Abstract
Introduction
There is no clear consensus as to what constitutes an obstructive ventilatory defect (OVD): Is it FEV1/FVC<lower limit of normal (LLN) or<0.70 (respectively, physiological and operational definitions)?
Aim
To determine, according to the two definitions, the percentage of subjects having an OVD among them explored in a lung function exploration laboratory.
Population and methods
This is a retrospective study including 4,730 subjects aged 17–85 years. Subjects were divided according to the presence [physio (+) or operat (+)] or absence [physio (−) or operat (−)] of an OVD, and into younger (<45 years, n=2,076), older (≥45 years, n=2,654), smokers (n=1,208), and non-smokers (n=3,522) groups.
Results
For the total sample, the younger and older groups [mean±SD of age (years), respectively, 46.7±14.1; 33.9±7.4, and 56.8±9.1], the ‘physiological definition’ detected, respectively, 13.46, 43.22, and 5.09% more OVD than the ‘operational one’ (p<0.05). In addition, the operational definition, compared with the physiological one, overdiagnosed OVD in 2.33 and 0.44% of smokers and non-smokers, respectively, and underdiagnosed it in 4.46% and 29.72% of smokers and non-smokers, respectively (p<0.05). Compared with the group ‘physio (−), operat (+)’, the ‘physio (+), operat (−)’ one was younger (74.2±4.7 years vs. 40.9±10.3 years) and had significantly higher FEV1 (62±13% vs. 78±17%) and FVC (71±15% vs. 93±19%).
Conclusion
The frequency of OVD much depends on the criteria used for its definition.
Keywords: obstructive ventilatory defect, FEV1/FVC, fixed threshold, lower limit of normal, spirometry, guideline
The prevalence of bronchial asthma and chronic obstructive pulmonary disease (COPD) is constantly increasing worldwide including African countries (1, 2). These two chronic diseases, often having in common an obstructive ventilatory defect (OVD), should be diagnosed more accurately by using spirometry (3, 4). However, there is no clear consensus as to what constitutes an OVD.
On the one hand, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) (5) opted for a ‘physiological definition’ based on a first-second forced expiratory volume/forced vital capacity (FEV1/FVC) ratio below the lower limit of normal (LLN) range. On the other hand, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) opted for an ‘operational definition’ based on a fixed threshold value of 0.70 (FEV1/FVC<0.70) (3). The last definition was recently criticised in an open letter addressed to GOLD committee members (6–10). The main criticism was that the FEV1/FVC ratio declines with increasing age and height, even in healthy lifelong non-smokers, whose LLN drops below a ratio of 0.70 from about 45 years of age (6–10). Therefore, the use of a fixed threshold value causes up to 50% overdiagnosis (misclassification) above that age (6–10). Authors and signatories of the open letter have asked GOLD committee members to abandon the ‘operational method’ in favour of the ‘physiological’ one (6–10).
The lack of a clear worldwide consensus about OVD definition could be a source of confusion and/or misdiagnosis for clinicians and respiratory researchers. This is the case in Africa, where the ‘operational definition’ is widely applied (11–15). For example, a recent paper published in the Libyan Journal of Medicine (11) was criticised (16) since authors have opted for the use of a fixed threshold of 0.70 to define the OVD.
Recently, the ATS/ERS in a paper entitled ‘Research Questions in COPD’ have recommended studies that evaluate the impact of age on the importance of identifying an OVD (17). Thus, the aim of the present study is to highlight, on a large sample, the potential errors engendered by applying the ‘operational definition’ instead of the ‘physiological’ one.
Population and methods
Study design
It is a retrospective study including anthropometric and spirometric data (n=4,516 records) from a local team's previous published studies during the last 10 years (18–34). Some included data (n=214 cases) were prospectively evaluated during January and February of 2015.
The Tunisian population comprises people of mainly Arab, Berber, and Turkish descent (26).
Inclusion and non-inclusion criteria
Only reproducible spirometric data of subjects aged more than 17 years were considered for analysis.
Collected data
Quantity of used cigarettes and/or narghile [respectively, in packets-years (PY) and narghiles-years (NY)], age (years), height (m), weight (kg), body mass index (BMI, kg/m2), FEV1 (L,%), FVC (L,%), and FEV1/FVC (absolute value).
Smoking status (smoker/non-smoker)
The subject was qualified as a smoker when the cigarette or narghile use was ≥5 PY or NY, respectively, or when the sum of cigarette and narghile use was ≥5 (20, 26).
Spirometric measurements
All spirometric measurements were performed according to the ATS/ERS 2005 guidelines (35–37). Local spirometric norms were applied (20, 26, 38).
Applied definitions
Subjects were divided into seven groups of 10-year age ranges, into two groups of younger (<45 years) or older (≥45 years) subjects (6–10, 39), and into two groups of smokers and non-smokers. According to the presence or absence of an OVD, subjects were divided into six groups:
Group I: Physiological definition ‘physio (+)’: FEV1/FVC<LLN.
Group II: Operational definition ‘operat (+)’: FEV1/FVC<0.7.
Group III: ‘physio (+), operat (−)’: FEV1/FVC<LLN and FEV1/FVC≥0.70.
Group IV: ‘operat (+), physio (−)’: FEV1/FVC<0.70 and FEV1/FVC≥LLN.
Group V: ‘physio (+), operat (+)’: FEV1/FVC<LLN and FEV1/FVC<0.70.
Group VI: ‘physio (−), operat (−)’: FEV1/FVC≥LLN and FEV1/FVC ≥0.70.
Statistical analyses
Quantitative and qualitative data were expressed, respectively, as mean±SD and as number (%). Chi-square test was used to compare percentages of included subjects between Groups I and II. Parametric (t-test) and non-parametric (Mann–Whitney U) tests were used to compare anthropometric and spirometric data between Groups III and VI. Analyses were carried out using Statistica statistical software (Statistica Kernel version 6; Stat Software, Maisons-Alfort, France). Significance was set at the 0.05 level.
