Abstract
Hydrotrifluoromethylation, vinylic trifluoromethylation, and iodotrifluoromethylation of simple alkenes have been achieved by using Togni reagent in the absence of any transition metal catalyst. These reactions were readily controllable by selection of appropriate salts and solvents. The addition of K2CO3 afforded the hydrotrifluoromethylation product, with DMF acting not only as a solvent, but also as the hydrogen source. In contrast, the use of tetra-n-butylammonium iodide (TBAI) in 1,4-dioxane resulted in vinylic trifluoromethylation, while the use of KI afforded the iodotrifluoromethylation product. The vinylic trifluoromethylation product was obtained by treatment of the iodotrifluoromethylation product with ammonium 2-iodobenzoate, indicating that it was formed through an elimination reaction of the in-situ-generated iodotrifluoromethylation product, and the solubility of the resulting 2-iodobenzoate salt plays a key role in the product switching. A radical-clock experiment showed that these reactions proceed via radical intermediates.
Keywords: additive effect, alkenes, difunctionalization, Togni reagent, trifluoromethylation
Introduction
Fluorine-containing functional groups are found in many types of organic molecules used in the pharmaceutical, agrochemical, and materials science fields.[1] Particular attention has been paid to trifluoromethylation reactions.[2] Carbonyl compounds have typically been used for the construction of the C(sp3)−CF3 bond by nucleophilic addition with Ruppert–Prakash reagent or electrophilic trifluoromethylation through enolate formation.[2a,2h–2l Trifluoromethylation of olefins with an electrophilic trifluoromethylating reagent[3] is another approach towards C(sp3)−CF3 bond formation.[4]
We have been investigating copper-catalyzed trifluoromethylation using Togni reagent.[5],[6] Recently, carbotrifluoromethylation through 1,2-aryl migration to afford a β-trifluoromethyl carbonyl moiety was achieved using Fe(OAc)2 as a catalyst (Scheme 1 a),[7] and the research groups of Wu and Tu independently reported a similar reaction using a copper catalyst.[8] These reactions appear to proceed through a neophyl-type rearrangement of an alkyl radical intermediate. During our studies, we obtained the hydrotrifluoromethylation product as a by-product when DMF was used as a solvent (Scheme 1 b). These preliminary results prompted us to examine trifluoromethylations via radical intermediates, including hydrotrifluoromethylation of simple alkenes.
Scheme 1.
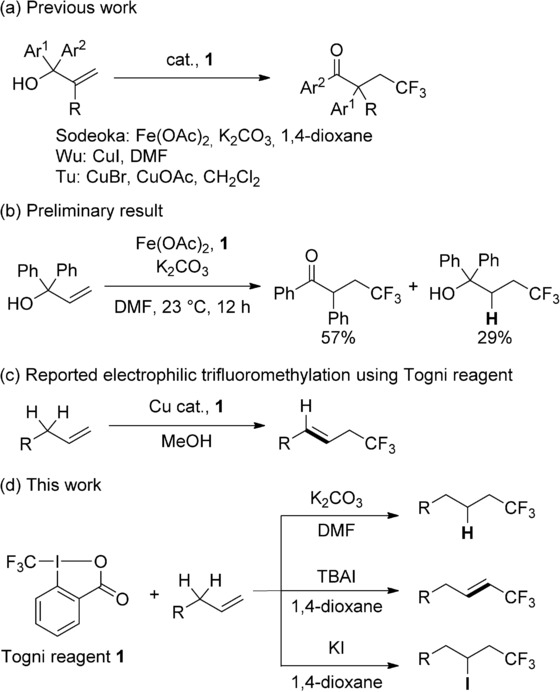
Trifluoromethylations of alkenes.
Seminal work on catalytic hydrotrifluoromethylation of unactivated olefins[9] has been reported by the research groups of Qing, Gouverneur, and Nicewicz in 2013.[10a–c] Qing and co-workers operated the reaction using Ag catalyst and 1,4-cyclohexadiene under oxidative conditions.[10a] Gouverneur used photoredox catalysis with a Ru complex and Umemoto's reagent in MeOH, which worked as not only a solvent, but also as a hydrogen source.[10b] Nicewicz and co-workers studied the photoredox catalysis of mesityl acridinium ion for this transformation using Langlois reagent (CF3SO2Na).[10c] Furthermore, during the preparation of this paper, Scaiano and co-workers reported a hydrotrifluoromethylation using a combination of Togni reagent and methylene blue under photo-irradiation, though the vinylic trifluoromethylation product was simultaneously formed.[10d] Attack of a free trifluoromethyl radical at a C=C bond and trapping of the resulting radical by a hydrogen source are essential for these reactions. On the other hand, Buchwald and Wang independently reported the deprotonative trifluoromethylation of unactivated olefins using a combination of CuI and Togni reagent in 2011 (Scheme 1 c).[11] In our study on the trifluoromethylation of allylsilane, we also found that deprotonative trifluoromethylation occurred when mono-substituted allylsilane was used as a substrate.[6c] The hydrotrifluoromethylation of simple alkenes having allylic protons using the Togni reagent is accordingly a challenging topic.
Moreover, the trifluoromethyl-substituted alkene unit is a key structural motif for pharmaceutical chemistry and materials science.[2] This unit has generally been synthesized from pre-functionalized substrate by cross-coupling reactions,[12] and direct trifluoromethylation of simple alkenes is still under development. Our first work directed toward this type of trifluoromethylation was reported in 2012. In the study of copper-catalyzed oxytrifluoromethylation of styrene derivatives, it was found that addition of p-toluenesulfonic acid directly afforded β-trifluoromethylstyrene derivatives instead of oxytrifluoromethylation products.[6d,i] Although many research groups have recently reported the vinylic trifluoromethylation of various types of alkenes, the reaction of simple alkenes with allylic protons is still rare.[13]
A typical example of alkene trifluoromethylation through an atom transfer-type radical reaction is halotrifluoromethylation, and this type of transformation has been studied for a long time due to the utility of the products.[14] Classically, CF3I has been used for the reaction and various radical initiators have been reported.[14] However, there are very few reports on halotrifluoromethylation using Togni reagent. Szabó and co-workers reported the halotrifluoromethylation of vinylsilane and 4-fluorostyrene with a stoichiometric amount of copper salts, albeit in moderate yield.[15]
This background suggests that product control in alkene trifluoromethylation using the Togni reagent is a challenging task, especially in the case of trifluoromethylations of simple alkenes bearing an allylic proton, in which deprotonative trifluoromethylation may occur competitively (Scheme 1 c). Here, we describe base-promoted hydrotrifluoromethylation and iodide ion-promoted vinylic trifluoromethylation and iodotrifluoromethylation of simple alkenes (Scheme 1 d).
Results and Discussion
To screen the reaction conditions, we employed 2 a as a test substrate (Table 1). First, the reaction with Fe(OAc)2 in DMF was examined, based on a preliminary result (Scheme 1 b). The desired hydrotrifluoromethylation product 3 a was formed in 53 % yield at 80 °C (Table 1, entry 1). However, the reaction of 2 a was slower than that of 1,1-diphenyl-2-propen-1-ol (Scheme 1 b), and only 5 % of 3 a was obtained when the reaction was carried out at 23 °C (Table 1, entry 2). The vinylic trifluoromethylation product 4 a was the major product in the absence of K2CO3 (Table 1, entry 3). To our surprise, the iron catalyst was not required for the hydrotrifluoromethylation. The reaction without Fe(OAc)2 provided mainly the hydrotrifluoromethylation product in 40 % yield (Table 1, entry 4). However, it was found that the iron catalyst somewhat accelerated the hydrotrifluoromethylation. The reaction using fine powder of K2CO3 afforded 3 a in 58 % (Table 1, entry 5). N,N-Dimethylacetamide (DMAc) and N,N-diethylformamide (DEF) were also good solvents. Although the reaction proceeded smoothly in these solvents, the yields of 4 a also increased and the product selectivities were low (Table 1, entries 6 and 7). Among the bases screened, K2CO3 gave the best result (Table 1, entries 4–5, 10–11). When the reaction was carried out with 3 equivalents of Togni reagent in 0.05 m DMF solution for 12 h, 3 a was obtained in 75 % yield, with only 2 % of 4 a (Table 1, entry 12). The reaction without any catalyst or base gave negligible amounts of 3 a and 4 a (Table 1, entry 13), suggesting that the base was essential for the hydrotrifluoromethylation.
Table 1.
Screening of reaction conditions for hydro-trifluoromethylation[a]
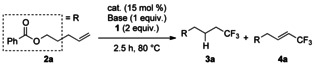 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Base | Solvent | Yield of3 a[%][b] | Yield of4 a[%][b] |
| 1 | Fe(OAc)2 | K2CO3 | DMF | 53 | 7 |
| 2[c] | Fe(OAc)2 | K2CO3 | DMF | 5 | 0 |
| 3 | Fe(OAc)2 | – | DMF | 9 | 16[d] |
| 4 | – | K2CO3 | DMF | 40 | 3 |
| 5 | – | K2CO3[e] | DMF | 58 | 7 |
| 6 | – | K2CO3 | DMAc[f] | 47 | 18[g] |
| 7 | – | K2CO3 | DEF[h] | 43 | 20[i] |
| 8 | – | K2CO3 | DCE | 1 | trace |
| 9 | – | K2CO3 | MeCN | 3 | trace |
| 10 | – | Cs2CO3 | DMF | 51 | 20[i] |
| 11 | – | KHCO3 | DMF | 28 | trace |
| 12[j] | – | K2CO3[e] | DMF | 75[k] | 2 |
| 13 | – | – | DMF | 2 | 2 |
[a] The reactions were carried out with Togni reagent 1 (2 equiv), catalyst (15 mol %), and base (1 equiv) in solvent (1.2 mL, 0.2 m) at 80 °C on a 0.25 mmol scale, unless otherwise noted. [b] Determined by 19F NMR analysis using fluorobenzene as an internal standard. [c] Run at 23 °C. [d] The E/Z ratio was 7:1. [e] Fine powder was used. [f] DMAc=N,N-dimethylacetamide. [g] The E/Z ratio was 8:1. [h] DEF=N,N-diethylformamide. [i] The E/Z ratio was 9:1. [j] Run with 3 equiv of 1 in DMF (0.05 m) for 12 h. [k] Yield of isolated product.
With the optimized conditions in hand, the substrate scope of the hydrotrifluoromethylation was investigated (Table 2). It was found that various functional groups, such as aryl halides, ester and carbonyl groups, were tolerated, and the corresponding products were obtained in good-to-high yields with high selectivity. Interestingly, gem-di-substituted alkene 2 g was smoothly converted into the corresponding product 3 g in 51 % yield.
Table 2.
Hydrotrifluoromethylation of alkenes[a]
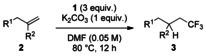 | |
|---|---|
 |
 |
 |
 |
 |
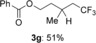 |
[a] The reactions were carried out with 1 (3 equiv) and K2CO3 (1 equiv) in DMF (5 mL) at 80 °C for 12 h on a 0.25 mmol scale. [b] Run in DMF/1,4-dioxane (1:10).
