Abstract
Background
Improvements in care for inflammatory bowel diseases (IBD) could utilize the Chronic Care Model (CCM), an evidence-based approach that has improved patient outcomes and reduced costs in other illnesses. Specific aims include: (1) To explore patient perception of chronic illness care in a large IBD cohort, (2) To determine whether demographic factors, medication adherence, quality of life, disease type and activity were associated with perception of chronic illness care.
Methods
We randomly selected 1000 participants from the CCFA Partners internet cohort to receive the validated Patient Assessment of Chronic Illness Care (PACIC) instrument, which measures patient experience with specific aspects of care congruent with the CCM on a scale of 1–5, with 5 being highest perception of care. We used descriptive and bivariate statistics to assess relationships.
Results
945 participants completed the PACIC [576 Crohn’s disease, 339 ulcerative colitis, 30 indeterminate or other, 74% female, mean age 45 (SD=15.1), mean PACIC 2.4 (SD=0.93)]. Recent gastroenterologist visit, hospitalization, surgery and current pouch/ostomy were all associated with significantly higher PACIC (p<0.05). PACIC correlated positively with quality of life (Pearson correlation=0.12, p=0.003) but not medication adherence or disease activity.
Conclusions
Reports of chronic illness care in this IBD cohort are in the same range as other illnesses. PACIC is positively associated with quality of life, so efforts to align care with the CCM may benefit this population. Subjects who had more sub-specialty interactions reported an increased perception of care, indicating the important role of direct patient contact.
Keywords: inflammatory bowel disease, chronic illness care, Patient Assessment of Chronic Illness Care (PACIC)
INTRODUCTION
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, relapsing illnesses affecting as many as 1.5 million individuals in the United States(1). IBD can negatively impact quality of life for patients(2) and carry a considerable cost burden for health care systems(3). Furthermore, there is a persisting gap in quality of care for IBD(4), as in many other illnesses. Ideally, improvements in care for IBD would improve patient outcomes and reduce costs while delivering high quality care across the United States.
The Chronic Care Model (CCM) is an evidence-based guide to quality improvement that has been studied in hundreds of settings since its development in 2001(5). The goal of the CCM is to establish proactive, planned care for chronic illnesses built upon productive interactions between active patients and proficient health care providers. The model provides specific recommendations for changes in the following six areas: health care organization, community resources, self-management support, delivery system design, decision support and clinical information systems. Implementation of CCM-based care has improved patient outcomes(6) and reduced costs(7) in diabetes, as well as many other diseases.
Based on findings in other illnesses, the CCM could be an effective framework for improving care for IBD. Additionally, one group has demonstrated decreased costs and health care utilization with a gastroenterologist-led shared care model for IBD that is congruent with some elements of the CCM(8). Before large-scale changes in health care delivery for IBD are implemented, however, appropriate tools for measuring CCM-based care need to be established. The Patient Assessment of Chronic Illness Care (PACIC) instrument is a short, validated survey that measures patient experience with specific actions and aspects of congruent with the CCM(9). To our knowledge, patient assessment of quality of care, as it pertains to the CCM, has not yet been evaluated in a large IBD population. The purpose of this study was (1) To explore patient perception of chronic illness care in a large, internet-based IBD cohort; (2) To determine whether demographic factors, medication adherence, quality of life, disease type and disease activity were associated with perception of chronic illness care.
METHODS
Study design
We performed a cross-sectional study of the relationship between patient assessment of chronic illness care and demographic factors, disease type and activity, medication adherence and quality of life, nested within a large, internet-based cohort study (CCFA Partners). CCFA Partners is an internet registry of IBD patients who complete twice-yearly online surveys about health history, disease management, and issues facing IBD patients(10). Inclusion criteria are self-reported IBD, age of 18 years or older and internet access. Since launching in 2011, CCFA Partners has over 12,000 participants.