Results
Anthropometric and spirometric data of 4,730 subjects were retained. Table 1 presents their data divided according to age ranges. Compared with the ‘physio (+)’ group, the ‘operat (+)’ group included significantly lower percentages of subjects (total samples data) only in age ranges <55 years. For the total sample (17–85 years), compared with the ‘physiological definition’, the ‘operational definition’ gives a significantly lower percentage of subjects having OVD, respectively, 19.15% vs. 16.57%. Thus, the ‘physiological definition’ detected 13.46% more OVD than the ‘operational definition’.
Table 1.
Anthropometric and spirometric data of included subjects divided according to age ranges (n=4,730)
| Anthropometric data | FEV1 | FVC | FEV1/FVC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age range (years) | Sex | Age (year) | Height (m) | Weight (kg) | BMI | (L) | % | (L) | % | Absolute value | Group I (FEV1/FVC<0.70) | Group II (FEV1/FVC<LLN) | Probability |
| M (n=189) | 21.5±2.1 | 1.75±0.07 | 71±12 | 23.2±3.3 | 4.27±0.53 | 89±10 | 4.97±0.65 | 90±10 | 0.86±0.05 | 0 (0.00) | 6 (3.19)* | 0.0169 | |
| 17–25 | F (n=111) | 21.0±1.9 | 1.65±0.08 | 61±10 | 22.6±3.6 | 3.40±0.60 | 90±12 | 3.86±0.67 | 88±11 | 0.88±0.05 | 0 (0.00) | 0 (0.00) | 1 |
| T (n=300) | 21.3±2.0 | 1.71±0.09 | 68±12 | 23.0±3.4 | 3.95±0.70 | 90±11 | 4.56±0.85 | 89±10 | 0.86±0.05 | 0 (0.00) | 6 (2.00)* | 0.0141 | |
| M (n=556) | 30.0±2.8 | 1.74±0.07 | 78±14 | 25.6±4.0 | 4.00±0.62 | 88±11 | 4.85±0.77 | 92±11 | 0.82±0.06 | 17 (3.05) | 47 (8.45)* | 0.003 | |
| 25–35 | F (n=182) | 30.5±2.9 | 1.65±0.08 | 68±12 | 25.2±4.0 | 3.30±0.91 | 94±17 | 3.91±0.91 | 95±17 | 0.84±0.05 | 3 (1.64) | 8 (4.39) | 0.2641 |
| T (n=738) | 30.1±2.8 | 1.72±0.08 | 75±14 | 25.5±4.0 | 3.82±0.71 | 90±13 | 4.62±0.90 | 93±13 | 0.83±0.06 | 20 (2.71) | 55 (7.45)* | 0.003 | |
| M (n=768) | 40.1±2.7 | 1.71±0.07 | 80±14 | 27.2±4.3 | 3.51±0.69 | 84±15 | 4.37±0.77 | 90±13 | 0.80±0.07 | 84 (10.93) | 118 (15.36)* | 0.0199 | |
| 35–45 | F (n=270) | 39.8±2.7 | 1.62±0.08 | 73±12 | 27.8±4.3 | 3.00±0.71 | 94±19 | 3.61±0.85 | 95±18 | 0.83±0.06 | 9 (3.33) | 20 (7.40)* | 0.0334 |
| T (n=1038) | 40.1±2.7 | 1.69±0.09 | 78±14 | 27.4±4.3 | 3.38±0.73 | 86±16 | 4.17±0.86 | 91±15 | 0.81±0.07 | 93 (8.58) | 138 (13.29)* | 0.036 | |
| M (n=870) | 49.6±2.8 | 1.71±0.06 | 78±14 | 26.6±4.5 | 2.93±0.88 | 75±21 | 3.83±0.83 | 84±16 | 0.75±0.13 | 197 (22.64) | 229 (26.32) | 0.1459 | |
| 45–55 | F (n=484) | 49.5±3.0 | 1.59±0.08 | 73±11 | 28.7±4.2 | 2.57±0.61 | 89±17 | 3.11±0.75 | 91±17 | 0.82±0.06 | 12 (2.47) | 20 (4.13) | 0.0685 |
| T (n=1354) | 49.6±2.8 | 1.67±0.09 | 76±13 | 27.4±4.5 | 2.80±0.81 | 80±21 | 3.58±0.88 | 86±17 | 0.77±0.11 | 209 (15.43) | 249 (18.38)* | 0.0356 | |
| M (n=458) | 58.7±2.7 | 1.68±0.07 | 75±14 | 26.7±4.7 | 2.44±0.87 | 67±23 | 3.30±0.84 | 78±18 | 0.72±0.14 | 157 (34.27) | 162 (35.37) | 0.7503 | |
| 55–65 | F (n=256) | 58.8±2.8 | 1.58±0.08 | 71±12 | 28.2±4.5 | 2.26±0.61 | 87±19 | 2.74±0.72 | 88±18 | 0.82±0.09 | 16 (6.25) | 19 (7.42) | 0.6465 |
| T (n=714) | 58.7±2.7 | 1.65±0.09 | 74±14 | 27.2±4.7 | 2.38±0.79 | 74±23 | 3.10±0.84 | 82±18 | 0.76±0.14 | 173 (24.22) | 181 (25.35) | 0.6605 | |
| M (n=328) | 68.5±2.6 | 1.67±0.07 | 71±12 | 25.5±4.4 | 1.71±0.78 | 54±25 | 2.59±0.84 | 67±21 | 0.64±0.14 | 197 (60.06) | 190 (57.92) | 0.6027 | |
| 65–75 | F (n=104) | 68.1±2.3 | 1.56±0.09 | 65±11 | 26.9±3.9 | 1.99±0.71 | 87±24 | 2.44±0.80 | 89±21 | 0.81±0.10 | 9 (8.65) | 9 (8.65) | 1 |
| T (n=432) | 68.4±2.6 | 1.64±0.09 | 69±12 | 25.8±4.4 | 1.78±0.77 | 62±28 | 2.55±0.83 | 73±23 | 0.68±0.15 | 206 (47.68) | 199 (46.06) | 0.5561 | |
| M (n=129) | 77.9±2.7 | 1.64±0.06 | 69±10 | 25.5±3.8 | 1.47±0.63 | 62±17 | 2.30±0.56 | 72±18 | 0.62±0.17 | 83 (64.34) | 78 (60.46) | 0.5087 | |