In the reaction of 2 a, compound 5 was formed in 56 % yield (Scheme 2 a), thus suggesting that the hydrogen source of this hydrotrifluoromethylation is DMF.[16] To obtain further information about this reaction, we also investigated the reaction in deuterated solvent (Scheme 2 b). As expected, deuterated product [D]3 a was obtained in 18 % yield, together with 34 % yield of the vinylic trifluoromethylation product 4 a. In this reaction, no H-adduct was observed. When the reaction was performed in 1:1 DMF/[D7]DMF under the standard conditions, the hydrogen/deuterium ratio in the product was 92:8. These results indicate that hydrogen atom abstraction needs high energy and would be involved in the rate-determining step of this reaction.
Scheme 2.
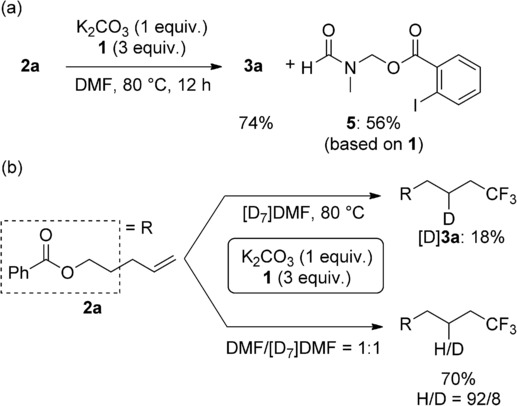
Hydrogen source in the hydrotrifluoromethylation.
Inspired by the production of 4 a as a by-product during the hydrotrifluoromethylation (Table 1), we next planned to find optimum conditions for the vinylic trifluoromethylation of simple alkenes using the Togni reagent. Fortunately, TBAI was found to promote the target vinylic trifluoromethylation.[17] The results of screening of reaction conditions (reaction solvent, iodide ion source) are summarized in Table 3. The reaction in DMF gave 4 a in 18 % yield, together with a trace amount of hydrotrifluoromethylation product 3 a (entry 1). Among the solvents tested (Table 3, entries 1–4), 1,4-dioxane was found to be the best solvent for this reaction (Table 3, entry 4), but the yield was still not satisfactory. Since NMR studies suggested the formation of CF3I during the reaction, Togni reagent appears to be decomposed by iodide ion. Thus, the amount of TBAI was decreased. It is noteworthy that 4 a was selectively obtained in 78 % yield when the reaction was operated with 0.3 equivalents of TBAI for 12 h (Table 3, entry 5). In this case, the E/Z ratio of the product was as high as 9:1. Other iodide sources were also examined (Table 3, entries 6–9). Molecular iodine (I2) did not work as a mediator for the trifluoromethylation (Table 3, entry 6). In contrast to TBAI, NaI in 1,4-dioxane selectively provided iodotrifluoromethylation product 6 a in 50 % yield (Table 3, entry 7). KI and CsI were found to be better mediators, selectively affording 6 a in 61 % and 72 % yields, respectively (Table 3, entries 8 and 9). Contrary to the result in 1,4-dioxane, the reactions using KI or CsI in DMF again selectively provided vinylic trifluoromethylation product 4 a (Table 3, entries 10 and 11). A higher yield of 6 a was obtained when 1.5 equivalents of Togni reagent was used with CsI (Table 3, entry 12). The reaction with KI smoothly proceeded even at 60 °C, and 6 a was obtained in 92 % yield when the reaction time was prolonged to 9 h (Table 3, entry 13).
Table 3.
Screening of the reaction conditions using iodide salts[a]
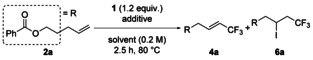 | |||||
|---|---|---|---|---|---|
| Entry | Iodide salts | Equiv | Solvent | Yield of4 a[%][b] | Yield of6 a[%][b] |
| 1 | TBAI | 1.0 | DMF | 21[c] | 0 |
| 2 | TBAI | 1.0 | MeCN | 19[d] | trace |
| 3 | TBAI | 1.0 | MeOH | 16[e] | 3 |
| 4 | TBAI | 1.0 | 1,4-dioxane | 37[f] | 0 |
| 5[g,h,i] | TBAI | 0.3 | 1,4-dioxane | 78[e,j] | 0 |
| 6 | I2 | 1.0 | 1,4-dioxane | 0 | 3 |
| 7 | NaI | 1.0 | 1,4-dioxane | trace | 50 |
| 8 | KI | 1.0 | 1,4-dioxane | trace | 61 |
| 9 | CsI | 1.0 | 1,4-dioxane | trace | 72 |
| 10 | KI | 1.0 | DMF | 22[c] | 0 |
| 11 | CsI | 1.0 | DMF | 30[k] | 0 |
| 12[g] | CsI | 1.1 | 1,4-dioxane | 0 | 88 |
| 13[g,l] | KI | 1.1 | 1,4-dioxane | 0 | 92[j] |
[a] The reactions were carried out with Togni reagent 1 (1.2 equiv) and additive on a 0.25 mmol scale, unless otherwise noted. [b] Determined by 19F NMR analysis using fluorobenzene as an internal standard. [c] The E/Z ratio was 6:1. [d] The E/Z ratio was 17:2. [e] The E/Z ratio was 7:1. [f] The E/Z ratio was 8:1. [g] Run with 1.5 equiv of Togni reagent 1. [h] Run for 12 h. [i] Run in 0.5 m solution. [j] Yield of isolated product. [k] The E/Z ratio was 13:2. [l] Run at 60 °C for 9 h.
Having established the reaction conditions, we next investigated the substrate scope for the vinylic trifluoromethylation using TBAI in 1,4-dioxane (Table 4). Similar to that of the hydrotrifluoromethylation, various functional groups were tolerated in this reaction. Although the E/Z ratio depended on the substrate, the reaction with mono-substituted alkenes proceeded in a highly stereoselective manner. It should be noted that no deprotonative trifluoromethylation product was detected under these reaction conditions.[11]
Table 4.
Vinylic trifluoromethylation of alkenes using TBAI[a]
 | |
|---|---|
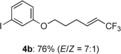 |
 |
 |
 |
 |
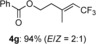 |
[a] The reactions were carried out with 1 (1.5 equiv) and TBAI (0.3 equiv) in 1,4-dioxane (0.5 mL) at 80 °C for 12 h on a 0.25 mmol scale.
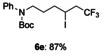
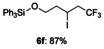
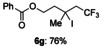
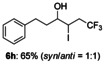
The reactions of these substrates with KI were also examined (Table 5). The iodotrifluoromethylation products were obtained in good to high yields under these reaction conditions. As expected, functional group tolerance was broad. Although an internal alkene was unsuitable for this iodotrifluoromethylation due to generation of regio- and stereo-isomers, not only mono-substituted alkenes 2 b–2 f, but also gem-disubstituted alkene 2 g were successfully converted into iodotrifluoromethylation products 6 b–6 g. Interestingly, allylic alcohol 2 h could be transformed into γ-trifluoromethyl-β-iodo-alcohol 6 h without epoxide formation in 65 % yield, albeit in a non-stereoselective manner (syn/anti=1:1); this product was expected to be difficult to obtain, and has synthetic potential as a building block.
Table 5.
Iodotrifluoromethylation of alkenes using KI[a]
 | |
|---|---|
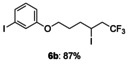 |
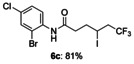 |
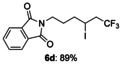 |
 |
 |
 |
 | |
[a] The reactions were carried out with 1 (1.5 equiv) and KI (1.1 equiv) in 1,4-dioxane (1.2 mL) at 60 °C for 9 h on a 0.25 mmol scale.
Encouraged by these results, we next examined the use of KBr [Eq. (1)]. The reaction in 1,4-dioxane resulted in very low conversion. To our surprise, the iodotrifluoromethylation product was obtained in moderate yield when the reaction was carried out in alcoholic solvent and no bromotrifluoromethylation product was detected. Although the reason for this is still unclear, the iodide source is presumably 2-iodobenzoate derived from Togni reagent. Indeed, benzoic acid was formed during the reaction.
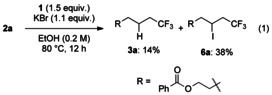 |
To extend these protocols, 4-chlorostyrene 7 was examined as a substrate (Scheme 3). Conjugated olefins tend to polymerize under radical conditions. However, contrary to our expectation, the vinylic trifluoromethylation and iodotrifluoromethylation products 8 and 9 were obtained in good yields, however, hydrotrifluoromethylation gave a complex mixture. Unfortunately, an electron-rich substrate, 4-methoxystyrene, gave only the oxytrifluoromethylation product even with these protocols.[6d] Therefore, the electron-rich substrates are not suitable for the present reaction. In our previous copper-catalyzed conditions for oxy-trifluoromethylation and preparation of β-trifluoromethylstyrene,[6d,h,15a] electron-deficient styrene derivatives were unsuitable as substrates. Thus, the current reaction conditions provide a complementary method for transformation of styrene derivatives to β-trifluoromethylated products.
Scheme 3.
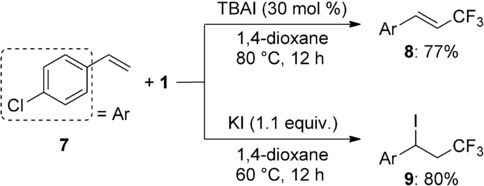
Trifluoromethylations of a styrene derivative.
To establish the reason for the observed product switching depending on the iodide salts, crown ether was added to the reaction using KI in 1,4-dioxane. In this case, vinylic trifluoromethylation product 4 a was selectively obtained in 70 % yield (Scheme 4 a). Based on this result, we anticipated that the solubility of 2-iodobenzoate would be crucial for the selectivity, and a vinylic trifluoromethylation product would be obtained through iodotrifluoromethylation and an E2 reaction. Thus, treatment of 6 a with potassium-2-iodobenzoate in DMF and 1,4-dioxane and the reaction with ammonium-2-iodobenzoate were examined under the described conditions (Scheme 4 b). As expected, the reaction with the barely soluble potassium salt in 1,4-dioxane gave a trace amount of 4 a, whereas an almost quantitative yield of 4 a was obtained in DMF. The ammonium salt efficiently transformed 6 a to 4 a.
Scheme 4.
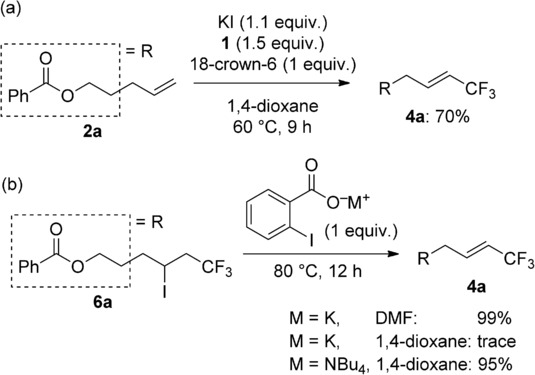
Formation of vinylic trifluoromethylation product.