Patient selection
Between February 4, 2012 and February 25, 2012, 1000 participants were randomly selected to receive the Patient Assessment of Chronic Illness Care (PACIC) instrument(9) in addition to the standard 6-month follow-up survey for the CCFA Partners study.
Instruments
The 6-month follow-up CCFA Partners survey was released to all eligible CCFA Partners participants and included questions on demographics, health care, smoking history, IBD characteristics, medications, family history, disease history, daily activity, quality of life and health status. Additional modules, including the PACIC, were released to varying numbers of participants. All instruments have been previously validated and are described below. All measures were reported for the time between baseline and follow up surveys (approximately 6 months) unless otherwise specified by the instrument.
The Patient Assessment of Chronic Illness Care (PACIC) instrument consists of 20 questions divided into 5 subscales (Patient Activation, Delivery System Design/Decision Support, Goal Setting, Problem Solving/Contextual Counseling, and Follow-up/Coordination) which overlap all dimensions of the CCM that could be perceived by the patient(9). The PACIC measures the patient experience with actions or aspects of care that are consistent with the CCM within the past 6 months. It is scored by averaging the weighted values of all responses for the overall score or questions pertinent to each of the subscales. Results are expressed as a decimal value from 1–5, with 1 being the lowest perception of chronic illness care and 5 being the highest. The PACIC has been validated multiple times(11) and has been used in a variety of diseases. This instrument is the most appropriate instrument for measuring the patient experience of receiving integrated chronic care based on its psychometric characteristics, perceived applicability and relevance(12).
The short Crohn’s Disease Activity Index (sCDAI) was shown to be comparable to the original and widely-employed Crohn’s Disease Activity Index(13) in validity, reliability and responsiveness(14). It contains 15 questions and results are expressed as inactive disease (≤150), mild disease (151–199) and moderate to severe disease (≥200).
The Simple Clinical Colitis Activity Index (SCCAI) was validated in a longitudinal cohort study of patients undergoing colonoscopy, and was found to have excellent psychometric validity and moderate to good performance of validity(15). A score of ≤2 is associated with remission(16) and a score of ≥5 defines a relapse of UC(17).
The Manitoba IBD Index (MIBDI) is a validated single-item indicator of IBD activity over an extended period of time (6 months for this study)(18). Responses were categorized into well or rarely active (a–b), occasionally or sometimes active (c–d) and often or constantly active (e–f).
The short IBD questionnaire (SIBDQ) is a validated, continuous measure of health-related quality of life that has been shown to correlate with disease activity in the IBD population(19). Scores range from 10 to 70 with 10 associated with low health-related quality of life and 70 being optimum.
The Morisky medication adherence scale (MMAS) consists of 8 questions regarding different aspects of medication adherence for various time periods(20). Each question is worth 1 point and results are expressed as the sum. Responses are categorized into low (1–5), medium (6–7) and high (8) adherence.
Statistical analysis
All analyses were performed using SAS version 9.3 (Cary, NC). The population was characterized using descriptive statistics, including proportions, means and standard deviations, for overall IBD and stratified for CD and UC. Outcomes were compared using bivariate statistics, including the Student’s t test. The associations between PACIC scores and other continuous measures were described by Pearson correlation. Confidence intervals were 95% and p<0.05 was considered statistically significant. The study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
RESULTS
Study population
Of the 1000 participants selected to receive the PACIC survey, 979 (98%) started the survey and 945 (97% of those who started) completed the instrument [576 CD, 339 UC, 74% women, mean age 45 (SD=15.1)] (Table 1). Thirty participants reported indeterminate colitis or IBD unspecified and hereafter are included with UC. The majority of the respondents reported White race (94.9%). Nearly all were residents of the United States (96.9%). In general, respondents were well-educated, with 40.6% having completed a college degree and 32.2% with graduate-level education. A small percentage reported recent hospitalizations (9.0%), surgeries (4.2%) and current pouch (4.4%) or ostomy (6.4%).
Table 1.
