Abstract
Background
Transplantation of enteric neural stem cells (ENSC) holds promise as a potential therapy for enteric neuropathies, including Hirschsprung disease. Delivery of transplantable cells via laparotomy has been described, but we propose a novel, minimally-invasive endoscopic method of cell delivery.
Methods
ENSC for transplantation were cultured from dissociated gut of postnatal donor mice. Twelve recipient mice, including Ednrb−/− mice with distal colonic aganglionosis, underwent colonoscopic injection of ENSC under direct vision using a 30-gauge Hamilton needle passed through a rigid cystoureteroscope. Cell engraftment, survival, and neuroglial differentiation were studied 1–4 weeks after the procedure.
Key Results
All recipient mice tolerated the procedure without complications and survived to sacrifice. Transplanted cells were found within the colonic wall in 9 of 12 recipient mice with differentiation into enteric neurons and glia.
Conclusions & Inferences
Endoscopic injection of ENSC is a safe and reliable method for cell delivery, and can be used to deliver a large number of cells to a specific area of disease. This minimally-invasive endoscopic approach may prove beneficial to future human applications of cell therapy for neurointestinal disease.
Keywords: cell therapy, endoscopy, enteric neural stem cells, enteric neuropathies, Hirschsprung disease
Introduction
Neural stem cell therapy offers an innovative approach for the treatment of enteric neuropathies, such as Hirschsprung disease (1, 2). Enteric neural stem cells (ENSC) have been isolated from the gastrointestinal tracts of rodents and humans (3) and shown to improve gastrointestinal motility following transplantation into mouse models of enteric neuropathy (4).
Previous studies transplanted ENSC into rodents via laparotomy with injection or implantation of cells into the gut wall (4–6). A less invasive approach would be attractive for future clinical applications. Moreover, given the limited ability of transplanted ENSC to migrate in vivo (7), efficient delivery methods are needed to transplant sufficient cell numbers to treat large areas of disease.
We have developed a novel, minimally-invasive method of endoscopic microinjection to deliver ENSC to the aganglionic colon of mice with Hirschsprung disease.
Material and Methods
Cell preparation
Donor ENSC were isolated from the intestine of 2–4 week old Actb-DsRed mice with a C57BL/6J background (Tg(CAG-DsRed*MST)1Nagy/J; Jackson Labs, Bar Harbor, ME, USA), in which all cells express DsRed (8). The longitudinal muscle layer containing the myenteric plexus was dissected, then mechanically and enzymatically dissociated with dispase (250 μg ml−1; StemCell Technologies, Vancouver, Canada) and collagenase XI (1 mg ml−1; Sigma Aldrich, St. Louis, MO, USA) at 37°C for 1 hour. Cells were filtered through a 40 μm cell strainer to obtain a single cell suspension, which was cultured at 50,000 cells ml−1in Neurocult Basal Medium (StemCell Technologies) supplemented with 20 ng ml−1 epidermal growth factor (StemCell Technologies), 10 ng ml−1 basic fibroblast growth factor (StemCell Technologies), 0.0002% Heparin (StemCell Technologies), and 100 U ml−1 Penicillin-Streptomycin (Life Technologies, Carlsbad, CA, USA) for 7–10 days to form enteric neurospheres. Neurospheres were passaged every 7–10 days with Accutase (StemCell Technologies) dissociation at 37°C for 30 minutes followed by re-plating. For transplantation, neurospheres were resuspended in phosphate buffered saline (Life Technologies) at 1000 cells μL−1.
Colonoscopic transplantation of ENSC
Recipients were either 6–8 week-old C57BL/6J (WT) mice or 2–3 week-old homozygous Ednrbtm1Ywa (Ednrb−/−) mice on a hybrid C57BL6/J-129Sv background (B6;129-Ednrbtm1Ywa/J, Jackson Labs). Ednrb−/− mice are characterized by white coat color and distal colonic aganglionosis similar to human Hirschsprung disease (9). Recipients were male and female. Animal protocols were approved by the Institutional Animal Care and Use Committee.