To obtain information regarding the mechanism of the present reactions, a radical-clock substrate 10 was exposed to iodotrifluoromethylation conditions [Eq. (2)].[18] The main product was 11 (67 % yield), which is expected to be produced by cyclopropane ring-opening reaction through a radical pathway. The structure of 11 was determined after removal of iodine by reduction with azobisisobutyronitrile (AIBN) and n-Bu3SnH (see the Supporting Information), and the diastereomeric ratio of 11 was 1:1. The cationic ring-opening product 12 was also produced, albeit in only 8 % yield. Possible reaction pathways to give 12 would be 1) simple iodotrifluoromethylation and then cyclopropane ring-opening reaction with C−I bond cleavage, and 2) direct trifluoromethylation with cyclopropane ring-opening reaction. In any case, the radical pathway product 11 was mainly obtained, suggesting that the reaction intermediate should have a radical character.[19]
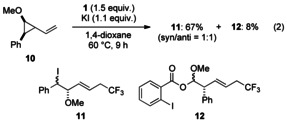 |
Conclusions
We have demonstrated that three distinct products, hydrotrifluoromethylated, vinylic trifluoromethylated, and iodotrifluoromethylated, can be selectively obtained by the reaction of simple alkenes with Togni reagent; a high level of product control is achieved simply by changing the salt and solvent. The combination of K2CO3 with DMF provided the hydrotrifluoromethylation product, with DMF as the hydrogen source. On the other hand, iodide ion was found to work as a catalyst or a promoter for vinylic trifluoromethylation and iodotrifluoromethylation. Vinylic trifluoromethylation was catalyzed by TBAI, and the use of KI resulted in iodotrifluoromethylation. Interestingly, KI could also mediate vinylic trifluoromethylation when the reaction was operated in the presence of 18-crown-6 or in DMF, thereby suggesting that the vinylic trifluoromethylation proceeds through the iodotrifluoromethylation product. The reaction using radical-clock substrate 10 indicated that the reactions proceeded through a radical intermediate. Further studies of the reactions and mechanism are in progress.
Experimental Section
General Procedures
All reactions were carried out under an atmosphere of nitrogen. Togni reagent was prepared according to the literature method.[5] Dehydrated solvents (DMF, 1,4-dioxane, DMAc, MeCN, and MeOH) purchased from Kanto Chemical Co., Inc. were used. KI was purchased from Junsei Chemical Co., Ltd. and CsI and K2CO3 were purchased from Wako Pure Chemical Industries, Ltd. All other inorganic salts were obtained from commercial sources, and used as received. Other reagents were purified by usual methods. New alkenes were prepared using reported methods, and the results of characterization are given in the Supporting Information.
1H and 19F NMR spectra were measured on a JEOL JNM-ECS-400 spectrometer at 400 and 376 MHz, respectively. 13C NMR spectra were recorded on a JEOL JNM-ECS-400 spectrometer at 100 MHz. Chemical shifts were reported downfield from TMS (δ=0 ppm) or CDCl3 for 1H NMR. For 13C NMR, chemical shifts were reported in the scale relative to CDCl3. For 19F NMR, chemical shifts were reported in the scale relative to a CFCl3 external standard (0 ppm). Infrared spectra were measured on a Thermo Nicolet iS5; only diagnostic absorptions are listed below. ESI-MS was taken on a Bruker micrOTOF-QII-RSL and Varian QFT-7. FI-MS was taken on a JEOL JMS-T100GCV. Column chromatography was performed with silica gel N-60 (40–100 μm) purchased from Kanto Chemical Co., Inc. TLC analysis was performed on Silica gel 60 F254-coated glass plates (Merck). Visualization was accomplished by means of ultraviolet (UV) irradiation at 254 nm or by spraying a solution of 12-molybdo(VI)phosphoric acid in ethanol as the developing agent.
Typical experimental procedure for hydrotrifluoromethylation (Tables 1 and 2)
Togni reagent 1 (240 mg, 3 equiv) and K2CO3 (36.4 mg, 1 equiv) were added to a Schlenk flask. The flask was evacuated and backfilled with nitrogen. Then, degassed DMF (5 mL) and alkene 2 a (46.9 mg, 0.25 mmol) were added to the flask. The reaction mixture was stirred for 12 h at 80 °C, and then diluted with EtOAc (5 mL). The organic solution was washed with distilled water and brine, dried over Na2SO4, and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by column chromatography on silica gel (hexane/EtOAc=20:1) to give 3 a (47.8 mg, 75 %) as a colorless oil.
,6,6-trifluorohexyl benzoate (3 a)
Colorless oil; 47.8 mg, 75 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.50–1.57 (m, 2 H), 1.62–1.69 (m, 2 H), 1.78–1.84 (m, 2 H), 2.05–2.17 (m, 2 H), 4.34 (t, J=6.9 Hz, 2 H), 7.43–7.47 (m, 2 H), 7.57 (tt, J=1.8, 7.4 Hz, 1 H), 8.03–8.05 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=21.8 (q, J=2.9 Hz), 25.4, 28.5, 33.8 (q, J=28.9 Hz), 64.7, 127.2 (q, J=276.5 Hz), 128.5, 129.7, 130.4, 133.1, 166.8 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.3 ppm (t, J=10.8 Hz); IR (neat): 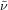 =2950, 1716, 1585, 1315, 1206, 834 cm−1; HRMS (ESI): Calcd. for [C13H15F3O2+Na]+: m/z=283.0916, Found: 283.0914.
=2950, 1716, 1585, 1315, 1206, 834 cm−1; HRMS (ESI): Calcd. for [C13H15F3O2+Na]+: m/z=283.0916, Found: 283.0914.
-[(6,6,6-trifluorohexyl)oxy]iodobenzene (3 b)
Colorless oil; 77.3 mg, 81 % (0.27 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.50–1.67 (m, 4 H), 1.76–1.83 (m, 2 H), 2.05–2.17 (m, 2 H), 3.93 (t, J=6.4 Hz, 2 H), 6.85 (ddd, J=0.9, 2.3, 8.3 Hz, 1H), 6.97–7.00 (m, 1 H), 7.23–7.29 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=21.8 (q, J=2.9 Hz), 25.4, 28.9, 33.8 (q, J=28.9 Hz), 67.8, 94.5, 114.3, 123.7, 127.3 (q, J=276.5 Hz), 130.0, 130.9, 159.7 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.3 ppm (t, J=10.8 Hz); IR (neat): 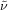 =2945, 1284, 1204, 1090, 1040, 970 cm−1; HRMS (FI): Calcd. for [C12H14F3IO]+: m/z=358.0041, Found:358.0022.
=2945, 1284, 1204, 1090, 1040, 970 cm−1; HRMS (FI): Calcd. for [C12H14F3IO]+: m/z=358.0041, Found:358.0022.
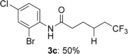
N-(2-bromo-4-chlorophenyl)-6,6,6-trifluorohexanamide (3 c)
White solid; 44.6 mg, 50 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.64–1.72 (m, 2 H), 1.80–1.87 (m, 2 H), 2.09–2.21 (m, 2 H), 2.47 (t, J=7.4 Hz, 2 H), 7.30 (dd, J=2.3, 8.7 Hz, 1 H), 7.50–7.59 (bs, 1 H), 7.55 (d, J=2.3 Hz, 1 H), 8.31 ppm (d, J=8.7 Hz, 1 H); 13C NMR (100 MHz, CDCl3): δ=21.7 (q, J=2.9 Hz), 24.4, 33.7 (q, J=28.9 Hz), 37.3, 113.5, 122.6, 127.1 (q, J=276.5 Hz), 128.6, 129.6, 131.8, 134.4, 170.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.2 ppm (t, J=10.8 Hz); IR (neat): 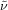 =2945, 1660, 1468, 1259, 1147, 1033 cm−1; HRMS (ESI): Calcd. for [C12H12BrClF3NO+Na]+: m/z=379.9635, Found: 379.9635.
=2945, 1660, 1468, 1259, 1147, 1033 cm−1; HRMS (ESI): Calcd. for [C12H12BrClF3NO+Na]+: m/z=379.9635, Found: 379.9635.
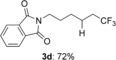
-(6,6,6-trifluorohexyl)isoindoline-1,3-dione (3 d)
Colorless oil; 50.9 mg, 72 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.39–1.46 (m, 2 H), 1.56–1.65 (m, 2 H), 1.68–1.75 (m, 2 H), 2.01–2.13 (m, 2 H), 3.70 (t, J=7.4 Hz, 2 H), 7.71–7.73 (m, 2 H), 7.84–7.86 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=21.6 (q, J=2.9 Hz), 26.0, 28.3, 33.7 (q, J=28.9 Hz), 37.7, 123.3, 127.2 (q, J=276.5 Hz), 132.2, 134.1, 168.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.3 ppm (t, J=10.8 Hz); IR (neat):  =2945, 1773, 1706, 1467, 1395, 1187 cm−1; HRMS (ESI): Calcd. for [C14H14F3NO2+Na]+: m/z=308.0869, Found: 308.0871.
=2945, 1773, 1706, 1467, 1395, 1187 cm−1; HRMS (ESI): Calcd. for [C14H14F3NO2+Na]+: m/z=308.0869, Found: 308.0871.
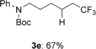
tert-butyl phenyl(6,6,6-trifluorohexyl)carbamate (3 e)
Colorless oil; 56.0 mg, 67 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.32–1.40 (m, 2 H), 1.42 (s, 9 H), 1.51–1.59 (m, 4 H), 1.98–2.10 (m, 2 H), 3.63 (t, J=7.4 Hz, 2 H), 7.18 (m, 2 H), 7.21 (tt, J=1.4, 7.4 Hz, 1 H), 7.31–7.36 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=21.8 (q, J=2.9 Hz), 26.0, 28.2, 28.5, 33.8 (q, J=28.9 Hz), 49.7, 80.2, 126.2, 127.3, 127.3 (q, J=276.5 Hz), 128.9, 142.6, 154.9 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.3 ppm (t, J=10.8 Hz); IR (neat): 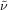 =3042, 1698, 1301, 1255, 1150, 1073 cm−1; HRMS (ESI): Calcd. for [C17H24F3NO2+Na]+: m/z=354.1651, Found: 354.1653.
=3042, 1698, 1301, 1255, 1150, 1073 cm−1; HRMS (ESI): Calcd. for [C17H24F3NO2+Na]+: m/z=354.1651, Found: 354.1653.
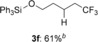
Triphenyl((5,5,5-trifluoropentyl)oxy)silane (3 f)
Colorless oil; 61.7 mg, 61 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3); 1H NMR (400 MHz, CDCl3): δ=1.63–1.68 (m, 4 H), 1.97–2.09 (m, 2 H), 3.81 (t, J=6.0 Hz, 2 H), 7.37–7.48 (m, 9 H), 7.60–7.62 ppm (m, 6 H); 13C NMR (100 MHz, CDCl3): δ=18.6 (q, J=2.9 Hz), 31.5, 33.5 (q, J=28.9 Hz), 63.1, 127.3 (q, J=276.5 Hz), 128.0, 130.2, 134.2, 135.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.3 ppm (t, J=10.8 Hz); IR (neat):  =3069, 1428, 1257, 1115, 1037, 738 cm−1; HRMS (FI): Calcd. for [C23H23F3OSi]+: m/z=400.1470, Found: 400.1487.
=3069, 1428, 1257, 1115, 1037, 738 cm−1; HRMS (FI): Calcd. for [C23H23F3OSi]+: m/z=400.1470, Found: 400.1487.