| 75–85 | F (n=25) | 78.4±2.3 | 1.48±0.04 | 62±7 | 28.3±2.9 | 1.38±0.23 | 80±14 | 1.58±0.28 | 74±15 | 0.87±0.05 | 0 (0.00) | 0 (0.00) | 1 |
| T (n=154) | 78.0±2.7 | 1.62±0.08 | 68±9 | 25.9±3.8 | 1.46±0.58 | 64±27 | 2.18±0.59 | 66±18 | 0.66±0.18 | 83 (53.89) | 78 (50.64) | 0.5985 | |
| M (n=3298) | 46.7±14.4 | 1.71±0.07 | 76±14 | 26.2±4.4 | 3.07±1.09 | 76±22 | 3.94±1.10 | 84±17 | 0.76±0.12 | 735 (22.28) | 830 (25.16)* | 0.0041 | |
| 17–85 | F (n=1432) | 46.6±13.4 | 1.60±0.08 | 70±12 | 27.4±4.5 | 2.69±0.79 | 90±18 | 3.23±0.93 | 91±18 | 0.83±0.07 | 49 (3.42) | 76 (5.30)* | 0.0064 |
| T (n=4730) | 46.7±14.1 | 1.67±0.09 | 75±14 | 26.6±4.5 | 2.96±1.02 | 90±22 | 3.72±1.10 | 86±18 | 0.78±0.12 | 784 (16.57) | 906 (19.15)* | 0.0114 | |
BMI, body mass index in kg/m2; F, Female; FEV1, first-second forced expiratory volume; FVC, forced vital capacity; LLN, lower limit of normal; M, male; T, total sample. Quantitative data are mean±SD. Groups I and II data are number (%).
Probability (chi-square) <0.05: ‘FEV1/FVC<0.70’ vs. ‘FEV1/FVC<LLN’.
For both younger (n=2,076) and older (n=2,654) groups [mean±SD of age (years): 33.9±7.4 and 56.8±9.1, respectively], compared with the ‘physiological definition’, the ‘operational definition’ gives statistically significant lower percentages of subjects having OVD, respectively, 9.59% vs. 5.44% and 26.64% vs. 25.28%. Thus, the ‘physiological definition’ detected 43.22 and 5.09% more OVD than the ‘operational definition’, respectively, in younger and older groups.
Table 2 presents the anthropometric and spirometric data of included subjects divided according to OVD definitions (Groups III−VI). Compared with the ‘operat (+), physio (−)’ group, the ‘physio (+), operat (−)’ group was younger, had significantly higher FEV1 and FVC, and included higher percentages of females and non-smokers. Among the 784 subjects ‘operat (+)’ (Table 1), 14 (2%) were ‘physiol (−)’ (Table 2). Among the 906 subjects ‘physio (+)’ (Table 1), 136 (15%) were ‘operat (−)’ (Table 2).
Table 2.
Anthropometric and spirometric data of included subjects divided according to OVD definitions (n=4,730)
| Group III ‘physio (+), operat (−)’ | Group IV ‘physio (−), operat (+)’ | Group V ‘physio (+), operat (+)’ | Group VI ‘physio (−), operat (−)’ | ||
|---|---|---|---|---|---|
| FEV1/FVC<LLN and FEV1/FVC≥0.70 (n=136) | FEV1/FVC<0.70 and FEV1/FVC≥LLN (n=14) | FEV1/FVC<0.70 and FEV1/FVC<LLN (n=770) | FEV1/FVC≥0.70 and FEV1/FVC≥LLN (n=3,810) | ||
| Sex | Male | 109 (80) | 14 (100)* | 721 (94) | 2,454 (64)* |
| Female | 27 (20) | 0 (0)* | 49 (16) | 1,356 (36)* | |
| Smoking status | Smoker | 39 (29) | 13 (93)* | 544 (71) | 544 (14)* |
| Non-smoker | 97 (71) | 1 (7)* | 226 (29) | 3,266 (86)* | |
| Age | (year) | 40.9±10.3 | 74.2±4.7a | 57.8±11.9 | 44.5±13.4b |
| Height | (m) | 1.71±0.09 | 1.67±0.02a | 1.69±0.07 | 1.67±0.09b |
| Weight | (kg) | 79±16 | 75±6 | 70±14 | 75±14b |
| BMI | (kg/m2) | 26.8±4.6 | 26.7±2.0 | 24.8±4.6 | 27.0±4.4b |
| FEV1 | (L) | 3.13±0.78 | 1.68±0.33a | 1.73±0.84 | 3.21±0.87b |
| (%) | 78±17 | 62±13a | 48±20 | 87±16b | |
| FVC | (L) | 4.37±1.07 | 2.49±0.49a | 2.99±1.10 | 3.85±1.04b |
| (%) | 93±19 | 71±15a | 71±22 | 89±15b | |
| FEV1/FVC (absolute value) | 0.71±0.01 | 0.67±0.00a | 0.56±0.10 | 0.83±0.05b | |
BMI, body mass index; FEV1, first-second forced expiratory volume; FVC, forced vital capacity; OVD, obstructive ventilatory defect; LLN, lower limit of normal. Data are mean±SD, except for sex and smoking status, where data are number (%).
Probability (Mann–Whitney U test)<0.05: Group III vs. Group IV.
Probability (t-test)<0.05: Group V vs. Group VI.
Probability (chi-square) <0.05: Group III vs. Group IV or Group V vs. Group VI.