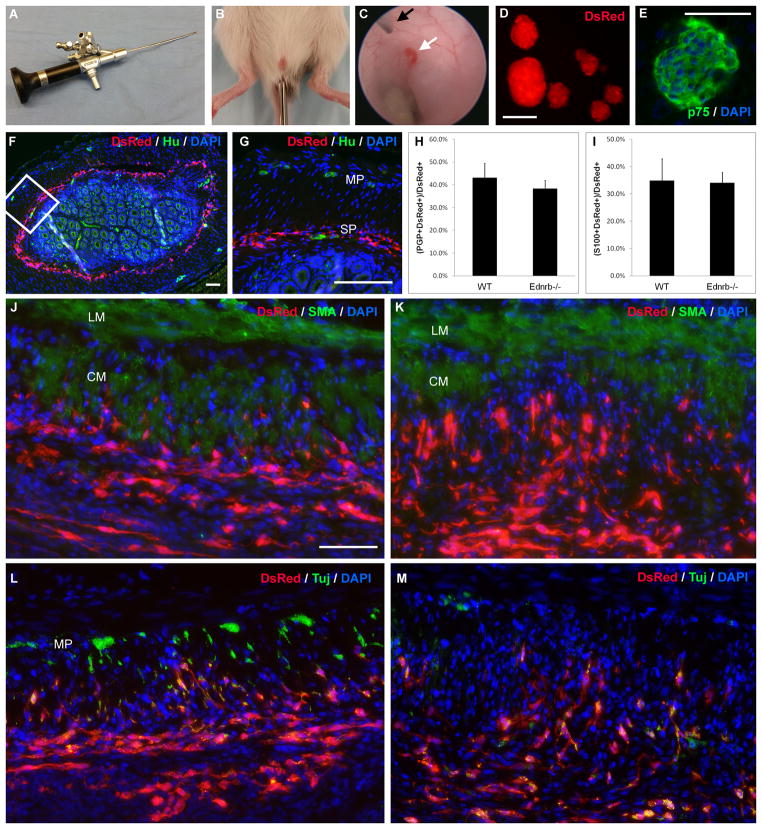
Recipient mice were anesthetized with isoflurane at 2% for induction and maintenance. A tap water enema was administered via 20-gauge angiocatheter to clear stool. The anus was gently dilated with forceps to accommodate the endoscope. A 4.5 Fr rigid 0 degree cystoureteroscope with a 2.4Fr working channel (product number: 4615.401; Richard Wolf Medical Instruments Corp., Vernon Hills, IL, USA) was introduced into the anus and advanced 2 cm under direct vision using a Karl Storz Tricom SL NTSC 202221 20 video camera (Karl Storz, El Segundo, CA, USA) (Fig. 1A,B). A 14-inch long, 30-gauge Hamilton needle with 45 degree bevel (VWR International, Radnor, PA, USA) was passed through the working channel and a single injection of 50 μL of a 1,000 cells μL−1 suspension delivered into the colon wall (Fig. 1C). India ink (10 μL) was injected to tattoo the injection site for later identification.
Figure 1. Enteric neuronal stem cells can be delivered by colonoscopy into the gut wall.
Colonoscopy is performed using a 4.5 Fr cystoureteroscope (A). The endoscope is introduced into the anus of WT or Ednrb−/− mice and advanced 2 cm (B). A 30-gauge Hamilton needle (C, black arrow) is passed through the working channel of the endoscope and 50 μL of a 1,000 cells μL−1 suspension is injected into the colon wall (C, white arrow). Enteric neurospheres generated from Actb-DsRed mice ubiquitously express DsRed (D) and also stain for neural progenitor marker, p75 (E). Immediately following endoscopic delivery of cells, there is circumferential spreading of DsRed-positive cells in the region of the submucosal plexus of the distal colon (F, inset magnified in G). Endogenous enteric neurons are labeled by neuronal marker, Hu, in a WT recipient (G). One week after injection, there is no significant difference in neuronal (H) or glial density (I) among transplanted cells in WT and Ednrb−/− recipients. Distribution of transplanted cells is similar in WT (J) and Ednrb−/− (K) recipients 1 week after transplantation. Fibers co-expressing neuronal marker, Tuj, extend into the muscularis propria both in WT (L) and Ednrb−/− (M) recipients. Endogenous myenteric ganglia are seen in WT colon (L). Scale bar is 100 μm. Scale bar is the same for J–M.
