxis to red meat in USA. Report of multiple cases of meat-allergy following ticks bites in Sydney, Australia. |
| 2010 | Range of evidence that ticks are responsible for the IgE response in the USA. |
| 2011 | Extensive evidence that the IgE response is not related to asthma despite cross-reacting with dog and cat. |
| 2014 | Open challenge tests confirm the delay in reactions to red meat. |
What we do know
IgE antibodies specific for the mammalian oligosaccharide galactose alpha-1, 3-galactose (alpha-gal) are common in a large area of the southeastern United States.
These IgE antibodies are causally associated with two novel forms of allergic reactions: i) Anaphylaxis or urticaria during the first infusion of cetuximab. Ii) Urticaria, Angioedema or Anaphylaxis starting 3–5 hours after eating red meat.
These IgE antibodies in the United States are caused predominantly if not exclusively by bites of larval or adult lone star ticks.
What is still unknown
Although deer are the major vector for the relevant ticks both in the USA and in Sweden, the increase in deer populations may not be the only or the major cause for an increase in the disease.
Can the IgE response to tick bites be explained simply by the normal contents of tick saliva or is it possible that some other symbiotic organism, e.g. a new Rickettsia species, is involved.
Although the best explanation for the delayed food responses to red meat is that it relates to the absorption of lipid particles it is not clear what form of particle carrying glyco-lipids or glyco-proteins is present in the circulation after 3–5 hours.
Abbreviations
- Ab
antibody
- Alpha-gal
galactose-alpha-1,3-galactose
- CBC
complete blood count
- DEET
N,N-Diethyl-meta-toluamide
- ED
emergency department
- EGFR
epidermal growth factor receptor
- Fab
fragment antigen binding
- Fc
fragment crystallizable
- mAb
monoclonal antibody
- RMSF
Rocky Mountain Spotted Fever
- TOF-MS
time-of-flight-mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. The New England journal of medicine. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3644–8. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 3.Maier S, Chung C, Morse M, Platts-Mills TA, Townes L, Mukhopadhyay P, et al. A retrospective analysis of cross-reacting cetuximab IgE antibody and its association with severe infusion reactions. Cancer Medicine. 2014 doi: 10.1002/cam4.333. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. The Journal of allergy and clinical immunology. 2005;115:1029–35. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 5.Landsteiner K. The specificity of serological reactions. Charles C Thomas; Baltimore, MD: 1936. [Google Scholar]
- 6.Milland J, Sandrin MS. ABO blood group and related antigens, natural antibodies and transplantation. Tissue antigens. 2006;68:459–66. doi: 10.1111/j.1399-0039.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 7.Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, et al. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:559–64. doi: 10.1073/pnas.0610012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochimica et biophysica acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. The Journal of experimental medicine. 1984;160:1519–31. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurtado-Guerrero R, Davies GJ. Recent structural and mechanistic insights into post-translational enzymatic glycosylation. Current opinion in chemical biology. 2012;16:479–87. doi: 10.1016/j.cbpa.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Analytical biochemistry. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Ayoub D, Jabs W, Resemann A, Evers W, Evans C, Main L, et al. Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. mAbs. 2013;5:699–710. doi: 10.4161/mabs.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunology and cell biology. 2005;83:674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 14.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. The Journal of allergy and clinical immunology. 2009;123:426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thall A, Galili U. Distribution of Gal alpha 1----3Gal beta 1----4GlcNAc residues on secreted mammalian glycoproteins (thyroglobulin, fibrinogen, and immunoglobulin G) as measured by a sensitive solid-phase radioimmunoassay. Biochemistry. 1990;29:3959–65. doi: 10.1021/bi00468a024. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131:e1545–52. doi: 10.1542/peds.2012-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apostolovic D, Tran T, Hamsten C, Starkhammar M, Cirkovic Velickovic T, van Hage M. Immunoproteomics of processed beef proteins reveal novel galactose-alpha-1,3-galactose containing allergens. Allergy. 2014 doi: 10.1111/all.12462. [DOI] [PubMed] [Google Scholar]
- 18.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. The Medical journal of Australia. 2009;190:510–1. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 19.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. The Journal of allergy and clinical immunology. 2011;127:1286–93. e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. The Journal of allergy and clinical immunology. 2013;132:1431–4. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, et al. Identification of galactose-alpha-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68:549–52. doi: 10.1111/all.12128. [DOI] [PubMed] [Google Scholar]
