Abstract
Over 230,000 new cases of invasive breast cancer are diagnosed annually within the USA. Recurrent breast cancer remains a mostly incurable disease with drug resistance, tumor latency and distant metastases driving breast tumor recurrence and morbidity. Understanding drug resistance is a critical component of combating breast cancer. Recently, the protein chaperone GRP78 and the unfolded protein response were implicated as drivers of drug resistance. Preclinical studies show inhibiting GRP78 can reverse drug resistance. Furthermore, drugs developed to target GRP78 show clinical promise in several ongoing clinical trials.
Breast cancer prevalence & drug resistance
One in eight American women will be diagnosed with breast cancer in their lifetime [1]. Advances in receptor profiling has lead to the identification of three predominant subclassifications of breast tumors: estrogen receptor positive (ER+); HER2 overexpression, and; ER negative, progesterone receptor (PR) negative and HER2 normal or also referred to as triple-negative breast cancers. Over 70% of all breast cancers express the estrogen receptor [2]. Due to the high prevalence of ER+ breast cancer, therapies targeting the estrogen receptor such as the estrogen receptor modulator tamoxifen or the selective estrogen receptor down regulator Faslodex (Fulvestrant, ICI 182,780) were developed to antagonize or degrade the estrogen receptor. Aromatase inhibitors (AIs) were developed to target the conversion of androgens into estrogens in the breast and elsewhere in the body; AIs have shown good clinical success [2]. Approximately 20% of all breast cancers have overexpression of HER2. Trastuzumab (Herceptin) is a monoclonal antibody developed for the treatment of HER2 overexpressing breast cancers. With a 34% response rate, Herceptin is successful as a single agent, and combination of Herceptin with other chemotherapeutic agents can exhibit increased clinical benefit [3]. Approximately 10–15% of all breast cancers are triple negative. Currently, patients with triple-negative breast cancer do not have targeted therapy options and are limited to regimens with cytotoxic drugs such as the anthracyclines. Even with initially effective therapies, drug resistance often occurs. Approximately 50% of ER+ breast tumors treated with endocrine-targeted drugs will never respond to therapy (de novo resistance). Moreover, most ER+ tumors that initially respond to tamoxifen will lose effectiveness over time (acquired resistance) [2]. Furthermore, most patients initially responding to Herceptin-based regimens generally acquire resistance within 1 year of treatment [3]. Thus drug resistance is a major cause of breast cancer mortality.
The unfolded protein response & GRP78
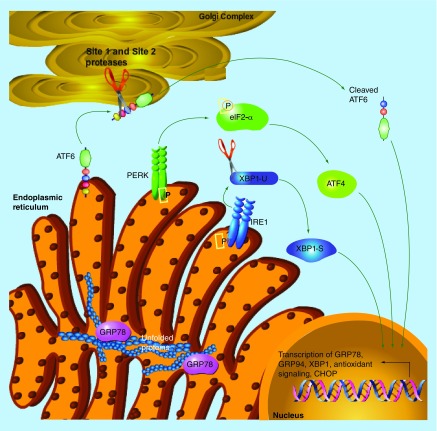
The unfolded protein response (UPR) is an endoplasmic reticulum stress pathway activated when unfolded and/or misfolded proteins accumulate within the lumen of the endoplasmic reticulum. An increased load of unfolded proteins within the endoplasmic reticulum lumen causes the protein chaperone GRP78 to be released from the three signaling control components of the UPR (IRE1 [ERN1], PERK [EIF2AK3], and ATF6), allowing activation of the pathway [4]. PERK and IRE1 are each homodimerized and activated following autophosphorylation. ATF6 translocates to the Golgi complex where it is cleaved to form the highly active transcription factor cleaved-ATF6. PERK phosphorylates eIF2α resulting in the halt of cap-dependent protein translation in a homeostatic attempt to relieve the burden of unfolded proteins within the endoplasmic reticulum [4]. PERK activation also results in the formation of the transcription factor ATF4 and DNA-damage inducible transcript 3 (CHOP [DDIT3]). IRE1 promotes the unconventional splicing of X-box binding protein 1 and phosphorylates c-Jun terminal kinase (see Figure 1 depicting UPR activation). Once the unfolded protein accumulation in the endoplasmic reticulum has been reduced, GRP78 binds and inactivates the three UPR signaling control components resulting in the cessation of signaling. Although activation of UPR is initially prosurvival, extended duration of UPR promotes apoptosis [4].
Figure 1. . Overview of the unfolded protein response.
