Abstract
Sodium-pumping rhodopsins (NaRs) are light-driven outward Na+ pumps. NaRs have a conserved Asn, Asp, and Gln motif (NDQ) in the third transmembrane helix (helix C). The NDQ motif is thus expected to play a crucial role in the operation of the Na+ pump. Herein, we studied the photocycles of the NDQ-motif mutants of Krokinobacter rhodopsin 2 (KR2), the first discovered NaR, by flash photolysis, to obtain insight into the mechanism of Na+ transport. For example, the KR2 N112A mutant did not accumulate the transient red-shifted Na+-bound state, suggesting that Asn112 is vital for the binding of Na+ ions. Additionally, Q123A and Q123V mutants showed significantly slower Na+ uptake and recovery of the initial state. Overall, the Gln123 residue was found to contribute to the optimization of the kinetics of sodium-ion uptake and release. These results demonstrate that the cooperative operation of the three residues of the NDQ motif are important in the operation of the Na+ pump.
Keywords: kinetics, photolysis, proteins, rhodopsin, sodium pump
Microbial rhodopsins are heptahelical transmembrane photoreceptive proteins widely found in eubacteria, archaea, and some eukaryotes.[1] The most ubiquitous rhodopsins are light-driven outward H+ pumps, such as bacteriorhodopsin (BR)[2] from archaea and proteorhodopsin (PR) from eubacteria.[3] However, until recently light-driven pumps of non-proton cations had never been found. The absence of pumps of this type seemed to be reasonable because the chromophore retinal, located in the middle of rhodopsin and forming a protonated Schiff base linkage with the protein moiety (opsin), is likely to inhibit the transport of non-proton cations. However, a novel sodium-ion-pumping rhodopsin was identified in the genome of the marine flavobacterium Krokinobacter eikastus in 2013.[4] This protein, called Krokinobacter rhodopsin 2 (KR2), outwardly pumps Na+ ions upon illumination. Interestingly, KR2 pumps H+ ions in the presence of larger cations such as K+, Rb+, and Cs+. Given this unexpected finding of a Na+ pump, the molecular mechanism of how Na+ is transported over the protonated Schiff base is intriguing.
A clue to the function of KR2 may exist in the amino acid sequence of the protein. After the discovery of KR2, more than five homologous proteins, termed sodium pump rhodopsins (NaRs), were reported.[5] All NaRs have a common feature in the sequence of the third transmembrane helix (helix C). H+ pumps have conserved acidic residues acting as H+ acceptors and donors (Asp85 and Asp96 of BR, respectively). The H+ acceptor, which also functions as a Schiff base counterion, interacts with a conserved Thr residue (Thr89 of BR).[6] These three residues, which play an important role in H+ transport, are referred to as the DTD(E) motif because the H+ donor is Glu for PR. In contrast, in NaR, Asn and Gln residues replace the H+ acceptor and donor within the H+ pumps, respectively (Figure 1). Separately, in NaRs, a new Asp residue is introduced at the Thr89 position of BR.[4, 5] These three residues, collectively referred to as the NDQ motif, are characteristic of NaR and are considered to play a crucial role in Na+ transport.
Figure 1.
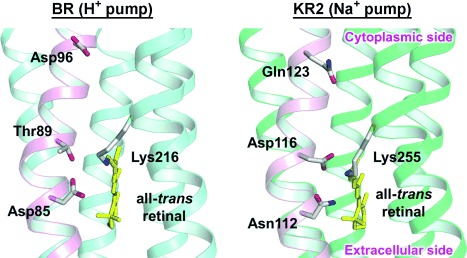
The structure of the DTD(E) and NDQ motifs in helix C (given in pink) of BR and KR2, respectively, based on their crystal structure. PDB codes: 1M0L (BR) and 3X3C (KR2).[5, 7]
The roles of the three residues of the KR2 NDQ motif (Asn112, Asp116, and Gln123) have been previously partially studied.[4, 5] The Asn112-to-Ala mutant (denoted N112A) pumps protons even in the solvent containing Na+.[4] In the D116N mutant, both Na+ and H+ pump functions were completely lost.[4] Recent crystal structures of KR2 at acidic and neutral pH values displayed a change in the orientation of Asp116, suggesting that Asp116 interacts with the Schiff base in the dark, whereas protonated Asp116 forms hydrogen bonds with Asn112 and Ser70 in the M state.[4] The effect of mutation for Gln123 was less significant than those of Asn112 and Asp116. The Q123A mutant showed sodium- and proton-pump activities in NaCl and KCl solutions, respectively, although they were lower than those of wild-type (WT) KR2.[4] These results suggest that the residues in the NDQ motif play specific roles in the function of NaR. To obtain more detailed information, herein we studied the photocycles of the mutants in the NDQ motif of KR2 and also of a newly designed Q123V mutant.