Sample characteristics, n = 945
| Characteristic | Mean (SD) or percent | |
|---|---|---|
|
| ||
| Demographics | Age, years | 44.9 (15.2) |
|
| ||
| Female | 73.9% | |
|
| ||
| United States resident | 96.9% | |
|
| ||
| Race/ethnicity | ||
| White | 94.9% | |
| African American | <1% | |
| Asian | 1.4% | |
| Native Hawaiian/Pacific Islander | <1% | |
| American Indian/Alaskan Native | <1% | |
| More than one race | 2.1% | |
| Other | <1% | |
|
| ||
| Hispanic | 1.8% | |
|
| ||
| Education completed | ||
| Less than 12th grade | 1.1% | |
| 12th grade | 7.1% | |
| Some college | 19.1% | |
| College graduate | 40.6% | |
| Graduate school | 32.2% | |
|
| ||
| Current smoker | 5.3% | |
|
| ||
| Disease characteristics | CD1 | 60.9% |
|
| ||
| UC2 | 35.9% | |
|
| ||
| Indeterminate or other | 3.2% | |
|
| ||
| ≥1 hospitalizations in the past 6 months | 9.0% | |
|
| ||
| ≥1 bowel surgeries in the past 6 months | 4.2% | |
|
| ||
| Current Ileal or Koch pouch | 4.4% | |
|
| ||
| Current ostomy | 6.4% | |
|
| ||
| Health care setting | Has PCP (seen at least once in past 6 months) | 82.5% |
|
| ||
| Has GI specialist (seen at least once in past 6 months) | 85.0% | |
|
| ||
| GI specialist – University/academic setting | 16.4% | |
|
| ||
| GI specialist – Private practice | 72.2% | |
|
| ||
| Mean scores | Mean MMAS* | 6.3 (1.72) |
|
| ||
| Mean SIBDQ+ | 50.5 (11.1) | |
|
| ||
| Mean Manitoba (CD) | occasionally or sometimes active | |
|
| ||
| Mean sCDAI$ | 52.1 | |
|
| ||
| Mean Manitoba (UC) | occasionally or sometimes active | |
|
| ||
| Mean SCCAI# | 3.3 | |
Crohn’s disease,
ulcerative colitis,
Morisky medication adherence scale,
short IBD questionnaire,
short Crohn’s Disease Activity Index,
Simple Clinical Colitis Activity Index
Mean medication adherence score was 6.3 (SD=1.72) or moderate. A total of 213 participants had a low adherence score (36.2%), 199 medium adherence (33.8%) and 176 high adherence (29.9%). Mean quality of life as measured by the SIBDQ score was 51 (SD=11.1), which is consistent with reported means for mild or inactive IBD(2, 10, 21, 22). Mean short Crohn’s Disease Activity Index was 52.1, indicating inactive disease. The mean Manitoba IBD index for CD was 3.65, indicating occasionally or sometimes active disease. Mean UC disease activity as measured by the Simple Clinical Colitis Activity Index was 3.3, or between relapse and remission. The mean Manitoba index for UC was 3.89, indicating occasionally or sometimes active disease.
PACIC: Overall and subscale scores
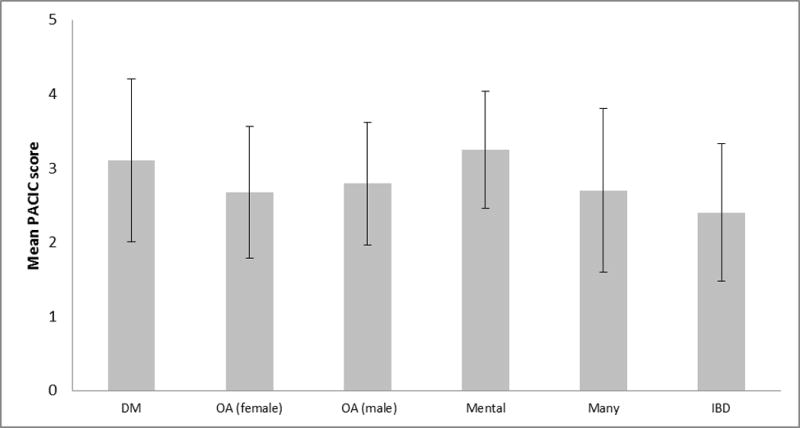
The mean PACIC score was 2.4 (SD = 0.93), which is within the range reported for other illnesses(23–26) (see Fig 1). The subscale domain of Patient Activation was significantly higher (p<0.0001) and the subscale domain of Follow-up/Coordination was significantly lower when compared across all other domains (p<0.0001).