GRP78 regulating cancer cell survival
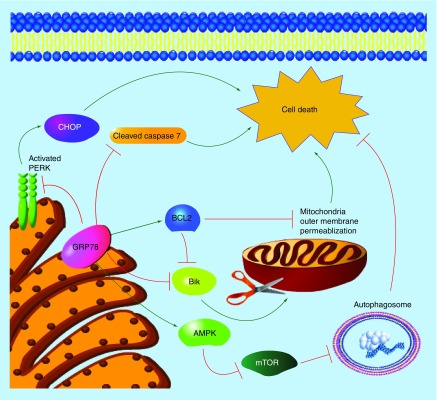
Our knowledge of the role of GRP78 in regulating cellular processes is expanding. A sole designation of GRP78 as a protein chaperone controlling UPR signaling is limiting because GRP78 is multifunctional [5]. For example, GRP78 can inhibit apoptosis by binding to procaspase 7, preventing its cleavage and subsequent activation of cell death signaling [6]. Moreover, GRP78 was shown to bind and inhibit the proapoptotic BCL2-family member Bik [7]. GRP78 overexpression in breast cancer cells elevates the antiapoptotic BCL2 family members BCL2, BCL-XL and BCL-W, suggesting a key role of GRP78 in inhibiting cell death [8]. GRP78 can also inhibit apoptosis through classical UPR signaling. Elevated GRP78 increases cellular capacity for maintaining low levels of unfolded proteins within the endoplasmic reticulum, thereby decreasing UPR stress and inhibiting proapoptotic UPR-mediated CHOP induction [4].
GRP78 overexpression resulted in the inhibition of mTOR and the promotion of prosurvival autophagy [9]. Autophagy, a cellular process of ‘self-eating’, removes misfolded proteins and old/damaged organelles through autolysis supplementing metabolism. Autophagy was shown to be a critical regulator of therapeutic resistance, indicating that GRP78 regulation of autophagy promotes drug resistance and survival [10]. GRP78 was also reported to bind to IGFBP3 to modulate autophagy and metabolism [11]. Figure 2 illustrates the multifaceted role of GRP78 in cell survival.
Figure 2. . Examples of prosurvival signaling mediated by GRP78.
GRP78 modulates therapeutic resistance
GRP78 is elevated in breast tumors when compared with normal tissue, and is often associated with therapeutic resistance [12,13]. Overexpression of GRP78 enabled estrogen-dependent MCF7/BUS cells to survive estrogen deprivation by binding and inhibiting the proapoptotic BCL2-family member Bik [7]. Moreover, long-term estrogen deprivation resulted in emergence of the estrogen-independent MCF7/BUS-10 cell line that exhibited increased basal levels of GRP78. These data suggested that elevated GRP78 might promote estrogen independence in this model that phenocopies AI resistance [14]. Increased GRP78 expression is observed in antiestrogen-resistant breast cancer cell lines and tumors, indicating a key role of GRP78 in modulating antiestrogen responsiveness [8]. In a rat model of mammary carcinogenesis, mammary tumors that acquired resistance to tamoxifen had higher levels of GRP78 when compared with mammary tumors that never responded (de novo resistant) or responded to tamoxifen, suggesting that GRP78 promotes acquired but not de novo tamoxifen resistance in rat ER+ mammary tumors [8]. Knockdown of GRP78 in antiestrogen-resistant MCF7/LCC9 (estrogen independent, Faslodex and tamoxifen resistant) and MCF7-RR (estrogen independent, tamoxifen resistant) cell lines resulted in their resensitization to Faslodex and/or tamoxifen. Overexpression of GRP78 in the antiestrogen-responsive parental cell lines MCF7 (estrogen dependent, antiestrogen sensitive) and MCF7/LCC1 (estrogen independent, antiestrogen sensitive) resulted in the loss of endocrine-targeted therapeutic effectiveness, clearly demonstrating the important role of GRP78 in regulating antiestrogen therapy resistance [8].
GRP78 expression is also associated with trastuzumab resistance. Proteomic profiling of trastuzumab sensitive or resistant SKBr3 breast cancer cells highlighted many putative protein mediators of drug resistance including GRP78 [15]. Moreover, activation of UPR in breast cancer cells by trastuzumab was shown to bypass drug-targeted inhibition of PI3K signaling, promoting resistance [16]. Trastuzumab resistance is also associated with the induction of autophagy [17], suggesting the GRP78-mediated autophagy that modulates antiestrogen responsiveness may also play a role in trastuzumab resistance.