Figure 2 a–e show the UV/Vis absorption spectra of WT KR2 and each mutant, which were heterologously expressed by E. coli, purified by histidine-tagged and cobalt NTA affinity chromatography, and solubilized in n-dodecyl-β-d-maltoside (DDM; 0.1 %) at pH 8 in the presence of NaCl (100 mm). WT KR2 had an absorption maximum (λmax) at λ=524 nm and the N112A, Q123A, and Q123V mutant showed similar λmax values (Figure 2 b–d). In contrast, the absorption maximum of D116N was largely red-shifted from WT KR2 (λmax=570 nm; Figure 2 e). Thus, Asp116 is deprotonated at pH 8 and acts as the counterion to the retinal Schiff base in KR2.[5]
Figure 2.
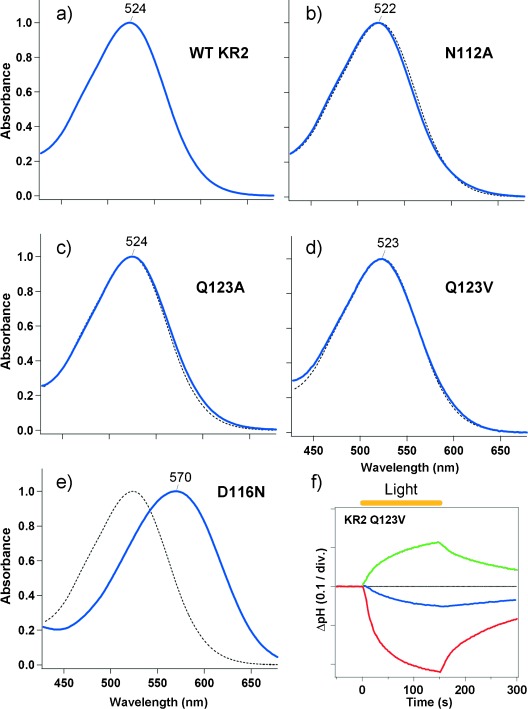
UV/Vis absorption spectra of a) WT KR2, b) N112A, c) Q123A, d) Q123V, and e) D116N. The spectra of the mutants (solid blue line) are overlaid with that of the WT (dotted line). Axis labels and scales in (a–e) are the same as given in (e). f) The ion-transport activity of Q123V in E. coli cells in 100 mm NaCl (blue) or KCl (red). The green line shows the pH change in a 100 mm NaCl suspension with 10 μm CCCP. One division (div.) in the vertical axis in (f) represents 0.1 units of pH change.