,5,5-trifluoro-3-methylpentyl benzoate (3 g)
Colorless oil; 32.4 mg, 51 % (0.24 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.21 (d, J=6.4 Hz, 3 H), 1.66–1.75 (m, 1 H), 1.89–2.27 (m, 4 H), 4.37 (td, J=6.4, 11.5 Hz, 1 H), 4.40 (td, J=6.4, 11.5 Hz, 1 H), 7.43–7.47 (m, 2 H), 7.57 (tt, J=1.4, 7.4 Hz, 1 H), 8.02–8.05 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=19.8, 25.2 (q, J=2.9 Hz), 35.4, 40.3 (q, J=27.0 Hz), 62.6, 127.1 (q, J=277.4 Hz), 128.5, 129.7, 130.3, 133.1, 166.7 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.2 ppm (t, J=10.8 Hz); IR (neat):  =2968, 1717, 1271, 1253, 1090, 710 cm−1; HRMS (ESI): Calcd. for [C13H15F3O2+Na]+: m/z=283.0916, Found: 283.0920.
=2968, 1717, 1271, 1253, 1090, 710 cm−1; HRMS (ESI): Calcd. for [C13H15F3O2+Na]+: m/z=283.0916, Found: 283.0920.
(N-methylformamido)methyl 2-iodobenzoate (5)
Yellow oil; 1H NMR (400 MHz, CDCl3): δ=3.07 (s, 3 H), 5.56 (s, 2 H), 7.18–7.22 (m, 1 H), 7.41–7.45 (m, 1 H), 7.80 (dd, J=1.4, 7.8 Hz, 1 H), 8.01–8.03 (m, 1 H), 8.44 ppm (s, 1 H); 13C NMR (100 MHz, CDCl3): δ=30.4, 74.9, 94.2, 128.2, 131.3, 133.4, 134.0, 141.7, 164.4, 166.1 ppm; IR (neat): 2878, 1723, 1682, 1239, 1012 742 cm−1; HRMS (FI): Calcd. for [C10H10NO3I]+: m/z=318.9705, Found: 318.9715.
Typical experimental procedure for vinylic trifluoromethylation (Tables 3 and 4)
Tetrabutylammonium iodide (TBAI) (28.0 mg, 0.3 equiv) and Togni reagent 1 (119 mg, 1.5 equiv) were added to a Schlenk flask. The flask was evacuated and backfilled with nitrogen. Then, degassed 1,4-dioxane (0.5 mL) and 2 a (46.7 mg, 0.25 mmol) were added to the flask. After stirring for 12 h at 80 °C, the reaction mixture was diluted with EtOAc (5 mL) and concentrated under reduced pressure and the residue was purified by column chromatography on silica gel (n-hexane/EtOAc=20:1) to give trifluoromethylated product 4 a (49.3 mg, 78 %, E/Z=9/1, the E/Z ratio was determined by 19F NMR analysis of the crude mixture) as a colorless oil. E isomer was isolated by further purification of a small portion of the product using HPLC (SenshuPak PEGASIL Silica SP100).
(E)-6,6,6-trifluorohex-4-en-1-yl benzoate (4 a)
Colorless oil; 49.3 mg, 78 %, E/Z=9:1 (0.25 mmol scale); E isomer was isolated by HPLC (n-hexane/EtOAc=10:1); E isomer: 1H NMR (400 MHz, CDCl3): δ=1.91–1.98 (m, 2 H), 2.32–2.38 (m, 2 H), 4.36 (t, J=6.4 Hz, 2 H), 5.69 (tqd, J=1.8, 6.4, 15.6 Hz, 1 H), 6.44 (qtd, J=2.3, 6.9, 15.6 Hz, 1 H), 7.43–7.47 (m, 2 H), 7.57 (tt, J=1.4, 7.4 Hz, 1 H), 8.02–8.05 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=27.3, 28.3, 64.0, 119.4 (q, J=32.7 Hz), 123.0 (q, J=269.7 Hz), 128.6, 129.7, 130.2, 133.2, 139.5 (q, J=6.7 Hz), 166.6 ppm; 19F NMR (376 MHz, CDCl3): δ=−64.0 ppm (tdd, J=2.3, 2.3, 6.4 Hz); IR (neat):  =2958, 1717, 1602, 1269, 1111, 710 cm−1; HRMS (ESI): Calcd. for [C13H13F3O2+Na]+: m/z=281.0760, Found: 281.0766; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.0 ppm (td, J=2.2, 8.7 Hz).
=2958, 1717, 1602, 1269, 1111, 710 cm−1; HRMS (ESI): Calcd. for [C13H13F3O2+Na]+: m/z=281.0760, Found: 281.0766; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.0 ppm (td, J=2.2, 8.7 Hz).
(E)-3-((6,6,6-trifluorohex-4-en-1-yl)oxy)iodobenzene (4 b)
Colorless oil; 68.1 mg, 76 %, E/Z=7:1 (0.25 mmol scale); E isomer was isolated by HPLC (n-hexane/EtOAc=25:1); E isomer: 1H NMR (400 MHz, CDCl3): δ=1.89–1.96 (m, 2 H), 2.32–2.39 (m, 2 H), 3.95 (t, J=6.0 Hz, 2 H), 5.69 (tqd, J=1.8, 6.4, 15.6 Hz, 1 H), 6.42 (qtd, J=2.3, 6.9, 15.6 Hz, 1 H), 6.85 (ddd, J=0.9, 2.3, 8.3 Hz, 1 H), 7.00 (dd, J=8.3, 8.3 Hz, 1 H), 7.24–7.30 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=27.8, 28.2, 67.0, 94.5, 114.3, 119.3 (q, J=33.7 Hz), 123.1 (q, J=268.8 Hz), 123.7, 130.1, 131.0, 139.7 (q, J=6.7 Hz), 159.4 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.9 ppm (tdd, J=2.3, 2.3, 6.4 Hz); IR (neat):  =2945, 1584, 1242, 1091, 1049, 971 cm−1; HRMS (FI): Calcd. for [C12H12F3IO]+: m/z=355.9885, Found: 355.9873; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.0 ppm (td, J=2.2, 8.7 Hz).
=2945, 1584, 1242, 1091, 1049, 971 cm−1; HRMS (FI): Calcd. for [C12H12F3IO]+: m/z=355.9885, Found: 355.9873; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.0 ppm (td, J=2.2, 8.7 Hz).
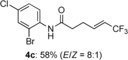
(E)-N-(2-bromo-4-chlorophenyl)-6,6,6-trifluorohex-4-enamide (4 c)
White solid; 52.3 mg, 58 %, E/Z=8:1 (0.25 mmol scale);1H NMR (400 MHz, CDCl3): δ=2.56–2.66 (m, 4 H), 5.69–5.77 (m, 1 H), 6.40–6.49 (m, 1 H), 7.30 (dd, J=2.3, 8.7 Hz, 1 H), 7.50–7.57 (bs, 1 H), 7.55 (d, J=2.3 Hz, 1 H), 8.29 ppm (d, J=8.7 Hz, 1 H); 13C NMR (100 MHz, CDCl3): δ=26.9, 35.8, 113.6, 120.1 (q, J=33.7 Hz), 122.7, 122.9 (q, J=269.7 Hz), 128.7, 129.8, 131.9, 134.3, 138.4 (q, J=6.7 Hz), 169.4 ppm; 19F NMR (376 MHz, CDCl3): δ=−64.1 ppm (m); IR (neat):  =3274, 1661, 1276, 1108, 1071, 741 cm−1; HRMS (ESI): Calcd. for [C12H10BrClF3NO+Na]+: m/z=377.9479, Found: 377.9480; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.2 ppm (td, J=2.2, 8.7 Hz).
=3274, 1661, 1276, 1108, 1071, 741 cm−1; HRMS (ESI): Calcd. for [C12H10BrClF3NO+Na]+: m/z=377.9479, Found: 377.9480; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.2 ppm (td, J=2.2, 8.7 Hz).
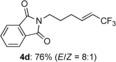
(E)-2-(6,6,6-trifluorohex-4-en-1-yl)isoindoline-1,3-dione (4 d)
White solid; 53.8 mg, 76 %, E/Z=8:1 (0.25 mmol scale); E isomer was isolated by HPLC (n-hexane/EtOAc=5:1); E isomer: 1H NMR (400 MHz, CDCl3): δ=1.86 (quintet, J=7.4 Hz, 2 H), 2.19–2.27 (m, 2 H), 3.73 (t, J=7.4 Hz, 2 H), 5.66 (tqd, J=1.8, 6.4, 15.6 Hz, 1 H), 6.36 (qtd, J=2.3, 6.9, 15.6 Hz, 1 H), 7.72–7.74 (m, 2 H), 7.84–7.86 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=27.0, 28.9, 37.3, 119.4 (q, J=33.7 Hz), 123.0 (q, J=268.8 Hz), 123.4, 132.1, 134.2, 139.2 (q, J=6.7 Hz), 168.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−64.0 ppm (tdd, J=2.3, 2.3, 6.4 Hz); IR (neat):  =1708, 1396, 1274, 1104, 972, 719 cm−1; HRMS (ESI): Calcd. for [C14H12F3NO2+Na]+: m/z=306.0712, Found: 306.0713. Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.1 ppm (td, J=2.2, 8.7 Hz).
=1708, 1396, 1274, 1104, 972, 719 cm−1; HRMS (ESI): Calcd. for [C14H12F3NO2+Na]+: m/z=306.0712, Found: 306.0713. Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.1 ppm (td, J=2.2, 8.7 Hz).
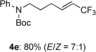
(E)-tert-butyl phenyl(6,6,6-trifluorohex-4-en-1-yl)carbamate (4 e)
Colorless oil; 64.1 mg, 80 %, E/Z=7:1 (0.24 mmol scale); E isomer was isolated by HPLC (n-hexane/EtOAc=10:1); E isomer: 1H NMR (400 MHz, CDCl3): δ=1.42 (s, 9 H), 1.69 (quintet, J=7.4 Hz, 2 H), 2.12–2.19 (m, 2 H), 3.66 (t, J=7.4 Hz, 2 H), 5.56 (tqd, J=1.8, 6.4, 15.6 Hz, 1 H), 6.34 (qtd, J=2.3, 6.9, 15.6 Hz, 1 H), 7.18 (m, 2 H), 7.21 (tt, J=1.4, 7.4 Hz, 1 H), 7.32–7.37 (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=27.0, 28.4, 28.8, 49.3, 80.4, 119.0 (q, J=32.8 Hz), 123.1 (q, J=268.8 Hz), 126.3, 127.2, 129.0, 139.8 (q, J=6.7 Hz), 142.4, 154.8 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.9 ppm (tdd, J=2.3, 2.3, 6.4 Hz); IR (neat):  =2977, 1694, 1597, 1299, 1272, 1088 cm−1; HRMS (ESI): Calcd. for [C17H22F3NO2+Na]+: m/z=352.1495, Found: 352.1498; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.0 ppm (td, J=2.2, 8.7 Hz).
=2977, 1694, 1597, 1299, 1272, 1088 cm−1; HRMS (ESI): Calcd. for [C17H22F3NO2+Na]+: m/z=352.1495, Found: 352.1498; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.0 ppm (td, J=2.2, 8.7 Hz).