Figure 1.
PACIC: Association with patient and provider characteristics
The following characteristics were associated with a significantly higher PACIC score: 1 or more visit with a gastroenterology (GI) specialist in the past 6 months, 1 or more hospitalization or surgery in the past 6 months and current pouch or ostomy (p<0.05) (Table 2). The following characteristics were not associated with a significant difference in PACIC score: demographic factors (including gender, age, education level), disease type (CD vs. UC) or duration, 1 or more visit with a primary care physician in the past 6 months and GI health care setting (university/academic vs. private practice). Recent GI visit significantly increased the Patient Activation (p=0.0003) and Problem Solving/Contextual Counseling (p=0.0008) subdomains. Recent hospitalization or surgery and current pouch or ostomy increased all subdomains.
Table 2.
Mean Patient Assessment of Chronic Illness Care (PACIC) overall scale and subscale scores (SD) by population characteristics, n = 945
| Characteristic | Patient activation | Delivery system design/decision support | Goal setting | Problem solving/contextual counseling | Follow up/coordination | Overall | p* | |
|---|---|---|---|---|---|---|---|---|
| Entire study population | 3.14 (1.30) | 2.68 (1.03) | 2.06 (0.98) | 2.78 (1.31) | 1.84 (0.9) | 2.40 (0.93) | n/a | |
| Sex | Male | 3.02 (1.25) | 2.78 (0.95) | 2.06 (0.94) | 2.78 (1.24) | 1.87 (0.89) | 2.41 (0.89) | 0.891 |
| Female | 3.19 (1.31) | 2.64 (1.06) | 2.06 (1.00) | 2.77 (1.33) | 1.83 (0.90) | 2.40 (0.94) | ||
| Age, years | 18–39 | 3.21 (1.29) | 2.62 (0.97) | 2.08 (0.97) | 2.85 (1.28) | 1.82 (0.89) | 2.42 (0.90) | 0.08 |
| 40–64 | 3.14 (1.31) | 2.69 (1.05) | 2.03 (0.97) | 2.72 (1.31) | 1.82 (0.85) | 2.38 (0.92) | ||
| ≥65 | 2.96 (1.25) | 2.82 (1.17) | 2.15 (1.06) | 2.78 (1.37) | 2.01 (1.08) | 2.46 (1.03) | ||
| Disease type | CD | 3.20 (1.31) | 2.72 (1.04) | 2.09 (0.98) | 2.81 (1.32) | 1.89 (0.91) | 2.45 (0.94) | 0.076 |
| UC | 3.06 (1.28) | 2.61 (1.02) | 2.01 (0.98) | 2.71 (1.29) | 1.76 (0.87) | 2.34 (0.90) | ||
| Disease duration, years | 0–5 | 3.11 (1.30) | 2.58 (1.02) | 2.01 (0.93) | 2.68 (1.26) | 1.74 (0.83) | 2.33 (0.88) | 0.23 |
| 6–10 | 3.34 (1.22) | 2.73 (1.00) | 2.17 (1.00) | 2.88 (1.32) | 1.89 (0.96) | 2.50 (0.91) | ||
| 11–20 | 3.21 (1.32) | 2.70 (1.06) | 2.06 (0.98) | 2.91 (1.30) | 1.83 (0.87) | 2.44 (0.92) | ||
| >20 | 3.02 (1.32) | 2.73 (1.06) | 2.05 (1.01) | 2.70 (1.34) | 1.90 (0.94) | 2.39 (0.97) | ||
| Education level | High school or less | 3.24 (1.32) | 2.83 (1.18) | 2.29 (1.22) | 2.99 (1.39) | 2.03 (1.04) | 2.59 (1.07) | 0.071 |