The pump activities of WT KR2 and the mutants, except for Q123V, have already been reported (the results are reproduced in Figure S1 in the Supporting Information).[4] In NaCl, WT KR2 showed an increase in solution pH value upon light illumination, resulting from a secondary H+ uptake against the additional membrane potential caused by an outward pumping of Na+ ions. This pH change was enhanced by the addition of the protonophore CCCP (carbonyl cyanide m-chlorophenylhydrazone). In KCl, a decrease in pH value was detected caused by outward pumping of H+ ions. N112A showed a similar decrease in pH value caused by an outward pumping of H+ ions, even in the presence of NaCl (Figure S1).[4] This indicates that Asn112 is vital for Na+ transport, but not for the transport of H+ ions. D116N shows no pump activity at all (Figure S1).[4] The pump function of Q123A was similar to WT KR2 but its efficiency was lower (Figure S1).[4]
In this study, we assayed the pump activity of Q123V, which showed a decrease in pH value in NaCl (Figure 2 f, blue line), indicating that Q123V pumps H+, even in NaCl. However, if CCCP is added, an increase in pH value was detected (Figure 2 f, green line). This result strongly suggests that Q123V pumps Na+ ions in a manner that competes with the pumping of protons. In KCl, a further decrease in pH value was detected (Figure 2 f, red line), presumably because H+ pumping did not compete with Na+. In our previous report, we observed only separately functioning Na+ or H+ pumps in WT and KR2 mutants.[4] Thus, the result reported herein for Q123V is the first example of competitive Na+ and H+ pumping. Competitive Na+ and H+ pumps in Q123V suggest a common transport mechanism, and the selection of ions is determined by kinetic factors in the cytoplasmic domain. WT KR2 may be more selective for Na+ ions than for H+ ions.
We next measured transient absorption changes for KR2 mutants as described previously.[4] Briefly, the proteins were reconstituted into lipid bilayers (1,2-dioleoyl-sn-glycero-3-phosphocholine, DOPC) in a solution containing NaCl (100 mm) and Tris HCl (50 mm; pH 8.0). The sample was excited by the second harmonic of a nanosecond-pulsed Nd3+:YAG laser (λ=532 nm). The change in absorption of the sample upon laser excitation was probed by the output of a Xe lamp.
The transient-absorption spectroscopic results for WT KR2, D116N, and Q123A have been previously reported.[4, 5] The WT KR2 photocycle has four photointermediates (K, L, M, and O; Figure S2a)[4, 8] and is expressed according to Equation (1):
| (1) |
The photocycle of D116N showed only the K-like intermediate, which decays to the initial state with τ=12.7 ms (Figure S2b).[4] Q123A shows a similar photocycle to WT (Figure S2c), but the decay rate of L/M and O were 5.6 and 2.6 times lower than those of WT KR2 (Table 1).[4]
Table 1.
Decay time constants of L/M (τL/M) and O (τO) intermediates of WT KR2 and its mutants
| Protein | τL/M [ms] | τL/M/τL/M,WT[a] | τO [ms] | τO/τO,WT[a] |
|---|---|---|---|---|
| WT KR2 | 1.0±0.1[c] | – | 8.7±0.6[b, c] | – |
| KR2 N112A | 1200±400 | 1200±400[b] | nd | – |
| KR2 D116N | nd | – | nd | – |
| KR2 Q123A | 5.6±0.4 | 5.6±0.7[d] | 22.4±1.7 | 2.6±0.3[d] |
| KR2 Q123V | 16.3±1.5 | 16.3±2.2 | 157±6 | 18.2±1.5 |
In this study, we also measured transient-absorption changes of N112A and Q123V (Figure 3). The change in the transient absorption profile of N112A was much longer than WT and other mutants, and it persisted for more than 8 s (Figure 3 a). Furthermore, no accumulation of the O photointermediate was detected. The long-lived M state had a lifetime 1200 times longer than that of WT (Table 1). A very slow photocycle in N112A, without the formation of the O state, is similar to that of H+-pumping WT KR2 in KCl solution (Figure S2d).[4] Therefore, the N112A photocycle is consistent with functioning exclusively as a H+ pump even in NaCl solution. On the other hand, the transient absorption change of Q123V was similar to that of Q123A. However, the decays of L/M and O were even slower than WT, 16.3 and 157 ms, respectively (Table 1). This suggests that Gln123 optimizes the protein structure to achieve rapid Na+ uptake (L/M-to-O state conversion) and release (O-state decay), and replacement with a hydrophobic residue lowers its efficiency.
Figure 3.