CM, circular muscle; LM, longitudinal muscle; MP, myenteric plexus; SP, submucosal plexus
Recipients were sacrificed 1–4 weeks following transplantation and the distal colons fixed in 4% paraformaldehyde. For cryosection, tissue was incubated in 15% sucrose at 4°C overnight, and then in 15% sucrose containing 7.5% gelatin at 37°C for 1 hour. Tissue was embedded in 15% sucrose containing 7.5% gelatin at room temperature and rapidly frozen at −40°C in methylbutane. Sections were cut at 12 μm thickness with a Leica CM3050 S cryostat (Leica Microsystems, Buffalo Grove, IL, USA).
Immunohistochemistry
Immunohistochemistry was performed as described (10). Tissue was permeabilized with 0.1% Triton X-100 and blocked with 10% donkey serum for 1 hour. Primary antibodies included: human antineuronal nuclear antibody-1 (Hu; 1:16,000; generous gift from Dr. Vanda Lennon), rabbit anti-p75 neurotrophin receptor (p75; 1:500; Promega, Madison, WI, USA), rabbit anti- protein gene protein 9.5 (PGP; 1:400; Cedarlane, Burlington, NC, USA), rabbit anti-S100 calcium binding protein B (S100; 1:100; NeoMarkers, Fremont, CA, USA), rabbit anti-α-smooth muscle antibody (SMA; 1:100; Abcam, Cambridge, MA, USA), rabbit anti-neuronal nitric oxide synthase (NOS; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-calretinin (CR; 1:200; Life Technologies), and goat anti-choline acetyltransferase (ChAT; 1:50; Millipore, Billerica, MA, USA). Secondary antibodies included: donkey anti-rabbit Alexa Fluor 488, donkey anti-goat Alexa Fluor 488, and donkey anti-human Alexa Fluor 488 (Life Technologies). Alex Fluor 488-conjugated neuronal class III β-tubulin (Tuj; 1:500; Biolegend, San Diego, CA, USA) was also used. Cell nuclei were stained with DAPI (Vector Labs, Burlingame, CA, USA). Images were taken using a Nikon Eclipse 80i microscope.
To quantify neuronal and glial cell density in the transplanted cell populations, 6 representative sections from 4 WT and 2 Ednrb−/− recipients each were stained for PGP and S100, respectively. The number of positive cells was counted with ImageJ software. Results were compared statistically using the Chi-squared test (α<0.05).
Results
Neurospheres from Actb-DsRed mice expressed DsRed in all cells (Fig. 1D) and were immunoreactive for neural crest cell marker, p75 (Fig. 1E).
Twelve mice underwent colonoscopic transplantation of ENSC, including 8 WT (6–8 weeks old) and 4 Ednrb−/− (2–3 week-old) mice. Average procedure time was 10 minutes. All mice tolerated the procedure with no post-operative complications and survived to sacrifice. Transplanted cells were found in 9 of 12 recipients (in 6 WT, in 3 Ednrb−/−), and cells were seen up to 4 weeks after transplantation (in WT).
Transplanted cells spread circumferentially within the submucosal plexus (Fig. 1F,G) immediately after injection. Transplanted cells spread longitudinally for a distance of up to 1 mm 1 week after injection. DsRed+ cells were present at 1 week in both ganglionic WT colon (Fig. 1J,L) and aganglionic Ednrb−/− colon (Fig. 1K,M) with similar patterns and densities of neurofiber extension into the muscularis propria. Neuronal cell density did not differ significantly between WT and Ednrb−/− recipients at 1 week (43.0 ± 6.3% vs. 38.2 ± 3.6%, respectively; p = NS). Glial cell density was also not significantly different (34.8 ± 8.0% vs. 34.0 ± 3.8%, respectively; p = NS). Due to the limited lifespan of Ednrb−/− mice, graft survival beyond 1 week was not assessed.