Cytotoxic chemotherapy resistance has also been linked with GRP78 and UPR signaling. Elevated levels of GRP78 were shown to inhibit doxorubicin effectiveness and promote chemoresistance. Moreover, in a patient cohort of 127 invasive breast cancer patients treated with anthracycline therapy, increased GRP78 expression was positively correlated with a shorter time to reoccurrence and increased hazard ratio [18]. These data imply a clinical role of GRP78 modulating chemotherapeutic responsiveness in breast cancer.
Drugs targeting GRP78
Various drugs were shown or developed to inhibit GRP78. These drugs fall into these various categories: natural products; modified bacterial toxins; nonplatinum-based metallodrugs or monoclonal antibodies. Natural products such as the soy phytoestrogen genistein, the green tea flavonoid epigallocatechin gallate and the Magnolia grandiflora derivative honokiol were shown to inhibit GRP78. Genistein blocks the binding of transcription factors to the promoter region of GRP78 blocking transcription [5]. Epigallocatechin gallate and honokiol bind to the ATPase domain of GRP78 inhibiting its actions and promoting cell stress-mediated apoptosis of cancer cells [19].
Bacterial derivatives from Streptomyces versipellis, versipelostatin and its second-generation analog, prunustatin A, inhibited GRP78 by blocking its transcription. Interestingly, the GRP78 inhibitory action of versipelostatin was only active in instances of low glucose and cellular stress, suggesting that versipelostatin may effectively target hypoxic tumor tissue [20]. Another bacterial product, subtilase cytotoxin (SubAB), derived from Shiga toxigenic Escherichia coli strain degrades GRP78, resulting in nonresolving endoplasmic reticulum stress and UPR-mediated apoptosis [21].
The new nonplatinum metallodrug NKP-1339 was shown to inhibit GRP78 and is currently undergoing clinical trials [22]. The preliminary success of NKP-1339 is due to the reduction of the ruthenium core being favored in hypoxic tissues targeting the tumor, disrupting redox homeostasis and promoting mitochondrial-mediated apoptosis [23]. NKP-1339 mechanism of inhibiting GRP78 is currently under investigation.
Targeting cell surface GRP78
Multiple reports of GRP78 localizing on the plasma membrane of cancer cells imply a role of GRP78 outside the endoplasmic reticulum. Recent work suggests GRP78 associates with GPI-anchored proteins thereby enabling GRP78 to exist as a peripheral protein on the plasma membrane of cancer cells [24]. Localization of GRP78 on the plasma membrane of cells is also associated with Cripto, α2-macroglobin and β1-integrin interaction promoting survival and inhibiting apoptosis. Cell surface GRP78/Cripto binding can inhibit TGF-β apoptotic signaling and activate progrowth MAP kinase signaling [25]. Cell surface GRP78/α2-macroglobin complex was reported to activate protein kinase B (Akt) proliferation signaling pathways [26]. Cell surface GRP78 was shown to activate PI3K signaling, suggesting a key role of GRP78 mediating various pro-tumorigenic cell growth pathways [27]. Furthermore, extracellular β1-integrin interaction with cell surface GRP78 was shown to activate focal adhesion kinase and promote cell invasion and metastasis [28], indicating the need for therapeutics to target cell surface GRP78 localization.
Small peptide inhibitors or antibody therapies have been developed to recognize and inhibit cell surface GRP78 activities. Studies utilizing GRP78 binding peptide conjugated to a known proapoptotic moiety selectively killed cell surface expressing GRP78 breast cancer cells. Moreover, administration of the peptide moiety inhibited primary tumor growth and prevented lung and bone micrometastases in a preclinical model of breast cancer [29]. These data suggest that GRP78 peptide inhibitors may be successful in the treatment of breast cancers.
The use of antibodies to target cell surface receptors for the treatment of cancer has been successful. Human monoclonal antibodies targeted against GRP78, such as MAb159 and PAT-SM6, showed responsiveness in the effective treatment of multiple myeloma, prostate cancer and leukemia in preclinical models [30,31]. The antibodies induced cell surface GRP78 endocytosis and targeted the tumor with high specificity. Humanized GRP78 antibodies effectively reduced tumor size, burden and metastatic potential, demonstrating the success of targeting cell surface GRP78 for the treatment of cancer [31]. PAT-SM6 is currently undergoing clinical trials as single agent therapeutic agent targeting GRP78 for the treatment of multiple myeloma [32]. Completion of these studies will help evaluate the clinical potential of targeting cell surface GRP78 in possible combination with other drugs to reverse or prevent the development of therapeutic resistance in breast cancers.