| Some college or higher | 3.14 (1.30) | 2.67 (1.02) | 2.04 (0.95) | 2.76 (1.30) | 1.82 (0.87) | 2.39 (0.91) | ||
| Recent interactions | ≥1 primary care | 3.13 (1.29) | 2.69 (1.03) | 2.07 (0.98) | 2.78 (1.31) | 1.87 (0.91) | 2.41 (0.92) | 0.648 |
| No primary care | 3.35 (1.30) | 2.73 (1.05) | 2.11 (0.97) | 2.85 (1.36) | 1.77 (0.84) | 2.45 (0.92) | ||
| ≥1 specialist | 3.23 (1.26) | 2.72 (1.00) | 2.09 (0.97) | 2.87 (1.30) | 1.89 (0.90) | 2.46 (0.91) | 0.004 | |
| No specialist | 2.80 (1.39) | 2.59 (1.13) | 1.98 (1.04) | 2.46 (1.30) | 1.67 (0.88) | 2.21 (0.97) | ||
| Health care setting | University or academic | 3.33 (1.22) | 2.70 (1.08) | 2.23 (1.04) | 2.97 (1.37) | 1.99 (0.95) | 2.55 (0.99) | 0.060 |
| Private practice | 3.14 (1.29) | 2.71 (1.01) | 2.05 (0.98) | 2.77 (1.30) | 1.81 (0.99) | 2.40 (0.91) | ||
| Health care utilization | ≥1 hospitalization | 3.27 (1.31) | 2.84 (1.10) | 2.31 (1.00) | 3.03 (1.33) | 2.16 (0.96) | 2.64 (0.95) | 0.014 |
| None | 3.13 (1.30) | 2.66 (1.03) | 2.04 (0.98) | 2.75 (1.30) | 1.81 (0.89) | 2.38 (0.92) | ||
| ≥1 bowel surgeries | 3.71 (1.25) | 3.04 (0.98) | 2.42 (1.08) | 3.43 (1.26) | 2.51 (1.05) | 2.93 (0.93) | <0.001 | |
| No surgeries | 3.12 (1.30) | 2.66 (1.03) | 2.04 (0.98) | 2.75 (1.30) | 1.81 (0.88) | 2.40 (0.92) | ||
| Pouch/ostomy status | Current pouch | 3.67 (1.11) | 3.07 (1.10) | 2.62 (1.28) | 3.40 (1.31) | 2.34 (1.13) | 2.93 (1.03) | <0.001 |
| No pouch | 3.12 (1.30) | 2.66 (1.03) | 2.04 (0.96) | 2.75 (1.30) | 1.82 (0.88) | 2.38 (0.91) | ||
| Current ostomy | 3.35 (1.22) | 2.99 (1.13) | 2.40 (1.10) | 3.19 (1.21) | 2.23 (1.01) | 2.75 (0.99) | 0.003 | |
| No ostomy | 3.13 (1.31) | 2.66 (1.02) | 2.04 (0.97) | 2.75 (1.31) | 1.81 (0.89) | 2.38 (0.92) |
p value for t test comparing overall PACIC scores by population characteristics
PACIC: Association with disease activity, quality of life and medication adherence
Quality of life, as measured by SIBDQ, was found to have a modest but significant positive association with PACIC score (Pearson correlation = 0.119, p = 0.003). The associations between PACIC and disease activity as measured by a number of instruments were small and inconsistent (Table 3). For CD, PACIC was not associated with current disease activity, as measured by sCDAI and MIDBI. For UC, PACIC was significantly negatively associated with current disease measured by SCCAI (categorical p = 0.0107; continuous Pearson correlation = −0.144, p = 0.005); however, PACIC was not significantly associated with MIBDI for UC (p = 0.0726). Medication adherence did not correlate with PACIC score (p>0.05).