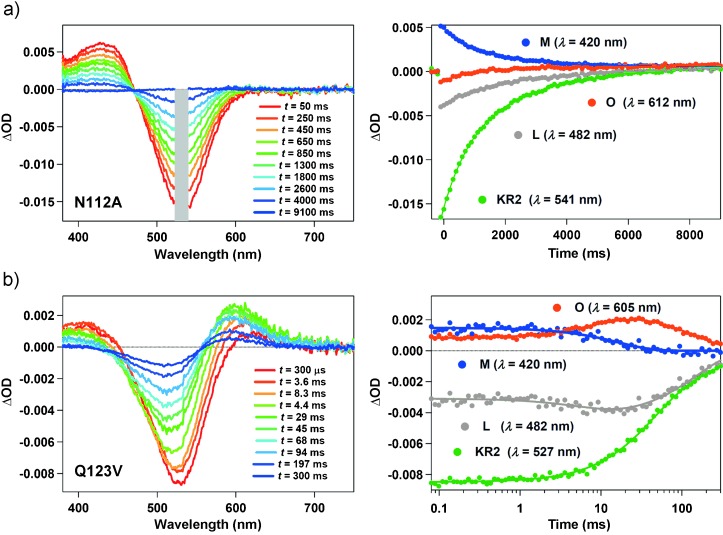
Transient absorption spectra (left) and transient absorption spectral changes at specific wavelengths (right, filled circles) of a) N112A and b) Q123V. The solid lines in the right panels show the multiexponential fit to the data. OD=optical density. As a notch filter is inserted on the pathway of the probe light to remove scattered laser beam, the absorption change in the region indicated by a gray rectangle could not be detected for N112A.
In the present study, we investigated the spectroscopic properties and photoreaction processes of mutants of the KR2 NDQ motif. The highly red-shifted spectrum of D116N (Figure 2 e) shows that Asp116 is deprotonated and acts as the Schiff base counterion in the dark.[5] The photocycle of D116N showed only the K-like intermediate which recovered to the initial state without any further transition to the L/M state. This implies that Asp116 is the H+ acceptor from the retinal Schiff base upon formation of the M state, and that subsequent photoreaction processes, including formation of L/M and O states, do not occur when H+ transfer is inhibited by this mutation (Figure 4 a).
Figure 4.
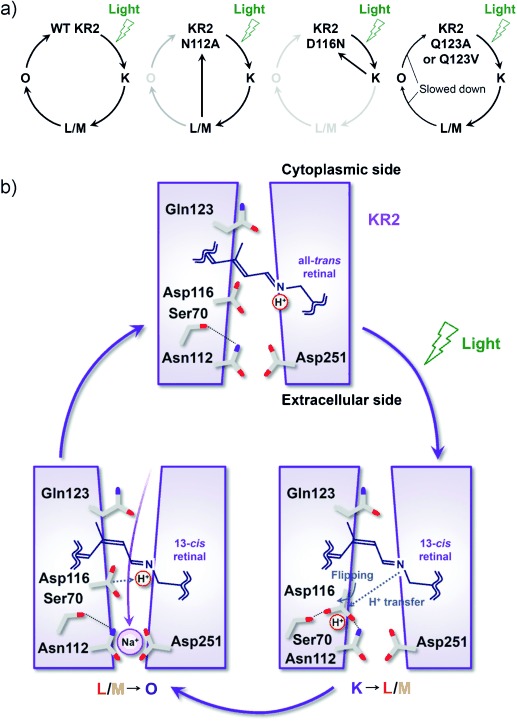
a) A schematic model of the photocycles of WT KR2 and its mutants. b) The Na+-transport mechanism of KR2 suggested from the results of the present and previous studies.[4, 5, 9]
In contrast, the N112A photocycle showed only the blue-shifted L/M state (Figure 3 a), and the formation of the following O state was inhibited (Figure 4 a). It was revealed that Na+ uptake occurs upon O-state formation, in which the O state is the Na+-bound state near the retinal Schiff base.[4, 9] The absence of the O photointermediate in N112A suggests that Asn112 plays a critical role in the Na+ binding required for O-state formation. Based on the present results, we show a schematic model of the Na+-ion pump mechanism during the KR2 photocycle in Figure 4 b. H+ transfer to Asp116 and Na+ binding to Asn112 is the main process for the formation of the (L/)M and O states, respectively. The crystallographic study of KR2 at acidic and neutral pH values suggested that deprotonated Asp116 interacts with the retinal Schiff base in the dark, whereas protonated Asp116 forms hydrogen bonds with Asp112 and Ser70.[5] Following this rearrangement of the side chain of Asp116, a H+ ion from the retinal Schiff base is sequestered from the Na+-conducting pathway (Figure 4 b, K→L/M), and Na+ uptake occurs from the cytoplasmic medium to the binding site near the retinal Schiff base and Asn112 (Figure 4 b, L/M→O).[4, 5] The dissociation constant of Na+ binding in the O intermediate was estimated to be approximately 60 μm, where Asp251 (homologue of Asp212 of BR) is involved in the binding.[9] Therefore, both Asp112 and Asp251 participate in Na+ binding in the O state, and Na+ may be bound between them (Figure 4 b). In this case, multiple-coordination might occur between Na+ ions and carbonyl and/or carboxylate groups of these residues. In fact, such multiple coordination of Na+ ions with amino acid residues was detected in the Na+ binding site of the dark state of KR2[10] and V-type Na+-ATPase.[11]