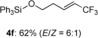
(E)-triphenyl((5,5,5-trifluoropent-3-en-1-yl)oxy)silane (4 f)
White solid; 63.3 mg, 62 %, E/Z=6:1 (0.25 mmol scale); E isomer was isolated by HPLC (n-hexane/EtOAc=25:1); E isomer: 1H NMR (400 MHz, CDCl3): δ=2.38–2.44 (m, 2 H), 3.89 (t, J=6.0 Hz, 2 H), 5.66 (tqd, J=1.4, 6.4, 15.6 Hz, 1 H), 6.39 (qtd, J=2.3, 6.9, 15.6 Hz, 1 H, 7.37–7.47 (m, 9 H), 7.60–7.62 ppm (m, 6 H); 13C NMR (100 MHz, CDCl3): δ=34.8, 62.1, 120.4 (q, J=33.7 Hz), 123.0 (q, J=268.8 Hz), 128.1, 130.3, 133.9, 135.5, 137.6 ppm (q, J=6.7 Hz); 19F NMR (376 MHz, CDCl3): δ=−64.1 ppm (tdd, J=2.3, 2.3, 6.4 Hz); IR (neat):  =1429, 1115, 1029, 972, 736, 699 cm−1; HRMS (ESI): Calcd. for [C23H21F3OSi+Na]+: m/z=421.1206, Found: 421.1214; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.2 ppm (d, J=8.7 Hz).
=1429, 1115, 1029, 972, 736, 699 cm−1; HRMS (ESI): Calcd. for [C23H21F3OSi+Na]+: m/z=421.1206, Found: 421.1214; Z isomer: 19F NMR (376 MHz, CDCl3): δ=−58.2 ppm (d, J=8.7 Hz).
(E)-5,5,5-trifluoro-3-methylpent-3-en-1-yl benzoate (4 g)
Colorless oil; 59.5 mg, 94 %, E/Z=2:1 (0.25 mmol scale); stereoisomers were isolated by HPLC (n-hexane/EtOAc=10:1); E isomer: 1H NMR (400 MHz, CDCl3): δ =1.98–2.00 (m, 3 H), 2.55–2.58 (m, 2 H), 4.47 (t, J=6.4 Hz, 2 H), 5.57 (qq, J=1.4, 8.3 Hz, 1 H), 7.43–7.47 (m, 2 H), 7.57 (tt, J=1.4, 7.4 Hz, 1 H), 8.00–8.03 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=17.8, 38.6, 62.0, 116.8 (q, J=33.7 Hz), 123.3 (q, J=270.7 Hz), 128.6, 129.7. 130.0, 133.3, 147.5 (q, J=5.8 Hz), 166.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−57.5 ppm (m); IR (neat):  =2958, 1719, 1602, 1266, 1101, 710 cm−1; HRMS (ESI): Calcd. for [C13H13F3O2+Na]+: m/z=281.0760, Found: 281.0766; Z isomer: 1H NMR (400 MHz, CDCl3): δ =1.95–1.97 (m, 3 H), 2.76 (t, J=6.4 Hz, 2 H), 4.46 (t, J=6.4 Hz, 2 H), 5.59 (q, J=8.3 Hz, 1 H), 7.42–7.46 (m, 2 H), 7.57 (tt, J=1.4, 7.4 Hz, 1 H), 8.02–8.04 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=23.9, 32.3, 62.4, 117.5 (q, J=33.7 Hz), 123.2 (q, J=271.6 Hz), 128.5, 129.7. 130.1, 133.2, 147.5 (q, J=5.8 Hz), 166.6 ppm; 19F NMR (376 MHz, CDCl3): δ=−56.7 ppm (d, J=8.7 Hz); IR (neat):
=2958, 1719, 1602, 1266, 1101, 710 cm−1; HRMS (ESI): Calcd. for [C13H13F3O2+Na]+: m/z=281.0760, Found: 281.0766; Z isomer: 1H NMR (400 MHz, CDCl3): δ =1.95–1.97 (m, 3 H), 2.76 (t, J=6.4 Hz, 2 H), 4.46 (t, J=6.4 Hz, 2 H), 5.59 (q, J=8.3 Hz, 1 H), 7.42–7.46 (m, 2 H), 7.57 (tt, J=1.4, 7.4 Hz, 1 H), 8.02–8.04 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=23.9, 32.3, 62.4, 117.5 (q, J=33.7 Hz), 123.2 (q, J=271.6 Hz), 128.5, 129.7. 130.1, 133.2, 147.5 (q, J=5.8 Hz), 166.6 ppm; 19F NMR (376 MHz, CDCl3): δ=−56.7 ppm (d, J=8.7 Hz); IR (neat):  =1721, 1678, 1603, 1269, 1104, 710 cm−1; HRMS (ESI): Calcd. for [C13H13F3O2+Na]+: m/z=281.0760, Found: 281.0770.
=1721, 1678, 1603, 1269, 1104, 710 cm−1; HRMS (ESI): Calcd. for [C13H13F3O2+Na]+: m/z=281.0760, Found: 281.0770.
(E)-β-trifluoromethyl-4-chlorostyrene (8)
Colorless oil; 37.4 mg, 77 % (0.24 mmol scale); 1H NMR (400 MHz, CDCl3): δ=6.18 (qd, J=6.4, 16.0 Hz, 1 H), 7.11 (qd, J=2.3, 16.0 Hz, 1 H), 7.36–7.41 ppm (m, 4 H); 13C NMR (100 MHz, CDCl3): δ=116.6 (q, J=33.7 Hz), 123.6 (q, J=268.8 Hz), 128.9, 129.4, 132.0, 136.1, 136.6 ppm (q, J=6.7 Hz); 19F NMR (376 MHz, CDCl3): δ=−63.3 ppm (dd, J=2.3, 6.4 Hz); IR (neat):  =2929, 1492, 1273, 1093, 970, 807 cm−1; HRMS (FI): Calcd. for [C9H6ClF3]+: m/z=206.0110, Found: 206.0105.
=2929, 1492, 1273, 1093, 970, 807 cm−1; HRMS (FI): Calcd. for [C9H6ClF3]+: m/z=206.0110, Found: 206.0105.
Typical experimental procedure for the iodotrifluoromethylation (Tables 3 and 5)
Togni reagent 1 (118 mg, 1.5 equiv) and KI (46.6 mg, 1.1 equiv) were added to a Schlenk flask. The flask was evacuated and backfilled with nitrogen. Then, degassed 1,4-dioxane (1.2 mL) and 2 a (46.8 mg, 0.25 mmol) were added to the flask. The reaction mixture was stirred for 9 h at 60 °C, and then diluted with EtOAc (5 mL). After filtration to remove the insoluble solid, the filtrate was concentrated under reduced pressure and the residue was purified by column chromatography on silica gel (n-hexane/EtOAc=20:1) to give the trifluoromethylated product 6 a (87.4 mg, 92 %) as a colorless oil.
,6,6-trifluoro-4-iodohexyl benzoate (6 a)
Colorless oil; 87.4 mg, 92 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.86–2.13 (m, 4 H), 2.75–3.02 (m, 2 H), 4.24–4.30 (m, 1 H), 4.38 (t, J=6.0 Hz, 2 H), 7.43–7.47 (m, 2 H), 7.57 (tt, J=1.4, 7.4 Hz, 1 H), 8.02–8.05 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=20.6 (q, J=2.9 Hz), 29.0, 36.3, 45.0 (q, J=28.9 Hz), 63.7, 125.6 (q, J=279.4 Hz), 128.6, 129.7, 130.2, 133.2, 166.6 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.8 ppm (t, J=10.1 Hz); IR (neat):  =2958, 1716, 1584, 1270, 1216, 829 cm−1; HRMS (ESI): Calcd. for [C13H14F3IO2+Na]+: m/z=408.9883, Found: 408.9910.
=2958, 1716, 1584, 1270, 1216, 829 cm−1; HRMS (ESI): Calcd. for [C13H14F3IO2+Na]+: m/z=408.9883, Found: 408.9910.
-[(6,6,6-trifluoro-4-iodohexyl)oxy]iodobenzene (6 b)
Colorless oil; 105.7 mg, 87 % (0.25 mmol scale.); 1H NMR (400 MHz, CDCl3): δ=1.84–2.09 (m, 4 H), 2.75–3.02 (m, 2 H), 3.98 (t, J=6.0 Hz, 2 H), 4.22–4.29 (m, 1 H), 6.85 (ddd, J=0.9, 2.3, 8.3 Hz, 1 H), 6.99 (dd, J=8.3, 8.3 Hz, 1 H), 7.24–7.30 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=20.9, 29.4, 36.4, 45.0 (q, J=28.9 Hz), 66.8, 94.5, 114.3, 123.7, 125.6 (q, J=278.4 Hz), 130.1, 130.9, 159.4 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.8 ppm (t, J=10.1 Hz); IR (neat):  =2928, 1284, 1241, 1111, 1060, 989 cm−1; HRMS (FI): Calcd. for [C12H13F3I2O]+: m/z=483.9008, Found: 483.8993.
=2928, 1284, 1241, 1111, 1060, 989 cm−1; HRMS (FI): Calcd. for [C12H13F3I2O]+: m/z=483.9008, Found: 483.8993.
N-(2-bromo-4-chlorophenyl)-6,6,6-trifluoro-4-iodohexanamide (6 c)
Colorless oil; 97.3 mg, 81 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=2.06–2.16 (m, 1 H), 2.24–2.32 (m, 1 H), 2.58–2.66 (m, 1 H), 2.70–2.78 (m, 1 H), 2.80–3.05 (m, 2 H), 4.27–4.34 (m, 1 H), 7.30 (dd, J=2.3, 8.7 Hz, 1 H), 7.56 (d, J=2.3 Hz, 1 H), 7.57–7.66 (bs, 1 H), 8.29 ppm (d, J=8.7 Hz, 1 H); 13C NMR (100 MHz, CDCl3): δ=20.7, 34.9, 37.7, 45.2 (q, J=28.9 Hz), 113.6, 122.7, 125.5 (q, J=278.4 Hz), 128.6, 129.8, 131.9, 134.3, 169.3 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.6 ppm (t, J=10.1 Hz); IR (neat): 3295, 1661, 1469, 1284, 1210, 1110 cm−1; HRMS (ESI): Calcd. for [C12H11BrClF3INO+Na]+: m/z=505.8602, Found: 505.8612.
-(6,6,6-trifluoro-4-iodohexyl)isoindoline-1,3-dione (6 d)
Colorless oil; 90.4 mg, 89 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl): δ=1.78–1.88 (m, 3 H), 1.94–2.03 (m, 1 H), 2.69–2.96 (m, 2 H), 3.72–3.76 (m, 2 H), 4.19–4.26 (m, 1 H), 7.70–7.75 (m, 2 H), 7.84–7.88 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=20.2 (q, J=2.9 Hz), 28.8, 36.8, 36.8, 44.9 (q, J=27.9 Hz), 123.4, 125.5 (q, J=278.4 Hz), 132.1, 134.1, 168.4 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.8 ppm (t, J=10.1 Hz); IR (neat):  =2940, 1771, 1705, 1468, 1396, 1143 cm−1; HRMS (ESI): Calcd. for [C14H13F3INO2+Na]+: m/z=433.9835, Found: 433.9845.
=2940, 1771, 1705, 1468, 1396, 1143 cm−1; HRMS (ESI): Calcd. for [C14H13F3INO2+Na]+: m/z=433.9835, Found: 433.9845.