While targeting cell surface GRP78 through the utilization of antibodies and peptide inhibitors shows clinical promise for the treatment of cancers, antiestrogen-resistant LCC9 breast cancer cells treated with N-terminal recognizing GRP78 antibody, previously shown to inhibit cell surface GRP78, had no significant effect on endocrine therapy sensitivity [8]. These data suggest that the endoplasmic reticulum protein chaperone activity of GRP78 plays a critical role in antiestrogen resistance, while cell surface GRP78 expression may be less important for determining drug responsiveness. But, this does not rule out the importance of cell surface GRP78 signaling in determining the efficacy of other chemotherapeutic agents.
Conclusion & future perspective
The majority of breast cancer mortality is associated with the development of drug resistance in recurrent disease. GRP78 can play a critical role in the formation of drug resistance in breast cancer. Drugs targeting GRP78 in preclinical animal models show great promise for the treatment of cancer. Ongoing clinical trials with several GRP78-targeting agents will test the validity of GRP78 inhibition as a mechanism for cancer treatment. Combining current breast cancer treatment modalities with GRP78-inhibiting drugs may decrease tumor burden, metastases and drug resistance. Further experimentation is needed to determine the effectiveness of GRP78-targeting drugs and successful drug combinations; however, preclinical data suggest promising results.
Key terms.
Anthracycline: Anthracycline-based drugs are a bacterial derived class of chemotherapeutics, such as doxorubicin and epirubicin, that inhibit cellular DNA replication by intercalating DNA/RNA strands and causing DNA strand breaks through inhibition of topoisomerase II.
Unfolded protein response: An endoplasmic reticulum stress pathway triggered by the accumulation of unfolded/misfolded proteins within the lumen of the endoplasmic reticulum. GRP78 is a key regulator of this pathway.
GRP78: A protein chaperone involved in regulating protein folding and assembly, cellular stress signaling, apoptosis, growth and calcium homeostasis.
Antiestrogen resistance: Lack of responsiveness to therapies targeting the estrogen receptor-α, such as the selective estrogen receptor modulators (tamoxifen) or selector estrogen receptor downregulators (Faslodex). ER+ breast tumors may never respond to antiestrogen therapy (de novo resistance) or lose therapeutic responsiveness over time (acquired resistance).
Executive summary.
GRP78 is upregulated in many different types of cancers, including breast cancer.
Overexpression of GRP78 is associated with drug resistance in breast cancer.
Inhibiting GRP78 can restore therapeutic responsiveness.
Drugs such as epigallocatechin gallate, versipelostatin and NKP-1339 can inhibit intracellular GRP78.
Antibodies such as PAT-SM6 or MAb159 can inhibit cell surface GRP78 signaling and are currently in clinical trials for the treatment of cancer.
Footnotes
Financial & competing interests disclosure
KL Cook is supported by a DOD Breast Cancer Research Program Postdoctoral Fellowship (BC112023). This research was also supported in part by awards from the US Department of Health and Human Services (R01-CA131465 and U54-CA149147) to R Clarke. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Riggins RB, Bouton AH, Liu MC, Clarke R. Antiestrogens, aromatase inhibitors, and apoptosis in breast cancer. Vitam. Horm. 2005;71:201–237. doi: 10.1016/S0083-6729(05)71007-4. [DOI] [PubMed] [Google Scholar]
- 3.Nahta R, Esteva FJ. Her2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8(6):215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke R, Cook KL, Hu R, et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72(6):1321–1331. doi: 10.1158/0008-5472.CAN-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook KL, Clarke PA, Clarke R. Targeting GRP78 and antiestrogen resistance in breast cancer. Future Med. Chem. 2013;5(9):1047–1057. doi: 10.4155/fmc.13.77. [DOI] [PubMed] [Google Scholar]