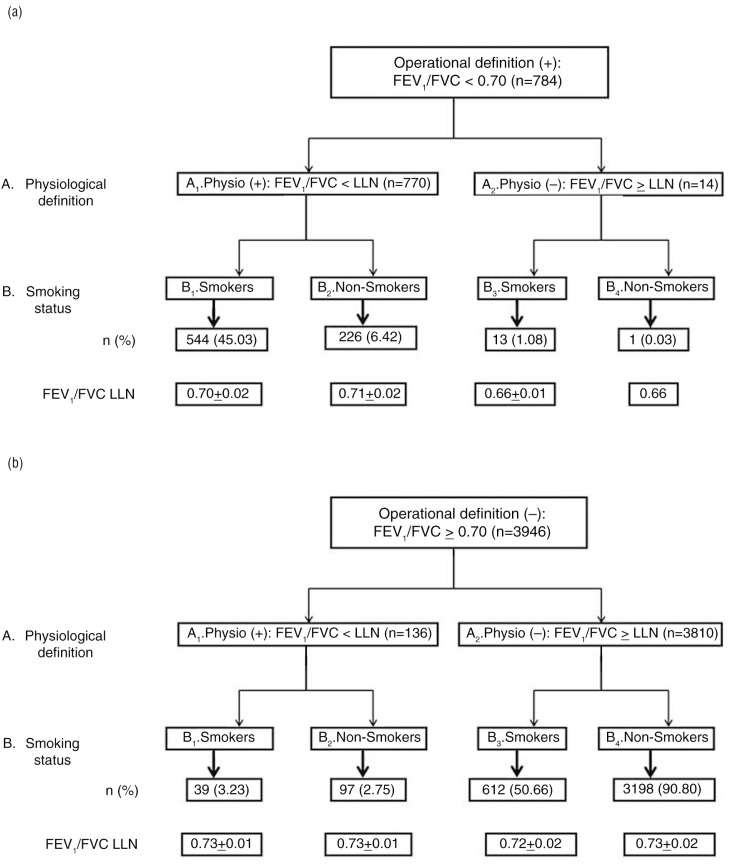
Figure 1 shows the step-by-step distribution of included subjects with respect to OVD definitions [operat ‘+’ (Fig. 1a); operat ‘−’ (Fig. 1b)] and smoking status. Depending on the OVD definitions and smoking status, each subgroup was distinguished in different cells (A1–A2; B1–B4). The analysis of Fig. 1 revealed the following:
Among the 557 smokers ‘operat (+)’ [B1+B3 (Fig. 1a)], 13 were ‘physiol (−)’ [B3 (Fig. 1a)], and among the 227 non-smokers ‘operat (+)’ [B2+B4 (Fig. 1a)], only one was ‘physiol (−)’ (B4 (Fig. 1a)). Thus, the ‘operational definition’ overdiagnosed OVD in 2.33% of smokers and in 0.44% of non-smokers.
Among the 583 smokers ‘physiol (+)’ [B1 (Fig. 1a)+B1 (Fig. 1b)], 26 were ‘operat (−)’ [(B1 (Fig.1a)+B1 (Fig.1b)) – (B1+B3 (Fig.1a))], and among the 323 non-smokers ‘physiol (+)’ [B2 (Fig. 1a)+B2 (Fig. 1b)], 96 were ‘operat (−)’ [(B2 (Fig. 1a)+B2 (Fig. 1b)) – (B2+B4 (Fig. 1a))]. Thus, the ‘operational definition’ underdiagnosed OVD in 4.46% of smokers and in 29.72% of non-smokers.
Fig. 1.
Distribution of included subjects with respect to obstructive ventilatory defect definitions and smoking status: (a) operational definition ‘negative’; (b) operational definition ‘positive’.
FEV1, first-second forced expiratory volume; FVC, forced vital capacity; LLN, lower limit of normal; n, number; %: percentage of smokers with OVD; FEV1/FVC LLN values are mean±SD.
Discussion
The present study, involving 4,730 adults, shows that the percentage of subjects having an OVD is recommendation dependent, especially in the age ranges <55 years. For the total sample (17–85 years), the younger (<45 years) and the older (≥45 years) groups, the ‘physiological definition’ detected, respectively, 13.46, 43,22, and 5.09% more OVD than the ‘operational definition’. In addition, the ‘operational definition’, compared with the ‘physiological definition’, overdiagnosed OVD in 2.33 and 0.44% of smokers and non-smokers, respectively, and underdiagnosed it in 4.46% and 29.72% of smokers and non-smokers, respectively. Therefore, a subject could be diagnosed as having or not having an OVD depending on which definition was applied.
Brief history of OVD spirometric definitions
In 1983, the ERS defined OVD as FEV1/FVC<88 and<89% of its predicted value, respectively, in males and females (40). In 1991, the ATS defined it as FEV1/FVC<LLN (41). In 1994, BTS opted for the use of a fixed threshold (FEV1/FVC<0.70 and FEV1<80% of its predicted value) (42). In 2000, Viegi et al. (43) defined it as an FEV1/FVC<0.70. In 2001, GOLD adopted the BTS definition (44). In 2005, the ATS/ERS opted for the ‘physiological definition’ using the LLN concept (5), but slow vital capacity replaced FVC. In 2012, a new definition (FEV1/FVC<z-score) was proposed by the Global Lung Initiative (GLI) (45). All these definitions continue to be used around the world; hence, a worldwide clear consensus is needed (17, 39).
Table 3 displays results of some studies (46–51) with a similar aim to the present one.
Table 3.