- 22.Burke G, Wikel SK, Spielman A, Telford SR, McKay K, Krause PJ. Hypersensitivity to ticks and Lyme disease risk. Emerging infectious diseases. 2005;11:36–41. doi: 10.3201/eid1101.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagerstone KA, Clay WH. Overview of USDA animal damage control efforts to manage overabundant deer. Wildlife Society Bulletin. 1997;25:413–7. [Google Scholar]
- 24.Cote SD, Rooney TP, Tremblay J, Dussault C, Waller DM. Ecological impacts of deer overabundance. Annual Review of Ecology, Evolution, and Systematics. 2004;35:113–47. [Google Scholar]
- 25.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. The American review of respiratory disease. 1993;147:573–8. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 26.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. The New England journal of medicine. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 27.Erwin EA, Ronmark E, Wickens K, Perzanowski MS, Barry D, Lundback B, et al. Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. The Journal of allergy and clinical immunology. 2007;119:359–65. doi: 10.1016/j.jaci.2006.12.648. [DOI] [PubMed] [Google Scholar]
- 28.Commins SP, Kelly LA, Ronmark E, James HR, Pochan SL, Peters EJ, et al. Galactose- alpha-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. American journal of respiratory and critical care medicine. 2012;185:723–30. doi: 10.1164/rccm.201111-2017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perzanowski MS, Ronmark E, Nold B, Lundback B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. The Journal of allergy and clinical immunology. 1999;103:1018–24. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 30.Arkestal K, Sibanda E, Thors C, Troye-Blomberg M, Mduluza T, Valenta R, et al. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-alpha 1,3-galactose. The Journal of allergy and clinical immunology. 2011;127:1024–8. doi: 10.1016/j.jaci.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Aalberse RC, van Ree R. Cross-reactive carbohydrate determinants. Monographs in allergy. 1996;32:78–83. [PubMed] [Google Scholar]
- 32.van Ree R, Cabanes-Macheteau M, Akkerdaas J, Milazzo JP, Loutelier-Bourhis C, Rayon C, et al. Beta(1,2)-xylose and alpha(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. The Journal of biological chemistry. 2000;275:11451–8. doi: 10.1074/jbc.275.15.11451. [DOI] [PubMed] [Google Scholar]
- 33.Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P, et al. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. The Journal of allergy and clinical immunology. 2010;125:1300–7. e3. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. International archives of allergy and immunology. 2002;129:286–95. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- 35.Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. The Journal of allergy and clinical immunology. 2014;134:108–15. e11. doi: 10.1016/j.jaci.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonen B, O’Donnell P, Post TJ, Quinn TJ, Schulman ES. Very low density lipoproteins (VLDL) trigger the release of histamine from human basophils. Biochimica et biophysica acta. 1987;917:418–24. doi: 10.1016/0005-2760(87)90121-4. [DOI] [PubMed] [Google Scholar]
- 37.Schulman ES, Quinn TJ, Post TJ, O’Donnell P, Rodriguez A, Gonen B. Low density lipoprotein (LDL) inhibits histamine release from human mast cells. Biochemical and biophysical research communications. 1987;148:553–9. doi: 10.1016/0006-291x(87)90912-0. [DOI] [PubMed] [Google Scholar]
- 38.Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67:699–704. doi: 10.1111/j.1398-9995.2012.02799.x. [DOI] [PubMed] [Google Scholar]
- 39.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. The Journal of allergy and clinical immunology. 2014;134:755–9. e1. doi: 10.1016/j.jaci.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 40.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snow RE, Djukanovic R, Stevenson FK. Analysis of immunoglobulin E VH transcripts in a bronchial biopsy of an asthmatic patient confirms bias towards VH5, and indicates local clonal expansion, somatic mutation and isotype switch events. Immunology. 1999;98:646–51. doi: 10.1046/j.1365-2567.1999.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt E, Collins AM, Sewell WA, Harvey RJ. Antigen selection in IgE antibodies from individuals with chronic rhinosinusitis with nasal polyps. American journal of rhinology & allergy. 2010;24:416–21. doi: 10.2500/ajra.2010.24.3538. [DOI] [PubMed] [Google Scholar]
- 43.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–87. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moody DB. Immunology: how a T cell sees sugar. Nature. 2007;448:36–7. doi: 10.1038/nature05890. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Jackson KJ, Chen Z, Gaeta BA, Siba PM, Pomat W, et al. IgE sequences in individuals living in an area of endemic parasitism show little mutational evidence of antigen selection. Scandinavian journal of immunology. 2011;73:496–504. doi: 10.1111/j.1365-3083.2011.02525.x. [DOI] [PubMed] [Google Scholar]
- 46.Kleine-Tebbe J, Heinatz A, Graser I, Dautel H, Hansen GN, Kespohl S, et al. Bites of the European pigeon tick (Argas reflexus): Risk of IgE-mediated sensitizations and anaphylactic reactions. The Journal of allergy and clinical immunology. 2006;117:190–5. doi: 10.1016/j.jaci.2005.08.056. [DOI] [PubMed] [Google Scholar]