N-phenyl-N-(6,6,6-trifluoro-4-iodohexyl) tert-butyl carbamate (6 e)
Colorless oil; 100.2 mg, 87 % (0.25 mmol scale.); 1H NMR (400 MHz, CDCl3): δ=1.42 (s, 9 H), 1.59–1.68 (m, 1 H), 1.74–1.84 (m, 3 H), 2.68–2.96 (m, 2 H), 3.64–3.74 (m, 2 H), 4.16–4.22 (m, 1 H), 7.16–7.19 (m, 2 H), 7.21 (tt, J=1.4, 7.4 Hz, 1 H), 7.32–7.37 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=21.0 (q, J=2.9 Hz), 28.4, 28.7, 36.8, 45.1 (q, J=27.9 Hz), 48.6, 80.4, 125.6 (q, J=278.4 Hz), 126.3, 127.2, 129.0, 142.3, 154.9 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.7 ppm (t, J=10.1 Hz); IR (neat):  =2977, 1693, 1252, 1208, 1145, 757 cm−1; HRMS (ESI): Calcd. for [C17H23F3INO2+Na]+: m/z=480.0618, Found: 480.0621.
=2977, 1693, 1252, 1208, 1145, 757 cm−1; HRMS (ESI): Calcd. for [C17H23F3INO2+Na]+: m/z=480.0618, Found: 480.0621.
Triphenyl [(5,5,5-trifluoro-3-iodopentyl)oxy]silane (6 f)
Colorless oil; 115.4 mg, 87 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ =1.92–2.08 (m, 2 H), 2.76–2.99 (m, 2 H), 3.86–3.99 (m, 2 H), 4.44–4.51 (m, 1 H), 7.38–7.48 (m, 9 H), 7.61–7.63 ppm (m, 6 H); 13C NMR (100 MHz, CDCl3): δ=17.9 (q, J=2.9 Hz), 42.3, 45.1 (q, J=28.9 Hz), 63.2, 125.7 (q, J=278.4 Hz), 128.1, 130.3, 133.8, 135.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.5 ppm (t, J=10.1 Hz); IR (neat):  =3068, 2874, 1428, 1255, 1114, 699 cm−1; HRMS (FI): Calcd. for [C23H22F3IOSi]+: m/z=526.0437, Found: 526.0431.
=3068, 2874, 1428, 1255, 1114, 699 cm−1; HRMS (FI): Calcd. for [C23H22F3IOSi]+: m/z=526.0437, Found: 526.0431.
,5,5-trifluoro-3-iodo-3-methylpentyl benzoate (6 g)
Colorless oil; 74.3 mg, 76 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=2.20–2.21 (m, 3 H), 2.24–2.37 (m, 2 H), 3.08 (q, J=10.6 Hz, 2 H), 4.56–4.68 (m, 2 H), 7.43–7.47 (m, 2 H), 7.58 (tt, J=1.4, 7.4 Hz, 1 H), 8.03–8.05 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=35.8, 40.0, 45.4, 51.2 (q, J=27.0 Hz), 65.5, 125.3 (q, J=280.3 Hz), 128.6, 129.7, 130.0, 133.3, 166.5 ppm; 19F NMR (376 MHz, CDCl3): δ=−60.0 ppm (t, J=10.8 Hz); IR (neat):  =2966, 1717, 1602, 1251, 1108, 710 cm−1; HRMS (FI): Calcd. for [C13H14F3IO2]+: m/z=385.9991, Found: 386.0016.
=2966, 1717, 1602, 1251, 1108, 710 cm−1; HRMS (FI): Calcd. for [C13H14F3IO2]+: m/z=385.9991, Found: 386.0016.
,6,6-trifluoro-4-iodo-1-phenylhexan-3-ol (6 h)
Colorless oil; 55.1 mg, 65 %, syn/anti=1:1 (0.24 mmol scale); 1H NMR (400 MHz, CDCl3): δ=1.61–1.64 (m, 1 H), 1.78–1.96 (m, 4 H), 1.97–1.99 (m, 1 H), 2.67–2.84 (m, 6 H), 2.85–2.95 (m, 2 H), 2.98–3.12 (m, 1 H), 3.42–3.47 (m, 1 H), 4.27–4.32 (m, 2 H), 7.19–7.24 (m, 6 H), 7.29–7.33 ppm (m, 4 H); 13C NMR (100 MHz, CDCl3): δ=29.5 (q, J=2.89 Hz), 31.8, 31.9, 32.0, 36.3, 39.9 (q, J=28.9 Hz), 40.7, 42.1 (q, J=28.9 Hz), 72.4, 74.6, 125.8 (q, J=278.4 Hz), 125.9 (q, J=278.4 Hz), 126.4, 126.4, 128.5, 128.5, 128.7, 128.7, 140.9, 141.1 ppm; 19F NMR (376 MHz, CDCl3): δ=−63.9 (t, J=10.1 Hz), −64.2 ppm (t, J=10.1 Hz); IR (neat):  =3441, 1603, 1429, 1251, 1141, 751 cm−1; HRMS (FI): Calcd. for [C12H14F3IO]+: m/z=358.0041, Found: 358.0032.
=3441, 1603, 1429, 1251, 1141, 751 cm−1; HRMS (FI): Calcd. for [C12H14F3IO]+: m/z=358.0041, Found: 358.0032.
-(4-chlorophenyl)-1-iodo-3,3,3-trifluoropropane (9)
Colorless oil; 65.8 mg, 80 % (0.25 mmol scale); 1H NMR (400 MHz, CDCl3): δ=3.09–3.29 (m, 2 H), 5.25–5.28 (m, 1 H), 7.28–7.36 ppm (m, 4 H); 13C NMR (100 MHz, CDCl3): δ=16.3 (q, J=2.9 Hz), 45.8 (q, J=28.9 Hz), 124.9 (q, J=279.4 Hz), 128.3, 129.3, 134.4, 140.9 ppm; 19F NMR (376 MHz, CDCl3): δ=−64.3 ppm (t, J=9.4 Hz); IR (neat):  =3028, 1490, 1244, 1202, 1092, 725 cm−1; HRMS (FI): Calcd. for [C9H7ClF3I]+: m/z=333.9233, Found: 333.9224.
=3028, 1490, 1244, 1202, 1092, 725 cm−1; HRMS (FI): Calcd. for [C9H7ClF3I]+: m/z=333.9233, Found: 333.9224.
Reaction with KI and 1-(2-methoxy-3-phenylcyclopropyl)ethene 10 [Eq. (2)]
Togni reagent 1 (121 mg, 1.5 equiv) and KI (47.2 mg, 1.1 equiv) were added to a Schlenk flask. The flask was evacuated and backfilled with nitrogen. Then, degassed 1,4-dioxane (1.2 mL) and 10 (44.8 mg, 0.26 mmol) were added to the flask. The reaction mixture was stirred for 9 h at 60 °C, diluted with EtOAc (5 mL), and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by column chromatography on silica gel (n-hexane/EtOAc=20:1) to give the radical pathway product 11 (63.8 mg, 67 %) as the major product, together with the cationic pathway product 12 (10.1 mg, 8 %).
-iodo-1-phenyl-2-methoxy-6,6,6-trifluorohex-3-ene (11)
Colorless oil; 63.8 mg, 67 %, syn/anti=1:1; 1H NMR (400 MHz, CDCl3): δ=2.63–2.82 (m, 2 H), 2.88 (dq, J=6.0, 10.6 Hz, 2 H), 3.29 (s, 3 H), 3.40 (s, 3 H), 3.78 (t, J=7.4 Hz, 1 H), 3.82 (t, J=6.4 Hz, 1 H), 4.96 (d, J=7.4 Hz, 1 H), 5.08 (d, J=6.4 Hz, 1 H), 5.43–5.48 (m, 1 H), 5.53–5.61 (m, 1 H), 5.59–5.71 (m, 2 H), 7.20–7.30 (m, 6 H), 7.34–7.38 (m, 2 H), 7.39–7.42 ppm (m, 2 H); 13C NMR (100 MHz, CDCl3): δ=35.3, 36.2, 37.0 (q, J=29.9 Hz), 37.1 (q, J=29.9 Hz), 57.2, 57.3, 85.4, 85.5, 123.1 (q, J=2.9 Hz), 124.0 (q, J=2.9 Hz), 125.5 (q, J=276.5 Hz), 125.8 (q, J=276.5 Hz), 128.2, 128.4, 128.5, 128.6, 128.7, 128.9, 134.5, 135.4, 140.3, 140.7 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.1 (t, J=10.8 Hz), −66.3 ppm (t, J=10.8 Hz); IR (neat):  =2933, 1252, 1133, 1101, 972, 696 cm−1; HRMS (FI): Calcd. for [C13H14F3IO]+: m/z=370.0041, Found: 370.0036.
=2933, 1252, 1133, 1101, 972, 696 cm−1; HRMS (FI): Calcd. for [C13H14F3IO]+: m/z=370.0041, Found: 370.0036.
(E)-1-methoxy-2-phenyl-6,6,6-trifluorohex-3-en-1-yl 2-iodobenzoate (12)
Colorless oil; 10.1 mg, 8 %, syn/anti=1:1; 1H NMR (400 MHz, CDCl3): δ=2.76–2.86 (m, 2 H), 2.81–2.92 (m, 2 H), 3.47 (s, 3 H), 3.54 (s, 3 H), 3.82 (dd, J=6.0, 7.4 Hz, 1 H), 3.84 (dd, J=6.0, 8.7 Hz, 1 H), 5.50 (dtd, J=1.4, 7.4, 15.6 Hz, 1 H), 5.58 (td, J=6.9, 15.6 Hz, 1 H), 6.04 (dd, J=8.7, 15.6 Hz, 1 H), 6.12–6.19 (m, 2 H), 6.23 (d, J=6.0 Hz, 1 H), 7.13 (dt, J=1.8, 7.8 Hz, 1 H), 7.17 (dt, J=1.4, 7.8 Hz, 1 H), 7.26–7.40 (m, 12 H), 7.49 (dd, J=1.4, 7.8 Hz, 1 H), 7.73 (dd, J=1.8, 7.8 Hz, 1 H), 7.97 (dd, J=0.9, 7.8 Hz, 1 H), 8.01 ppm (dd, J=0.9, 7.8 Hz, 1 H); 13C NMR (100 MHz, CDCl3): δ=37.5 (q, J=29.9 Hz), 37.6 (q, J=29.9 Hz), 53.2, 53.5, 58.0, 58.1, 94.3, 94.6, 101.7, 101.8, 121.6 (q, J=2.9 Hz), 121.7 (q, J=3.8 Hz), 125.8 (q, J=276.5 Hz), 126.0 (q, J=276.5 Hz), 127.4, 127.4, 128.0, 128.1, 128.8, 128.8, 131.0, 131.2, 133.0, 133.2, 134.1, 134.6, 135.1, 135.5, 138.5, 138.6, 141.4, 141.7, 166.0, 166.1 ppm; 19F NMR (376 MHz, CDCl3): δ=−66.2 (t, J=10.8 Hz), −66.2 ppm (t, J=10.8 Hz); IR (neat):  =2937, 1732, 1287, 1252, 1138, 743 cm−1; HRMS (ESI): Calcd. for [C20H18F3IO3+Na]+: m/z=513.0145, Found: 513.0146.
=2937, 1732, 1287, 1252, 1138, 743 cm−1; HRMS (ESI): Calcd. for [C20H18F3IO3+Na]+: m/z=513.0145, Found: 513.0146.