Table 3.
Association of mean Patient Assessment of Chronic Illness Care (PACIC) score and disease activity measured by 3 indices (sCDAI, SCCAI and Manitoba)
| Mean PACIC (SD) | p | ||
|---|---|---|---|
|
sCDAI n = 542 |
inactive, ≤150 | 2.42 (0.92) | 0.159 |
| mild disease, 151–199 | 2.83 (1.08) | ||
| moderate to severe disease, ≥200 | 2.63 (0.95) | ||
|
SCCAI n = 369 |
inactive, ≤2 | 2.46 (0.95) | 0.011 |
| mild disease, 2.1–4.9 | 2.33 (0.89) | ||
| moderate to severe disease, ≥5 | 2.12 (0.80) | ||
|
Manitoba IBD Index – CD n = 574 |
well or rarely active (a–b) | 2.41 (0.95) | 0.696 |
| occasionally or sometimes active (c–d) | 2.42 (0.93) | ||
| often or constantly active (e–f) | 2.49 (0.92) | ||
|
Manitoba IBD Index – UC n = 369 |
well or rarely active (a–b) | 2.20 (0.82) | 0.073 |
| occasionally or sometimes active (c–d) | 2.29 (0.84) | ||
| often or constantly active (e–f) | 2.46 (0.98) |
DISCUSSION
In this IBD population, overall PACIC scores were within the range reported for other illnesses. The Patient Activation subscale score was significantly higher and the Follow-Up/Coordination subscale score was significantly lower than all others (p<0.0001) for the entire study population. Although the value of individual subscale scores is debatable due to high internal consistency(11), these findings suggest that the CCFA Partners internet-based cohort of individuals with IBD is highly active, motivated population. Data suggest that perhaps that the greatest deficits in care for IBD may be in the arena of continuity of care. Studies of diabetes(24, 27), mental illness(23), osteoarthritis(25) and other chronic illnesses(26) also found Follow-Up/Coordination to be the lowest subscale score, and hence targets for quality improvement work. The significant, positive association between PACIC score and quality of life suggests that implementation of quality improvement in care for IBD based on the CCM could benefit this population; however, the correlation was weaker than expected (Pearson correlation = 0.12).
The association between sub-specialty care (such as visit with GI specialist, recent surgery or hospitalization or current pouch or ostomy) may result from increased opportunities for very sick patients to interface with their health care providers, resulting in increased perception of care. These findings may indicate an important role for direct patient contact in perception of quality of care, or could demonstrate a need to adjust for frequency of health care interactions in future studies using the PACIC instrument.
Associations between PACIC measurements of disease activity were inconsistent and only one was significant; thus, we fail to make any conclusive statements about the relationship between IBD activity and patient perception of care. Similarly, no relationship was found in a study of psychological support and disease activity with CD and UC patients(28). Disease duration was examined as a potential confounding factor, but no significant association was found between disease duration and PACIC score. Medication adherence was not found to be associated with PACIC, and this finding is consistent with studies in diabetes(29) and a study of many illnesses including diabetes, chronic pain, heart failure, asthma and coronary artery disease(26). While we found no statistically significant associations between demographic factors and PACIC scores, other reports show mixed associations with demographic factors. No association was found in a study of mental illnesses(23) and contradicting associations were found in different diabetes studies(9, 24), indicating that study population characteristics and specific health care settings may play an important role in patient perception of chronic illness care. It may not be possible to directly compare baseline PACIC scores in cross-sectional studies at different sites.
To our knowledge, this is the first reported instance of the PACIC instrument being used in an entirely self-administered, web-based setting. The high initiation (98%) and completion (97%) rates suggest no problems with the adaptation to a web-based format, completion of the survey and overall feasibility of an online version of the PACIC.