Results of some studies aiming to compare the OVD operational and physiological definitions
| First author | Miller (49) | Lau (48) | Szanto (51) | Roberts (50) | Aggarwal (46) | Present study |
|---|---|---|---|---|---|---|
| Year of study | NR | 2001–2003 | 2001–2002 | 2003–2004 | 1999–2008 | 2006–2015 |
| Country | United Kingdom USA New Zealand |
Hong Kong | Sweden | USA | India | Tunisia |
| Study design | Retrospective | Prospective | Retrospective | Retrospective | Retrospective | Retrospective/partially prospective |
| Sample size (male/female) | 11,413 (6,026/5,387) |
525 (525/0) |
574 (226/348) |
1,503 (796/707) |
27,307 (27,307/0) |
4,730 (3,298/1,432) |
| Inclusion criteria | White adults | Smokers Ex-smokers |
NR | NR | Male Age>15 years |
Age>17 years |
| Exclusion criteria | Non-white Lack of cooperation |
Asthma COPD Pulmonary tuberculosis Lung fibrosis Spinal abnormalities History of pleurodesis or chest tube insertion or thoracic surgery Acute illness (past 3 months) Respiratory tract infection (past 4 weeks) |
Lack of cooperation | Inaccurate tests Uninterpretable tests |
Age≤15 years | Lack of cooperation |
| Applied spirometric guidelines | ATS/ERS 2005 (35) | ATS-1995 (61) | ATS-1995 (61) | ATS-1995 (61) | ATS-1995 (61) ATS/ERS-2005 (35) |
.ATS/ERS-2005 (35) |
| Applied spirometric norms |
European norms (40) USA norms (62) |
Local norms (63) | ECSC (40) Local norms (64–66) |
Local norms (62, 67–69) | Local norms (70) | Local norms (20, 26, 38) |
| Age ranges (years) | 20–92 | 20–80 | 60–93 | 14–95 | 15–95 | 17–85 |
| Percentages of subjects with an OVD | ||||||
| Operational definition | 38 | 19 | 23 | 40 | 37 | 17 |
| Physiological definition | 32 | 14 | 10 | 37 (69) to 43 (74) | 33 | 19 |
| Significant difference between definitions | Yes | Yes | Yes | No | Yes | Yes |
COPD, chronic obstructive pulmonary disease; ECCS, European-Community for Coal and Steel; OVD, obstructive ventilatory defect; NR, not reported.
Discussion of the methodology
Study design
Like some other studies having similar aims (46, 49–54), the present one was retrospective. It was better to opt for a prospective study as done by some authors (47, 48). However, such studies require more time and human and economic resources (55, 56). In the present study, 5% of data were prospectively evaluated. In this prospective subgroup (n=214), similar results were found [statistically significant difference between the percentage of subjects with an OVD according to the ‘physiological’ 30.4% vs. the ‘operational’ (22.9%) definitions]. It was better to collect subjects’ medical data especially those about COPD and/or asthma (47, 48, 53). However, it seems that the diagnosis of COPD is also recommendation dependent, as shown in a recent local study (21).
Sample main characteristics
As carried out in some studies (46, 48–50), the present one included samples with a large age range (17–85 years). Szanto et al. (51) have opted for an elderly group aged ≥60 years. It is interesting to perform similar studies in children and adolescents who also need a clear consensus concerning OVD definition (45).
Discussion of the results
In the present study, the ‘physiological definition’ detected significantly 13.46% more OVD than the ‘operational definition’ (Table 1). On the one hand, this result was contrary to what was previously published, where the ‘physiological definition’ compared with the ‘operational definition’ significantly underdiagnosed OVD by 4% (46), 5% (48), and 6% (49) (Table 3). On the other hand, the present result was intermediate to the one published by Roberts et al. (50), where the differences between the two definitions were about −3 to 3%, depending on the applied spirometric norms. It is important to note that the differences reported by Roberts et al. (50) were not statistically significant (Table 3). The present study result cannot be compared with the one of Szanto et al. (51) (reporting a difference of 13% in favour of the ‘operational definition’) since they have studied only elderly subjects (Table 3). Some plausible explanations of the results’ divergence could be study design [retrospective vs. prospective (55, 56)], sample size (57) [low, e.g.,<2,000 (48, 50, 51) vs. high, e.g.,>4,000 (46, 49)], applied inclusion and exclusion criteria (58–60) [e.g. comorbidities (48)], applied spirometric guidelines [old (46, 48, 50, 51) (ATS-1995 (61)) vs. new (46, 49) (ATS/ERS-2005 (35)], and age ranges [large (46, 48–50) vs. narrow (51)].
The following sections will discuss the advantages and disadvantages of the two most applied definitions to diagnose OVD.
Operational definition
The advantage of the ‘operational definition’ (3) is that the diagnosis of OVD is made by reference to one easy to remember number (0.70), avoiding the use of reference values and calculations (43). Its use by general practitioners or specialists of other disciplines could be justified by its simplicity (43). These qualities lend it to practical use in the detection of COPD in any country (31). In addition, the use of the ‘operational definition’ is associated with increased death risk, whereas using the ‘physiological definition’ is not (71). The profile of subjects ‘operat (+), physio (−)’ is characterised by the predominance of males and smokers; the advanced age of included subjects; and lower FEV1, FVC, and FEV1/FVC (Group IV, Table 2).
However, the ‘operational definition’, the main objective of which is to favour screening, has its limits. First, the argument that the fixed cut-off of 0.70 is easy to remember cannot be justified because even inexpensive pocket spirometers compute the predicted FEV1 and FEV1/FVC, as well as the LLN (72). Secondly, ignoring that FEV1/FVC ratio changes with age (38, 50, 73) probably leads to and underestimates the OVD prevalence in the young subjects and overestimates its prevalence in the elderly subjects (74, 75). Thirdly, the fixed ratio does not delimit mild airways obstruction and its use introduces an important age and sex bias (39). However, the present study does not confirm the above hypothesis since in both younger and older groups, compared with the ‘physiological definition’, the ‘operational definition’ gives statistically significant lower percentages of subjects having OVD, respectively, 9.59% vs. 5.44% and 26.64% vs. 25.28%.
The use of a fixed threshold value for OVD diagnosis is based on the same reasoning for the diagnosis of other chronic diseases, such as diabetes or hypertension where it is conventional that using fixed cut points works well (72), so that a fixed threshold for FEV1/FVC should work equally well (76). However, the normal levels of blood pressures and glycaemia are maintained within a narrow range of the target value by physiological regulation systems. There is no such system that controls the level of FEV1/FVC and the absence of such a target value for FEV1/FVC should therefore be accepted (72).