Acknowledgments
This work was supported in part by Project Funding from RIKEN. We are grateful to Prof. Teiji Chihara and Prof. Hideki Kurokawa of Saitama University for helpful discussions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
References
- 1a.Kirsch P. Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications. Weinheim: Wiley-VCH; 2004. [Google Scholar]
- 1b.Bégué J-P, Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. Hoboken: Wiley-VHC; 2008. [Google Scholar]
- 1c.Ojima I. Medicinal Chemistry and Chemical Biology. Oxford: Wiley-Blackwell; 2009. Fluorine. [Google Scholar]
- 1d.Jeschke P. ChemBioChem. 2004;5:570–589. doi: 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]
- 1e.Böhm H-J, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Obst-Sander U, Stahl M. ChemBioChem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 1f.Shimizu M, Hiyama T. Angew. Chem. Int. Ed. 2005;44:214–231. doi: 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2005;117:218–234. [Google Scholar]
- 1g.Schlosser M. Angew. Chem. Int. Ed. 2006;45:5432–5446. doi: 10.1002/anie.200600449. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2006;118:5558–5572. [Google Scholar]
- 1h.Isanbor C, O'Hangan D. J. Fluorine Chem. 2006;127:303–319. [Google Scholar]
- 1i.Müller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 1j.Hagmann WK. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 1k.Gouverneur V, Müller K. Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications. London: Imperial College Press; 2012. Fluorine. [Google Scholar]
- 2a.Ma J-A, Cahard D. Chem. Rev. 2008;108:1–43. doi: 10.1021/cr800221v. For selected reviews on trifluoromethylation, see. [DOI] [PubMed] [Google Scholar]
- 2b.Roy S, Gregg BT, Gribble GW, Le V-D, Roy S. Tetrahedron. 2011;67:2161–2195. [Google Scholar]
- 2c.Tomashenko OA, Grushin VV. Chem. Rev. 2011;111:4475–4521. doi: 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]
- 2d.Wu X-F, Neumann H, Beller M. Chem. Asian J. 2012;7:1744–1754. doi: 10.1002/asia.201200211. [DOI] [PubMed] [Google Scholar]
- 2e.Studer A. Angew. Chem. Int. Ed. 2012;51:8950–8958. doi: 10.1002/anie.201202624. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:9082–9090. [Google Scholar]
- 2f.Ye Y, Snaford MS. Synlett. 2012;23:2005–2013. doi: 10.1055/s-0032-1316988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2g.Liu T, Shen Q. Eur. J. Org. Chem. 2012:6679–6687. [Google Scholar]
- 2h.Macé Y, Magnier E. Eur. J. Org. Chem. 2012:2479–2494. [Google Scholar]
- 2i.Liang T, Neumann CN, Ritter T. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:8372–8423. [Google Scholar]
- 2j.Merino E, Nevado C. Chem. Soc. Rev. 2014;43:6598–6608. doi: 10.1039/c4cs00025k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2k.Barata-Vallejo S, Lantano B, Postigo A. Chem. Eur. J. 2014;20:16806–16829. doi: 10.1002/chem.201404005. [DOI] [PubMed] [Google Scholar]
- 2l.Yang X, Wu T, Phipps R, Toste FD. Chem. Rev. 2015;115:826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Egami H, Sodeoka M. Angew. Chem. Int. Ed. 2014;53:8294–8308. doi: 10.1002/anie.201309260. For selected recent reviews on electrophilic trifluoromethylations, see. [DOI] [PubMed] [Google Scholar]
- 3b.Charpentier J, Früh N, Togni A. Chem. Rev. 2015;115:650–682. doi: 10.1021/cr500223h. [DOI] [PubMed] [Google Scholar]
- 4a.Xu J, Fu Y, Luo D-F, Jiang Y-Y, Xiao B, Liu Z-J, Gong T-J, Liu L. J. Am. Chem. Soc. 2011;133:15300–15303. doi: 10.1021/ja206330m. For selected recent reports on catalytic trifluoromethylation of alkenes, see. [DOI] [PubMed] [Google Scholar]
- 4b.Chu L, Qing F-L. Org. Lett. 2012;14:2106–2109. doi: 10.1021/ol300639a. [DOI] [PubMed] [Google Scholar]
- 4c.Mizuta S, Galicia-López O, Engle KM, Verhoog S, Wheelhouse K, Rassias G, Gouverneur V. Chem. Eur. J. 2012;18:8583–8587. doi: 10.1002/chem.201201707. [DOI] [PubMed] [Google Scholar]
- 4d.Mu X, Wu T, Wang H-Y, Guo Y-L, Liu G. J. Am. Chem. Soc. 2012;134:878–881. doi: 10.1021/ja210614y. [DOI] [PubMed] [Google Scholar]
- 4e.Zhu R, Buchwald SL. J. Am. Chem. Soc. 2012;134:12462–12465. doi: 10.1021/ja305840g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4f.Feng C, Loh T-P. Chem. Sci. 2012;3:3458–3462. [Google Scholar]
- 4g.Li Y, Studer A. Angew. Chem. Int. Ed. 2012;51:8221–8224. doi: 10.1002/anie.201202623. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:8345–8348. [Google Scholar]
- 4h.Yasu Y, Koike T, Akita M. Angew. Chem. Int. Ed. 2012;51:9567–9571. doi: 10.1002/anie.201205071. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:9705–9709. [Google Scholar]
- 4i.Kim E, Choi S, Kim H, Cho EJ. Chem. Eur. J. 2013;19:6209–6212. doi: 10.1002/chem.201300564. [DOI] [PubMed] [Google Scholar]
- 4j.Zhu R, Buchwald SL. Angew. Chem. Int. Ed. 2013;52:12655–12658. doi: 10.1002/anie.201307790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:12887–12890. [Google Scholar]
- 4k.Jiang X-Y, Qing F-L. Angew. Chem. Int. Ed. 2013;52:14177–14180. doi: 10.1002/anie.201307595. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:14427–14430. [Google Scholar]
- 4l.Lu D-F, Zhu C-L, Xu H. Chem. Sci. 2013;4:2478–2482. [Google Scholar]
- 4m.Kong W, Casimiro M, Merino E, Nevado C. J. Am. Chem. Soc. 2013;135:14480–14483. doi: 10.1021/ja403954g. [DOI] [PubMed] [Google Scholar]
- 4n.Gao P, Yan X-B, Tao T, Yang F, He T, Song X-R, Liu X-Y, Liang Y-M. Chem. Eur. J. 2013;19:14420–14424. doi: 10.1002/chem.201303025. [DOI] [PubMed] [Google Scholar]
- 4o.Wang F, Qi X, Liang Z, Chen P, Liu G. Angew. Chem. Int. Ed. 2014;53:1881–1886. doi: 10.1002/anie.201309991. [DOI] [PubMed] [Google Scholar]
- 4p.Wang F, Wang D, Mu X, Chen P, Liu G. J. Am. Chem. Soc. 2014;136:10202–10205. doi: 10.1021/ja504458j. [DOI] [PubMed] [Google Scholar]
- 5a.Eisenberger P, Gischig S, Togni A. Chem. Eur. J. 2006;12:2579–2586. doi: 10.1002/chem.200501052. For selected reports on Togni reagent, see. [DOI] [PubMed] [Google Scholar]
- 5b.Matoušek V, Pietrasiak E, Schwenk R, Togni A. J. Org. Chem. 2013;78:6763–6768. doi: 10.1021/jo400774u. [DOI] [PubMed] [Google Scholar]
- 5c.Fiederling N, Haller J, Schramm H. Org. Process Res. Dev. 2013;17:318–319. [Google Scholar]
- 6a.Shimizu R, Egami H, Nagi T, Chae J, Hamashima Y, Sodeoka M. Tetrahedron Lett. 2010;51:5947–5949. For our recent work on trifluoromethylations, see. [Google Scholar]
- 6b.Miyazaki A, Shimizu R, Egami H, Sodeoka M. Heterocycles. 2012;86:979–983. [Google Scholar]
- 6c.Shimizu R, Egami H, Hamashima Y, Sodeoka M. Angew. Chem. Int. Ed. 2012;51:4577–4580. doi: 10.1002/anie.201201095. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:4655–4658. [Google Scholar]
- 6d.Egami H, Shimizu R, Sodeoka M. Tetrahedron Lett. 2012;53:5503–5506. [Google Scholar]
- 6e.Egami H, Kawamura S, Miyazaki A, Sodeoka M. Angew. Chem. Int. Ed. 2013;52:7841–7844. doi: 10.1002/anie.201303350. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:7995–7998. [Google Scholar]
- 6f.Egami H, Shimizu R, Kawamura S, Sodeoka M. Angew. Chem. Int. Ed. 2013;52:4000–4003. doi: 10.1002/anie.201210250. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:4092–4095. [Google Scholar]
- 6g.Egami H, Shimizu R, Sodeoka M. J. Fluorine Chem. 2013;152:51–55. [Google Scholar]
- 6h.Egami H, Ide T, Fujita M, Tojo T, Hamashima Y, Sodeoka M. Chem. Eur. J. 2014;20:12061–12065. doi: 10.1002/chem.201403447. [DOI] [PubMed] [Google Scholar]
- 6i.Egami H, Shimizu R, Usui Y, Sodeoka M. J. Fluorine Chem. 2014;167:172–178. [Google Scholar]
- 6j.Kawamura S, Egami H, Sodeoka M. J. Am. Chem. Soc. 2015;137:4865–4873. doi: 10.1021/jacs.5b02046. [DOI] [PubMed] [Google Scholar]
- Egami H, Shimizu R, Usui Y, Sodeoka M. Chem. Commun. 2013;49:7346–7348. doi: 10.1039/c3cc43936d. [DOI] [PubMed] [Google Scholar]
- 8a.Liu X, Xiong F, Huang X, Xu L, Li P, Wu X. Angew. Chem. Int. Ed. 2013;52:6962–6966. doi: 10.1002/anie.201302673. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:7100–7104. [Google Scholar]
- 8b.Chen Z-M, Bai W, Wang S-H, Yang B-M, Tu Y-Q, Zhang F-M. Angew. Chem. Int. Ed. 2013;52:9781–9785. doi: 10.1002/anie.201304557. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:9963–9967. [Google Scholar]
- 9a.Brookes CJ, Coe PL, Pedler AE. J. Chem. Soc. Perkin Trans. 1. 1978:202–209. [Google Scholar]
- 9b.Müller N. J. Org. Chem. 1986;51:263–265. [Google Scholar]
- 9c.Uneyama K, Watanabe S. J. Org. Chem. 1990;55:3909–3912. [Google Scholar]