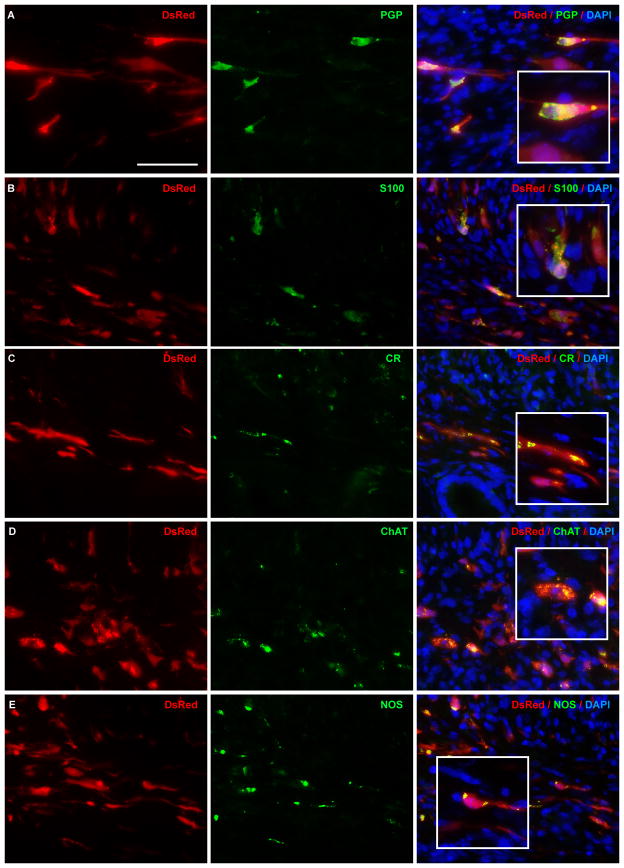
Cells transplanted to Ednrb−/− recipients after 1 week expressed markers of neurons (Fig. 2A), glia (Fig. 2B), and neuronal subtypes (Fig. 2C–E). Similar patterns of differentiation were observed in WT recipients.
Figure 2. Endoscopically transplanted enteric neuronal stem cells survive and differentiate in Ednrb−/− mouse colon in vivo.
Enteric neuronal stem cells from Actb-DsRed mice were colonoscopically injected into the distal colon of recipient Ednrb−/− mice. In vivo neuronal differentiation 1 week after transplantation is confirmed by co-localization of DsRed with neuronal marker, PGP (; A). Differentiation into glia and neuronal subtypes is shown by immunoreactivity to glial marker, S100 (B), and neuronal subtype markers: calretinin (CR; C), choline acetyltransferase (ChAT; D), neuronal nitric oxide synthase (NOS; E). Scale bar is 50 μm. Scale bar is the same for A–E.
Discussion
Endoscopic microinjection offers a safe, efficient and minimally invasive approach to optimize ENSC delivery to target regions of diseased enteric innervation. All recipient mice in our study survived the procedure without complications. Transplanted ENSC were successfully engrafted and gave rise to enteric neurons and glia in both ganglionic and aganglionic colon.
Endoscopy is a well-accepted and commonly used tool in the diagnosis and treatment of gastrointestinal diseases. Endoscopic mucosal biopsies have been used to isolate and culture human ENSC (11). Murine colonoscopy has been previously used in colorectal cancer research to implant orthotopic tumor cells into the colonic submucosa (12), but has not been previously described for delivery of ENSC. Compared to laparotomy, this approach is less invasive and can be readily applied to future human studies. In our experience, the procedure takes less time than laparotomy for cell delivery. Moreover, recipient mice experienced no intra-operative or post-operative complications. Overall, endoscopic delivery of ENSC appears to be safe and effective.
Others have described intraperitoneal cell injection in aganglionic mice (13) or intravascular delivery for central nervous system disease (14). However, in these approaches, cells distribute spontaneously to the gut wall without the ability for site-specific targeting. Ectopic spreading of ENSC to unwanted locations poses potential problems, such as risk for malignant transformation, altered host organ function, and decreased transplant efficiency. In contrast, endoscopic injection allows controlled delivery of cells to the desired location under direct vision, and multiple endoscopic microinjections could be used to target larger areas.