There are several strengths to this study of perception of chronic illness care in IBD. This study includes a large sample size, allowing for increased statistical power to describe associations between various clinical factors and perception of chronic illness care. Additionally, the population is quite diverse, as evidenced by the wide variability of demographic factors such as age, geographic location, education, health care setting and disease type, activity and duration. The limitations of this study include the cross-sectional design, which hinders our ability to draw causal conclusions regarding associations between PACIC and disease outcomes. PACIC scores may be more meaningful when used in a prospective study of PACIC scores at baseline with outcomes collected over time. Other weaknesses of this study include a lack of external generalizability, which is inherent to internet-mediated research, and the self-report IBD diagnoses within the CCFA Partners cohort.
In conclusion, patient-reported experiences of chronic illness care in this IBD cohort are similar to reports for other illnesses. Perception of chronic illness care is positively, albeit modestly, associated with quality of life. Efforts to align care with the chronic care model may benefit this population.
Acknowledgments
Grant support: This work was funded by NIH T35DK007386 (RLR) and the Crohn’s and Colitis Foundation of America.
Footnotes
Author contributions: RLR was involved in all aspects of the study, including study concept and design, analysis and interpretation of data, drafting and critical revision of the manuscript and obtained funding for this study. MDL was involved with study concept and design, analysis and interpretation of the data and critical revision of the manuscript. CFM was involved in study concept and design, analysis and interpretation of data, statistical analysis and critical revision of the manuscript. RSS was involved in study concept, critical revision of the manuscript and study supervision. WC was involved in data acquisition. KA was involved in study design, data analysis and critical revision of the manuscript. MDK was involved in all aspects of the study, including study concept and design, analysis and interpretation of data, critical revision of the manuscript and study supervision.
No relevant disclosures.
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Faust AH, Halpern LF, Danoff-Burg S, et al. Psychosocial Factors Contributing to Inflammatory Bowel Disease Activity and Health-Related Quality of Life. Gastroenterol Hepatol (N Y) 8:173–181. [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein CN, Longobardi T, Finlayson G, et al. Direct medical cost of managing IBD patients: A Canadian population-based study. Inflamm Bowel Dis. doi: 10.1002/ibd.21878. [DOI] [PubMed] [Google Scholar]
- 4.Kappelman MD, Palmer L, Boyle BM, et al. Quality of care in inflammatory bowel disease: a review and discussion. Inflamm Bowel Dis. 16:125–133. doi: 10.1002/ibd.21028. [DOI] [PubMed] [Google Scholar]
- 5.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 6.Warm EJ. Diabetes and the chronic care model: a review. Curr Diabetes Rev. 2007;3:219–225. doi: 10.2174/1573399076. [DOI] [PubMed] [Google Scholar]
- 7.Kuo S, Bryce CL, Zgibor JC, et al. Cost-effectiveness of implementing the chronic care model for diabetes care in a military population. J Diabetes Sci Technol. 5:501–513. doi: 10.1177/193229681100500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sack C, Phan VA, Grafton R, et al. A chronic care model significantly decreases costs and healthcare utilisation in patients with inflammatory bowel disease. J Crohns Colitis. 6:302–310. doi: 10.1016/j.crohns.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow RE, Wagner EH, Schaefer J, et al. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005;43:436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 10.Long MD, Kappelman MD, Martin CF, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): Methodology and initial results. Inflamm Bowel Dis. doi: 10.1002/ibd.22895. [DOI] [PubMed] [Google Scholar]