Physiological definition
Interpretation of spirometry data is usually based on comparisons of parameters measured in an individual patient or subject with reference values based on healthy subjects (77). Values below the LLN, defined by the 95% confidence interval, are considered as abnormal (5, 54, 77). The use of the LLN is an appropriate method to interpret spirometry data, since the clinical question is whether the calculated FEV1/FVC ratio is reduced (5, 41, 54). Problems arise, however, when FEV1/FVC lie near its LLN (5, 77). In this case, a literal interpretation of the functional defect is too simplistic and could fail to properly describe the functional status and other tests, such as the reversibility one, should be performed (5, 77). The ‘operational definition’, compared with the ‘physiological definition’, underestimated the OVD in 122 subjects (2.6%) (Table 1). The profile of subjects ‘physio (+), operat (−)’ is characterised by the predominance of females and non-smokers; the middle age of included subjects; and higher FEV1, FVC, and FEV1/FVC (Group III, Table 2).
One limitation to the application of the ‘physiological definition’ could be a lack of reference values for many countries, especially the low-income ones, such as some African countries (5). A Medline search performed on 12 September 2015 and applying the following keywords (‘reference equations’ and ‘spirometry’ and ‘Africa’ and ‘adult’), found 15 papers with norms published for only a few African countries (Tunisia, Algeria, South Africa, Sudan, Rwanda, Togo, Tanzania, Senegal, Ethiopia, and Ivory Coast). Another limitation is that errors in interpretation (with respect to both overestimation and underestimation of ventilatory defects) can occur if inappropriate reference equations are used (20, 26). In Europe and some other countries, the GLI reference equations are presently recommended (45). These reference equations have been expanded to include preschool children, and the LLN has been more precisely defined, using z-scores (45). Similar analyses are underway to update the spirometry reference equations for use in Africa with, for example, the project ‘Paediatric and Adult African Spirometry (PAAS)’ working group.
Implications for research
As advised by the ATS/ERS (5, 17), there is a need for more research related to OVD definition. Specifically, there is a need for higher quality prospective studies that could more clearly identify an unambiguous consensus on what constitutes an OVD taking account of the smoking status of subjects.
In conclusion, the prevalence of OVD very much depends on the criteria used for its definition. The present study provides powerful support for the view that the ‘physiological definition’ should be applied in order to avoid the risk of misdiagnosing a part of the population as free from pulmonary disease. The reasons of simplicity and ease of remembrance, advanced for the ‘operational definition’ seem unimportant compared with the objective of being able to properly detect OVD, especially in smokers (47). An OVD definition based on LLN derived from an appropriate spirometric reference equation would diminish the rate of misinterpretations. It will bring pulmonary medicine more in line with other medical disciplines and facilitate the correct interpretation of spirometry defects (49). Schooling societies and scientific organisations, such as GOLD (www.goldcopd.com/, accessed 12 September 2015), are recommended to return to evidence-based medicine and revise their guidelines.
Authors’ contributions
ZA and SR conceived the study, participated in its design, collected spirometric data, performed the statistical analysis, coordinated the study and helped to draft the manuscript. HBS conceived the study, participated in its design, performed the statistical analysis, coordinated the study, and approved the final version.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Daldoul H, Denguezli M, Jithoo A, Gnatiuc L, Buist S, Burney P, et al. Prevalence of COPD and tobacco smoking in Tunisia – results from the BOLD study. Int J Environ Res Public Health. 2013;10:7257–71. doi: 10.3390/ijerph10127257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nafti S, Taright S, El Ftouh M, Yassine N, Benkheder A, Bouacha H, et al. Prevalence of asthma in North Africa: the Asthma Insights and Reality in the Maghreb (AIRMAG) study. Respir Med. 2009;103(Suppl 2):S2–11. doi: 10.1016/S0954-6111(09)70022-8. [DOI] [PubMed] [Google Scholar]
- 3.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdf [cited 14 September 2015].
- 4.Global strategy for asthma management and prevention. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_2015.pdf [cited 14 September 2015].
- 5.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 6.Quanjer PH, Enright PL, Stocks J, Ruppel G, Swanney MP, Crapo RO, et al. Open letter to the members of the GOLD committee. Rev Mal Respir. 2010;27:1003–7. doi: 10.1016/j.rmr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Quanjer PH, Enright PL, Stocks J, Ruppel G, Swanney MP, Crapo RO, et al. Open letter to the members of the GOLD committee. Respiration. 2010;80:265–8. [Google Scholar]
- 8.Quanjer PH, Enright PL, Miller MR, Stocks J, Ruppel G, Swanney MP, et al. Open letter: the need to change the method for defining mild airway obstruction. Prim Care Respir J. 2010;19:288–91. doi: 10.4104/pcrj.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quanjer PH, Enright PL, Ruppel GL. The GOLD guidelines definition of mild airway obstruction. Respir Care. 2010;55:1397–8. [Google Scholar]
- 10.Quanjer PH, Enright PL, Miller MR, Stocks J, Ruppel G, Swanney MP, et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37:720–2. doi: 10.1183/09031936.00135110. [DOI] [PubMed] [Google Scholar]
- 11.Koubaa A, Triki M, Trabelsi H, Masmoudi L, Zeghal KN, Sahnoun Z, et al. Lung function profiles and aerobic capacity of adult cigarette and hookah smokers after 12 weeks intermittent training. Libyan J Med. 2015;10 doi: 10.3402/ljm.v10.26680. 26680, doi: http://dx.doi.org/10.3402/ljm.v10.26680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abd El-Azeem A, Hamdy G, Amin M, Rashad A. Pulmonary function changes in diabetic lung. Egypt J Chest Dis Tuberc. 2013;62:513–17. [Google Scholar]