- 6.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 2003;278(23):20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67(8):3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 8.Cook KL, Shajahan AN, Warri A, Jin L, Hilakivi-Clarke LA, Clarke R. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer Res. 2012;72(13):3337–3349. doi: 10.1158/0008-5472.CAN-12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook KL, Clarke R. Heat shock 70 KDA protein 5/glucose-regulated protein 78 “amp”ing up autophagy. Autophagy. 2012;8(12):1827–1829. doi: 10.4161/auto.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert Rev. Anticancer Ther. 2011;11(8):1283–1294. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grkovic S, O'reilly VC, Han S, Hong M, Baxter RC, Firth SM. IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene. 2012;32(19):2412–2420. doi: 10.1038/onc.2012.264. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez PM, Tabbara SO, Jacobs LK, et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res. Treat. 2000;59(1):15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 13.Scriven P, Coulson S, Haines R, Balasubramanian S, Cross S, Wyld L. Activation and clinical significance of the unfolded protein response in breast cancer. Br. J. Cancer. 2009;101(10):1692–1698. doi: 10.1038/sj.bjc.6605365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78-KDA glucose-regulated protein (GRP78): endocrine resistance factor in breast cancer, through release of B-cell lymphoma 2 (BCL-2) from BCL-2-interacting killer (BIK) J. Biol. Chem. 2011;286(29):25687–25696. doi: 10.1074/jbc.M110.212944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.G DIC, Marengo G, Albanese NN, et al. Proteomic profiling of trastuzumab (Herceptin(R))-sensitive and -resistant SKBR-3 breast cancer cells. Anticancer Res. 2013;33(2):489–503. [PubMed] [Google Scholar]
- 16.Kumandan S, Mahadevan NR, Chiu K, Delaney A, Zanetti M. Activation of the unfolded protein response bypasses trastuzumab-mediated inhibition of the PI-3K pathway. Cancer Lett. 2013;329(2):236–242. doi: 10.1016/j.canlet.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS ONE. 2009;4(7):e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66(16):7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 19.Martin S, Lamb HK, Brady C, et al. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br. J. Cancer. 2013;109(2):433–443. doi: 10.1038/bjc.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao P, Ueda JY, Kozone I, et al. New glycosylated derivatives of versipelostatin, the GRP78/BiP molecular chaperone down-regulator, from streptomyces versipellis 4083-SVS6. Org. Biomol. Chem. 2009;7(7):1454–1460. doi: 10.1039/b817312e. [DOI] [PubMed] [Google Scholar]
- 21.Yahiro K, Satoh M, Morinaga N, et al. Identification of subtilase cytotoxin (SubAB) receptors whose signaling, in association with SubAB-induced BiP cleavage, is responsible for apoptosis in HeLa cells. Infect. Immun. 2011;79(2):617–627. doi: 10.1128/IAI.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.http://clinicaltrials.gov/ct2/show/NCT01415297 ClinicalTrials Database: NCT01415297.
- 23.Trondl R, Heffeter P, Jakupec M, Berger W, Keppler B. NKP-1339, a first-in-class anticancer drug showing mild side effects and activity in patients suffering from advanced refractory cancer. BMC Pharmacol. Toxicol. 2012;13(Suppl. 1):A82. [Google Scholar]
- 24.Tsai YL, Zhang Y, Tseng CC, Stanciauskas R, Pinaud F, Lee AS. Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface. J. Biol. Chem. 2015;290(13):8049–8064. doi: 10.1074/jbc.M114.618736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol. Cell. Biol. 2008;28(2):666–677. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra UK, Pizzo SV. Receptor-recognized α(2)-macroglobulin binds to cell surface-associated GRP78 and activates mTORC1 and mTORC2 signaling in prostate cancer cells. PLoS ONE. 2012;7(12):e51735. doi: 10.1371/journal.pone.0051735. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhang Y, Tseng CC, Tsai YL, Fu X, Schiff R, Lee AS. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI(3,4,5)P3 production. PLoS ONE. 2013;8(11):e80071. doi: 10.1371/journal.pone.0080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Zhang L, Zhao Y, et al. Cell-surface GRP78 facilitates colorectal cancer cell migration and invasion. Int. J. Biochem. Cell Biol. 2013;45(5):987–994. doi: 10.1016/j.biocel.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Miao YR, Eckhardt BL, Cao Y, et al. Inhibition of established micrometastases by targeted drug delivery via cell surface-associated GRP78. Clin. Cancer Res. 2013;19(8):2107–2116. doi: 10.1158/1078-0432.CCR-12-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasche L, Duell J, Morgner C, et al. The natural human IgM antibody PAT-SM6 induces apoptosis in primary human multiple myeloma cells by targeting heat shock protein GRP78. PLoS ONE. 2013;8(5):e63414. doi: 10.1371/journal.pone.0063414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Li X, Gao W, et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth and metastasis. Clin. Cancer Res. 2013;19(24):6802–6811. doi: 10.1158/1078-0432.CCR-13-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.http://clinicaltrials.gov/ct2/show/NCT01727778 ClinicalTrials Database: NCT01727778.