- 11.Gugiu C, Coryn CL, Applegate B. Structure and measurement properties of the Patient Assessment of Chronic Illness Care instrument. J Eval Clin Pract. 16:509–516. doi: 10.1111/j.1365-2753.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 12.Vrijhoef HJ, Berbee R, Wagner EH, et al. Quality of integrated chronic care measured by patient survey: identification, selection and application of most appropriate instruments. Health Expect. 2009;12:417–429. doi: 10.1111/j.1369-7625.2009.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 14.Thia K, Faubion WA, Jr, Loftus EV, Jr, et al. Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 17:105–111. doi: 10.1002/ibd.21400. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PD, Leung J, Schwartz M, et al. The quantitative validation of non-endoscopic disease activity indices in ulcerative colitis. Aliment Pharmacol Ther. 2007;25:333–342. doi: 10.1111/j.1365-2036.2006.03205.x. [DOI] [PubMed] [Google Scholar]
- 16.Turner D, Seow CH, Greenberg GR, et al. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1081–1088. doi: 10.1016/j.cgh.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164–171. doi: 10.1080/00365520310000654. [DOI] [PubMed] [Google Scholar]
- 18.Clara I, Lix LM, Walker JR, et al. The Manitoba IBD Index: evidence for a new and simple indicator of IBD activity. Am J Gastroenterol. 2009;104:1754–1763. doi: 10.1038/ajg.2009.197. [DOI] [PubMed] [Google Scholar]
- 19.Irvine EJ. Quality of life in inflammatory bowel disease and other chronic diseases. Scand J Gastroenterol Suppl. 1996;221:26–28. doi: 10.3109/00365529609095550. [DOI] [PubMed] [Google Scholar]
- 20.Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Ananthakrishnan AN, Beaulieu DB, Ulitsky A, et al. Does primary sclerosing cholangitis impact quality of life in patients with inflammatory bowel disease? Inflamm Bowel Dis. 16:494–500. doi: 10.1002/ibd.21051. [DOI] [PubMed] [Google Scholar]
- 22.Lam MY, Lee H, Bright R, et al. Validation of interactive voice response system administration of the Short Inflammatory Bowel Disease Questionnaire. Inflamm Bowel Dis. 2009;15:599–607. doi: 10.1002/ibd.20803. [DOI] [PubMed] [Google Scholar]
- 23.Gensichen J, Serras A, Paulitsch MA, et al. The Patient Assessment of Chronic Illness Care questionnaire: evaluation in patients with mental disorders in primary care. Community Ment Health J. 47:447–453. doi: 10.1007/s10597-010-9340-2. [DOI] [PubMed] [Google Scholar]
- 24.Jackson GL, Weinberger M, Hamilton NS, et al. Racial/ethnic and educational-level differences in diabetes care experiences in primary care. Prim Care Diabetes. 2008;2:39–44. doi: 10.1016/j.pcd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Rosemann T, Laux G, Szecsenyi J, et al. The Chronic Care Model: congruency and predictors among primary care patients with osteoarthritis. Qual Saf Health Care. 2008;17:442–446. doi: 10.1136/qshc.2007.022822. [DOI] [PubMed] [Google Scholar]
- 26.Schmittdiel J, Mosen DM, Glasgow RE, et al. Patient Assessment of Chronic Illness Care (PACIC) and improved patient-centered outcomes for chronic conditions. J Gen Intern Med. 2008;23:77–80. doi: 10.1007/s11606-007-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szecsenyi J, Rosemann T, Joos S, et al. German diabetes disease management programs are appropriate for restructuring care according to the chronic care model: an evaluation with the patient assessment of chronic illness care instrument. Diabetes Care. 2008;31:1150–1154. doi: 10.2337/dc07-2104. [DOI] [PubMed] [Google Scholar]
- 28.Engel KL, Helvie K, Adler J, et al. Sa1297 The Relationship Between Perceived Psychological Support, Health-Related Quality of Life, and Disease Activity in Newly Diagnosed Inflammatory Bowel Disease Patients. Gastroenterology. 142:S-266. doi: 10.1089/heq.2020.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackey K, Parchman ML, Leykum LK, et al. Impact of the Chronic Care Model on medication adherence when patients perceive cost as a barrier. Prim Care Diabetes. 6:137–142. doi: 10.1016/j.pcd.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