- 13.Berriche O, Ben Mami F, Mhiri S, Achour A. Is the respiratory function altered during diabetes mellitus? Tunis Med. 2009;87:499–504. [PubMed] [Google Scholar]
- 14.Agodokpessi G, Ade G, Ahounou FJ, Gbenou DJ, Dansou HP, Gninafon M. Bronchoconstriction induced by exercise in the black African athlete. Mali Med. 2012;27:33–6. [PubMed] [Google Scholar]
- 15.Whittaker C. Chronic obstructive pulmonary disease. S Afr Fam Pract. 2013;55:20–5. [Google Scholar]
- 16.Ben Saad H. Methodological problems in the article comparing lung function profiles and aerobic capacity of adult cigarette and hookah smokers after 12 weeks intermittent training. Libyan J Med. 2015;10 doi: 10.3402/ljm.v10.27760. 28351, doi: http://dx.doi.org/10.3402/ljm.v10.28351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agusti A, Criner GJ, et al. An official American Thoracic Society/European Respiratory Society statement: research questions in COPD. Eur Respir J. 2015;45:879–905. doi: 10.1183/09031936.00009015. [DOI] [PubMed] [Google Scholar]
- 18.Ben Saad H, Ben Hassen I, Ghannouchi I, Latiri I, Rouatbi S, Escourrou P, et al. 6-Min walk-test data in severe obstructive-sleep-apnea-hypopnea-syndrome (OSAHS) under continuous-positive-airway-pressure (CPAP) treatment. Respir Med. 2015;109:642–55. doi: 10.1016/j.rmed.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Rouatbi S, Chouchene MA, Sfaxi I, Ben Rejeb M, Tabka Z, Ben Saad H. Fraction of exhaled nitric oxide (FeNO) norms in healthy Tunisian adults. Biomed Res Int. 2014;2014:269670. doi: 10.1155/2014/269670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Attar MN, Hadj Mabrouk K, Ben Abdelaziz A, Abdelghani A, Bousarssar M, Limam K, et al. Applicability of the old European respiratory society/European community for steel and coal reference equations for spirometry interpretation in Tunisian adult population. Tunis Med. 2014;92:574–80. [PubMed] [Google Scholar]
- 21.Ben Saad H, Ben Amor L, Ben Mdella S, Ghannouchi I, Ben Essghair M, Bougmiza I, et al. The diagnosis of COPD is recommendation dependent. Tunis Med. 2014;92:474–81. [PubMed] [Google Scholar]
- 22.Ben Saad H, Ben Amor L, Ben Mdalla S, Ghannouchi I, Ben Essghair M, Sfaxi R, et al. The importance of lung volumes in the investigation of heavy smokers. Rev Mal Respir. 2014;31:29–40. doi: 10.1016/j.rmr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Ben Saad H, Babba M, Boukamcha R, Ghannouchi I, Latiri I, Mezghenni S, et al. Investigation of exclusive narghile smokers: deficiency and incapacity measured by spirometry and 6-minute walk test. Respir Care. 2014;59:1696–709. doi: 10.4187/respcare.03058. [DOI] [PubMed] [Google Scholar]
- 24.Ben Moussa S, Sfaxi I, Tabka Z, Ben Saad H, Rouatbi S. Oxidative stress and lung function profiles of male smokers free from COPD compared to those with COPD: a case–control study. Libyan J Med. 2014;9 doi: 10.3402/ljm.v9.23873. 23873, doi: http://dx.doi.org/10.3402/ljm.v10.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Saad H, Khemiss M, Nhari S, Ben Essghaier M, Rouatbi S. Pulmonary functions of narghile smokers compared to cigarette smokers: a case-control study. Libyan J Med. 2013;8 doi: 10.3402/ljm.v8i0.22650. 22650, doi: http://dx.doi.org/10.3402/ljm.v10.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben Saad H, El Attar MN, Hadj Mabrouk K, Ben Abdelaziz A, Abdelghani A, Bousarssar M, et al. The recent multi-ethnic global lung initiative 2012 (GLI2012) reference values don't reflect contemporary adult's North African spirometry. Respir Med. 2013;107:20–8. doi: 10.1016/j.rmed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Ben Saad H, Khemis M, Bougmiza I, Prefaut C, Aouina H, Mrizek N, et al. Spirometric profile of narghile smokers. Rev Mal Respir. 2011;28:e39–51. doi: 10.1016/j.rmr.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Ben Saad H, Prefaut C, Tabka Z, Mtir AH, Chemit M, Hassaoune R, et al. 6-Minute walk distance in healthy North Africans older than 40 years: influence of parity. Respir Med. 2009;103:74–84. doi: 10.1016/j.rmed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Ben Saad H, Khemiss M, Bougmiza I, Prefaut C, Aouina H, Mrizek N, et al. Spirometric profile of narghile smokers. Rev Mal Respir. 2009;26:299–314. doi: 10.1016/s0761-8425(09)72587-2. [DOI] [PubMed] [Google Scholar]
- 30.Ben Saad H, Prefaut C, Tabka Z, Zbidi A, Hayot M. The forgotten message from gold: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulm Pharmacol Ther. 2008;21:767–73. doi: 10.1016/j.pupt.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Ben Saad H, Ben Attia Saafi R, Rouatbi S, Ben Mdella S, Garrouche A, Zbidi A, et al. Which definition to use when defining airflow obstruction? Rev Mal Respir. 2007;24:323–30. doi: 10.1016/s0761-8425(07)91064-5. [DOI] [PubMed] [Google Scholar]
- 32.Ben Saad H, Ben Attia Saafi R, Rouatbi S, Ben Mdella S, Garrouche A, Hadj Mtir A, et al. Which definition to use when defining reversibility of airway obstruction? Rev Mal Respir. 2007;24:1107–15. doi: 10.1016/s0761-8425(07)74260-2. [DOI] [PubMed] [Google Scholar]
- 33.Ben Saad H, Tfifha M, Harrabi I, Tabka Z, Guenard H, Hayot M, et al. Factors influencing pulmonary function in Tunisian women aged 45 years and more. Rev Mal Respir. 2006;23:324–38. doi: 10.1016/s0761-8425(06)71598-4. [DOI] [PubMed] [Google Scholar]
- 34.Sfaxi I, Ben Saad H, Rouatbi S. Fraction of exhaled nitric oxide in healthy elderly Tunisian subjects. Nitric Oxide. 2015;50:88–97. doi: 10.1016/j.niox.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 36.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 37.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 38.Tabka Z, Hassayoune H, Guenard H, Zebidi A, Commenges D, Essabah H, et al. Spirometric reference values in a Tunisian population. Tunis Med. 1995;73:125–31. [PubMed] [Google Scholar]
- 39.Quanjer PH, Cooper B, Ruppel GL, Swanney MP, Stocks J, Culver BH, et al. Defining airflow obstruction. Eur Respir J. 2015;45:561–2. doi: 10.1183/09031936.00126014. [DOI] [PubMed] [Google Scholar]