- 9d.Uneyama K. Tetrahedron. 1991;47:555–562. [Google Scholar]
- 9e.Uneyama K, Watanabe S, Tokunaga Y, Kitagawa K, Sato Y. Bull. Chem. Soc. Jpn. 1992;65:1976–1981. [Google Scholar]
- 9f.Billard T, Roques N, Langlois BR. Tetrahedron Lett. 2000;41:3069–3072. [Google Scholar]
- 9g.Tommasino T-B, Brondex A, Médebielle M, Thomalla M, Langlois BR. Synlett. 2002:1697–1699. [Google Scholar]
- 9h.Sato K, Omote M, Ando A, Kumadaki I. Org. Lett. 2004;6:4359–4361. doi: 10.1021/ol048134v. [DOI] [PubMed] [Google Scholar]
- 9i.Yajima T, Tonoi T, Nagano H, Tomita Y, Mikami K. Eur. J. Org. Chem. 2010:2461–2464. [Google Scholar]
- 9j.Erdbrink H, Peuser I, Gerling UIM, Lentz D, Koksch B, Czekelius C. Org. Biomol. Chem. 2012;10:8583–8586. doi: 10.1039/c2ob26810h. [DOI] [PubMed] [Google Scholar]
- 10a.Wu X, Chu L, Qing F-L. Angew. Chem. Int. Ed. 2013;52:2198–2202. doi: 10.1002/anie.201208971. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:2254–2258. [Google Scholar]
- 10b.Mizuta S, Verhoog S, Engle KM, Khotavivattana T, O'Duill M, Wheelhouse K, Rassias G, Médebielle M, Gouverneur V. J. Am. Chem. Soc. 2013;135:2505–2508. doi: 10.1021/ja401022x. [DOI] [PubMed] [Google Scholar]
- 10c.Wilger DJ, Gesmundo NJ, Nicewics DA. Chem. Sci. 2013;4:3160–3165. [Google Scholar]
- 10d.Pitre SP, McTiernan CD, Ismaili H, Scaiano JC. ACS catal. 2014;4:2530–2535. [Google Scholar]
- 11a.Parsons AT, Buchwald SL. Angew. Chem. Int. Ed. 2011;50:9120–9123. doi: 10.1002/anie.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2011;123:9286–9289. [Google Scholar]
- 11b.Wang X, Ye Y, Zhang S, Feng J, Xu Y, Zhang Y, Wang J. J. Am. Chem. Soc. 2011;133:16410–16413. doi: 10.1021/ja207775a. [DOI] [PubMed] [Google Scholar]
- 12a.Fuchikami T, Yatabe M, Ojima I. Synthesis. 1981:365–366. For selected reports on the synthesis of trifluoromethyl-substituted alkenes, see. [Google Scholar]
- 12b.Kitazume T, Ishikawa N. Chem. Lett. 1982:1453–1454. [Google Scholar]
- 12c.Fujita M, Hiyama T. Tetrahedron Lett. 1986;27:3655–3658. [Google Scholar]
- 12d.Umemoto T, Gotoh Y. Bull. Chem. Soc. Jpn. 1987;60:3307–3313. [Google Scholar]
- 12e.Shimizu M, Fujimoto T, Minezaki H, Hata T, Hiyama T. J. Am. Chem. Soc. 2001;123:6947–6948. [Google Scholar]
- 12f.Kobayashi T, Eda T, Tamura O, Ishibashi H. J. Org. Chem. 2002;67:3156–3159. doi: 10.1021/jo0111311. [DOI] [PubMed] [Google Scholar]
- 12g.Hanamoto T, Morita N, Shindo K. Eur. J. Org. Chem. 2003:4279–4285. [Google Scholar]
- 12h.Dubbaka SR, Vogel P. Chem. Eur. J. 2005;11:2633–2641. doi: 10.1002/chem.200400838. [DOI] [PubMed] [Google Scholar]
- 12i.Xu J, Luo D-F, Xiao B, Liu Z-J, Gong T-J, Fu Y, Liu L. Chem. Commun. 2011;47:4300–4302. doi: 10.1039/c1cc10359h. [DOI] [PubMed] [Google Scholar]
- 12j.Liu T, Shen Q. Org. Lett. 2011;13:2342–2345. doi: 10.1021/ol2005903. [DOI] [PubMed] [Google Scholar]
- 12k.Prakash GKS, Krishnan HS, Jog PV, Iyer AP, Olah GA. Org. Lett. 2012;14:1146–1149. doi: 10.1021/ol300076y. [DOI] [PubMed] [Google Scholar]
- 12l.Parsons AT, Senecal TD, Buchwald SL. Angew. Chem. Int. Ed. 2012;51:2947–2950. doi: 10.1002/anie.201108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:3001–3004. [Google Scholar]
- 12m.He Z, Luo T, Hu M, Cao Y, Hu J. Angew. Chem. Int. Ed. 2012;51:3944–3947. doi: 10.1002/anie.201200140. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:4010–4013. [Google Scholar]
- 12n.Omote M, Tanaka M, Ikeda A, Nomura S, Tarui A, Sato K, Ando A. Org. Lett. 2012;14:2286–2289. doi: 10.1021/ol300670n. [DOI] [PubMed] [Google Scholar]
- 12o.Hirotaki K, Kawazoe G, Hanamoto T. J. Fluorine Chem. 2015;171:169–173. [Google Scholar]
- 13a.Iqbal N, Choi S, Kim E, Cho EJ. J. Org. Chem. 2012;77:11383–11387. doi: 10.1021/jo3022346. [DOI] [PubMed] [Google Scholar]
- 13b.Feng C, Loh T-P. Angew. Chem. Int. Ed. 2013;52:12414–12417. doi: 10.1002/anie.201307245. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:12640–12643. [Google Scholar]
- 13c.Ilchenko NO, Janson PG, Szabó KJ. Chem. Commun. 2013;49:6614–6616. doi: 10.1039/c3cc43357a. [DOI] [PubMed] [Google Scholar]
- 13d.Wang X, Ye Y, Ji G, Xu Y, Zhang S, Feng J, Zhang Y, Wang J. Org. Lett. 2013;15:3730–3733. doi: 10.1021/ol4016095. [DOI] [PubMed] [Google Scholar]
- 13e.Wang X-P, Lin J-H, Zhang C-P, Xiao J-C, Zheng X. Beilstein J. Org. Chem. 2013;9:2635–2640. doi: 10.3762/bjoc.9.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13f.Fang Z, Ning Y, Mi P, Liao P, Bi X. Org. Lett. 2014;16:1522–1525. doi: 10.1021/ol5004498. [DOI] [PubMed] [Google Scholar]
- 13g.Cao X-H, Pan X, Zhou P-J, Zou J-P, Asekun OT. Chem. Commun. 2014;50:3359–3362. doi: 10.1039/c3cc49689a. [DOI] [PubMed] [Google Scholar]
- 13h.Besset T, Cahard D, Pannecoucke X. J. Org. Chem. 2014;79:413–418. doi: 10.1021/jo402385g. [DOI] [PubMed] [Google Scholar]
- 13i.Lin Q-Y, Xu X-H, Qing F-L. J. Org. Chem. 2014;79:10434–10446. doi: 10.1021/jo502040t. [DOI] [PubMed] [Google Scholar]
- 14a.Fuchikami T, Ojima I. Tetrahedron Lett. 1984;25:303–306. [Google Scholar]
- 14b.Maruoka K, Sano H, Fukutani Y, Yamamoto H. Chem. Lett. 1985;14:1689–1692. [Google Scholar]
- 14c.Takeyama Y, Ichinose Y, Oshima K, Uchimoto K. Tetrahedron Lett. 1989;30:3159–3162. [Google Scholar]
- 14d.Andrieux CP, Gelis L, Saveant J-M. Tetrahedron Lett. 1989;30:4961–4964. [Google Scholar]
- 14e.Kamigata N, Fukushima T, Terakawa Y, Yoshida M, Sawada H. J. Chem. Soc. Perkin Trans. 1. 1991:627–633. [Google Scholar]
- 14f.Tsuchii K, Imura M, Kamada N, Hirao T, Ogawa A. J. Org. Chem. 2004;69:6658–6665. doi: 10.1021/jo0495889. [DOI] [PubMed] [Google Scholar]
- 14g.Ignatowska J, Dmowski W. J. Fluorine Chem. 2007;128:997–1006. [Google Scholar]
- 14h.Yajima T, Nagano H. Org. Lett. 2007;9:2513–2515. doi: 10.1021/ol0707620. [DOI] [PubMed] [Google Scholar]
- 14i.Tonoi T, Nishikawa A, Yajima T, Nagano H, Mikami K. Eur. J. Org. Chem. 2008:1331–1335. [Google Scholar]
- 14j.Wallentin C-J, Nguyen JD, Finkbeiner P, Stephenson CRJ. J. Am. Chem. Soc. 2012;134:8875–8884. doi: 10.1021/ja300798k. [DOI] [PubMed] [Google Scholar]
- 14k.Oh SH, Malpani YR, Ha N, Jung Y-S, Han SB. Org. Lett. 2014;16:1310–1313. doi: 10.1021/ol403716t. [DOI] [PubMed] [Google Scholar]
- 14l.Hang Z, Li Z, Liu Z-Q. Org. Lett. 2014;16:3648–3651. doi: 10.1021/ol501380e. [DOI] [PubMed] [Google Scholar]
- 14m.Xu T, Cheung CW, Hu X. Angew. Chem. Int. Ed. 2014;53:4910–4914. doi: 10.1002/anie.201402511. [DOI] [PubMed] [Google Scholar]
- 15a.Janson PG, Ghoneim I, Ilchenko NO, Szabó KJ. Org. Lett. 2012;14:2882–2885. doi: 10.1021/ol3011419. [DOI] [PubMed] [Google Scholar]
- 15b.Ilchenko NO, Janson PG, Szabó KJ. J. Org. Chem. 2013;78:11087–11091. doi: 10.1021/jo401831t. [DOI] [PubMed] [Google Scholar]
- Compound 5 was obtained even in the reaction without substrate 2 a under the standard conditions, thus suggesting that DMF could independently react with the Togni reagent under basic conditions.
- 17a.Kieltsch I, Eisenberger P, Togni A. Angew. Chem. Int. Ed. 2007;46:754–757. doi: 10.1002/anie.200603497. Trifluoromethylations of other types of substrates using Togni reagent and TBAI were reported, see. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2007;119:768–771. [Google Scholar]
- 17b.Kong W, Casimiro M, Fuentes N, Merino E, Nevado C. Angew. Chem. Int. Ed. 2013;52:13086–13090. doi: 10.1002/anie.201307377. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:13324–13328. [Google Scholar]
- 17c.Zhang B, Mück-Lichtenfeld C, Daniliuc CG, Studer A. Angew. Chem. Int. Ed. 2013;52:10792–10795. doi: 10.1002/anie.201306082. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2013;125:10992–10995. [Google Scholar]
- 17d.Li L, Guo J-Y, Liu X-G, Chen S, Wang Y, Tan B, Liu X-Y. Org. Lett. 2014;16:6032–6035. doi: 10.1021/ol503067g. [DOI] [PubMed] [Google Scholar]
- 18a.Newcomb M, Chestney DL. J. Am. Chem. Soc. 1994;116:9753–9754. [Google Scholar]
- 18b.Toy PH, Newcomb M, Hollenderg PF. J. Am. Chem. Soc. 1998;120:7719–7729. [Google Scholar]
- 18c.Newcomb M, Toy PH. Acc. Chem. Res. 2000;33:449–455. doi: 10.1021/ar960058b. [DOI] [PubMed] [Google Scholar]
- 18d.Phipps RJ, McMurray L, Ritter S, Duong HA, Gaunt MJ. J. Am. Chem. Soc. 2012;134:10773–10776. doi: 10.1021/ja3039807. [DOI] [PubMed] [Google Scholar]
- The nature of the active species in the present reactions is not clear. Nevado proposed that Togni reagent was activated by coordination of an iodide ion, see Ref. [17b]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