In 25% of our recipients, transplanted cells were not found. In these mice, the delivery needle may have penetrated through the thin murine colon wall. Perforation is a risk of endoscopy, but in our small cohort, all mice survived to sacrifice and none exhibited signs of peritonitis despite suspected microperforation. Injection of cells into a specific gut layer presents a technical challenge in this small animal model, but would likely be easier in large animals. Endoscopic ultrasound or other combined imaging could also facilitate precise delivery. Another limitation of this technique is the rigid endoscope which limits access to the more proximal colon. This can be overcome in larger animals where a flexible endoscope can be employed.
Advances in our understanding of the enteric nervous system, neural progenitors, and cell therapy have moved us closer to transplanting ENSC to treat Hirschsprung and other neurointestinal diseases. Future use of this therapy in humans requires a safe and effective method of cell delivery, and endoscopy offers such an approach.
Key Messages.
Neural cell replacement therapy is a potential treatment for Hirschsprung and other neurointestinal diseases, but delivery of cells to the target area presents a challenge. Endoscopy allows the targeted delivery of a large number of cells in a minimally-invasive manner.
Our aim is to establish endoscopic cell delivery as a safe and reliable alternative to open surgical transplantation of ENSC.
Twelve mice underwent colonoscopic injection of ENSC. Engraftment and differentiation of transplanted cells were analyzed 1–4 weeks later.
All mice tolerated the procedure without complications. Transplanted ENSC differentiated into enteric neurons and glia and were found in 9 of 12 recipients, including 3 Ednrb−/− mice with distal colonic aganglionosis.
Acknowledgments
We thank Dr. Vanda Lennon, MD, PhD (Mayo Clinic, Rochester, MN, USA) for the kind gift of Hu antibody.
Funding
LSC is supported by a resident scholar award from the Society of University Surgeons. RH is supported by a grant from the REACHirschsprung Foundation. AMG is supported by the National Institutes of Health (DK080914).
Footnotes
Contributions
LSC, RH, and JBG performed the research and analyzed the data. HKG supplied the reagents and maintained the mouse colonies. LSC and NN performed immunohistochemistry. RH, JBG and AMG designed the study. LSC wrote the paper. All authors reviewed and approved the final manuscript.
Disclosure
The authors have no conflicts of interest.
References
- 1.Kulkarni S, Becker L, Pasricha PJ. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 61:613–621. doi: 10.1136/gut.2010.235614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotta R, Natarajan D, Thapar N. Potential of cell therapy to treat pediatric motility disorders. Semin Pediatr Surg. 2009;18:263–273. doi: 10.1053/j.sempedsurg.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–496. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817–1824. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 5.Dong YL, Liu W, Gao YM, et al. Neural stem cell transplantation rescues rectum function in the aganglionic rat. Transplant Proc. 2008;40:3646–3652. doi: 10.1016/j.transproceed.2008.06.107. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Wu RD, Dong YL, Gao YM. Neuroepithelial stem cells differentiate into neuronal phenotypes and improve intestinal motility recovery after transplantation in the aganglionic colon of the rat. Neurogastroenterol Motil. 2007;19:1001–1009. doi: 10.1111/j.1365-2982.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 7.Hotta R, Stamp LA, Foong JP, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 123:1182–1191. doi: 10.1172/JCI65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vintersten K, Monetti C, Gertsenstein M, et al. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 9.Hosoda K, Hammer RE, Richardson JA, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 10.Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, et al. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterol Motil. 25:61–69. e67. doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger M, Bareiss PM, Danker T, et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137:2063–2073. e2064. doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Zigmond E, Halpern Z, Elinav E, Brazowski E, Jung S, Varol C. Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One. 6:e28858. doi: 10.1371/journal.pone.0028858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martucciello G, Brizzolara A, Favre A, et al. Neural crest neuroblasts can colonise aganglionic and ganglionic gut in vivo. Eur J Pediatr Surg. 2007;17:34–40. doi: 10.1055/s-2007-964952. [DOI] [PubMed] [Google Scholar]
- 14.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]