- 40.Quanjer P. Standardized lung function testing. Report working party standardization of lung function tests, European community for coal and steel. Bull Europ Physiopathol Respir. 1983;19:1–95. [PubMed] [Google Scholar]
- 41.No authors listed. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 42.No authors listed. Guidelines for the measurement of respiratory function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med. 1994;88:165–94. [PubMed] [Google Scholar]
- 43.Viegi G, Pedreschi M, Pistelli F, Di Pede F, Baldacci S, Carrozzi L, et al. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest. 2000;117:339S–45S. doi: 10.1378/chest.117.5_suppl_2.339s. [DOI] [PubMed] [Google Scholar]
- 44.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 45.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal AN, Gupta D, Agarwal R, Jindal SK. Comparison of the lower confidence limit to the fixed-percentage method for assessing airway obstruction in routine clinical practice. Respir Care. 2011;56:1778–84. doi: 10.4187/respcare.01160. [DOI] [PubMed] [Google Scholar]
- 47.Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008;63:1040–5. doi: 10.1136/thx.2008.095554. [DOI] [PubMed] [Google Scholar]
- 48.Lau AC, Ip MS, Lai CK, Choo KL, Tang KS, Yam LY, et al. Variability of the prevalence of undiagnosed airflow obstruction in smokers using different diagnostic criteria. Chest. 2008;133:42–8. doi: 10.1378/chest.07-1434. [DOI] [PubMed] [Google Scholar]
- 49.Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest. 2011;139:52–9. doi: 10.1378/chest.10-0189. [DOI] [PubMed] [Google Scholar]
- 50.Roberts SD, Farber MO, Knox KS, Phillips GS, Bhatt NY, Mastronarde JG, et al. FEV1/FVC ratio of 70% misclassifies patients with obstruction at the extremes of age. Chest. 2006;130:200–6. doi: 10.1378/chest.130.1.200. [DOI] [PubMed] [Google Scholar]
- 51.Szanto O, Montnemery P, Elmstahl S. Prevalence of airway obstruction in the elderly: results from a cross-sectional spirometric study of nine age cohorts between the ages of 60 and 93 years. Prim Care Respir J. 2010;19:231–6. doi: 10.4104/pcrj.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22:268–73. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 53.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not <70% Chest. 2007;131:349–55. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal AN, Gupta D, Behera D, Jindal SK. Comparison of fixed percentage method and lower confidence limits for defining limits of normality for interpretation of spirometry. Respir Care. 2006;51:737–43. [PubMed] [Google Scholar]
- 55.Hess DR. Retrospective studies and chart reviews. Respir Care. 2004;49:1171–4. [PubMed] [Google Scholar]
- 56.Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113:c214–17. doi: 10.1159/000235241. [DOI] [PubMed] [Google Scholar]
- 57.Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Burgel PR. From COPD definitions to COPD phenotypes. Presse Med. 2014;43:1337–43. doi: 10.1016/j.lpm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Echave-Sustaeta JM, Comeche Casanova L, Cosio BG, Soler-Cataluna JJ, Garcia-Lujan R, Ribera X. Comorbidity in chronic obstructive pulmonary disease. Related to disease severity? Int J Chron Obstruct Pulmon Dis. 2014;9:1307–14. doi: 10.2147/COPD.S71849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinreich UM, Thomsen LP, Bielaska B, Jensen VH, Vuust M, Rees SE. The effect of comorbidities on COPD assessment: a pilot study. Int J Chron Obstruct Pulmon Dis. 2015;10:429–38. doi: 10.2147/COPD.S76124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.No authors listed. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 62.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 63.Ip MS, Ko FW, Lau AC, Yu WC, Tang KS, Choo K, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129:384–92. doi: 10.1378/chest.129.2.384. [DOI] [PubMed] [Google Scholar]
- 64.Berglund E, Birath G, Bjure J, Grimby G, Kjellmer I, Sandqvist L, et al. Spirometric studies in normal subjects. I. Forced expirograms in subjects between 7 and 70 years of age. Acta Med Scand. 1963;173:185–92. [PubMed] [Google Scholar]
- 65.Hedenstrom H, Malmberg P, Agarwal K. Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21:551–7. [PubMed] [Google Scholar]
- 66.Hedenstrom H, Malmberg P, Fridriksson HV. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 1986;91:299–310. doi: 10.3109/03009738609178670. [DOI] [PubMed] [Google Scholar]
- 67.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 68.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 69.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 70.Aggarwal AN, Gupta D, Jindal SK. Development of a simple computer program for spirometry interpretation. J Assoc Physicians India. 2002;50:567–70. [PubMed] [Google Scholar]
- 71.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62:237–41. doi: 10.1136/thx.2006.068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enright P, Brusasco V. Counterpoint: should we abandon FEV(1)/FVC<0.70 to detect airway obstruction? Yes. Chest. 2010;138:1040–2. doi: 10.1378/chest.10-2052. [DOI] [PubMed] [Google Scholar]
- 73.Ben Saad H, Rouatbi S, Raoudha S, Tabka Z, Laouani Kechrid C, Hassen G, et al. Vital capacity and peak expiratory flow rates in a North-African population aged 60 years and over: influence of anthropometric data and parity. Rev Mal Respir. 2003;20:521–30. [PubMed] [Google Scholar]
- 74.Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present and future. Eur Respir J. 2010;36:12–19. doi: 10.1183/09031936.00143209. [DOI] [PubMed] [Google Scholar]
- 75.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–51. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 76.Celli BR, Halbert RJ. Point: should we abandon FEV(1)/FVC <0.70 to detect airway obstruction? No. Chest. 2010;138:1037–40. doi: 10.1378/chest.10-2049. [DOI] [PubMed] [Google Scholar]
- 77.Crapo RO. Role of reference values in making medical decisions. Indian J Med Res. 2005;122:100–2. [PubMed] [Google Scholar]