The mutation of Gln123 (Q123A and Q123V) slowed down the L/M-to-O and O-to-KR2 processes (Table 1). The crystallographic structure of KR2 revealed a large cavity, the ion-uptake cavity, between the cytoplasmic surface of the protein and Gln123,[5, 10] which constitutes the Na+ transport pathway in the cytoplasmic half of the protein. Thus, the slower L/M-to-O conversion for the Gln123 mutants suggests that Gln123 optimizes the structure of the conduction pathway for efficient Na+ uptake. On the other hand, the O-to-KR2 process gives rise to the release of Na+ ions from the retinal binding site to the extracellular region. During this process, sodium ions are conveyed by interacting with the residues in the extracellular half of the protein but the Gln123 residue does not directly accompany it. However, the Q123A and Q123V mutations significantly decelerated decay of the O photointermediate. This may suggest that the Gln123 residue optimizes the structure of the extracellular half of the protein allosterically to achieve rapid Na+ release.
In this study, we report the role of the NDQ amino acid motif in the operation of the light-driven Na+-pump functionality of KR2. We showed that the Asn112 and Asp116 residues are prerequisites for the binding of Na+ ions and accepting protons from the retinal Schiff base in the O and M photointermediates, respectively. Additionally, incorporation of the Gln123 residue optimizes the protein structure to achieve rapid Na+ uptake and release. Notably, the Q123V mutant showed competing transport of Na+ and H+ ions, suggesting that the Gln123 residue is important for the selection of ions. It was recently reported that KR2 can serve as a next-generation optogenetic tool.[5] With this in mind, specific roles of the NDQ motif will be investigated for future development of novel optogenetic tools based on NaR.
Acknowledgments
This work was supported by JSPS KAKENHI Grant to K.I. (26708001, 26115706, and 26620005) and to H.K. (25104009, 15H02391).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H. Chem. Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oesterhelt D, Stoeckenius W. Nat. New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 3.Béjà O, et al. Science. 2000;289:1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Ono H, Abe-Yoshizumi R, Yoshizawa S, Ito H, Kogure K, Kandori H. Nat. Commun. 2013;4:1678. doi: 10.1038/ncomms2689. [DOI] [PubMed] [Google Scholar]
- 5.Kato HE, et al. Nature. 2015;521:48–53. doi: 10.1038/nature14322. [DOI] [PubMed] [Google Scholar]
- 6.Kandori H, Yamazaki Y, Shichida Y, Raap J, Lugtenburg J, Belenky M, Herzfeld J. Proc. Natl. Acad. Sci. USA. 2001;98:1571–1576. doi: 10.1073/pnas.98.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schobert B, Cupp-Vickery J, Hornak V, Smith S, Lanyi J. J. Mol. Biol. 2002;321:715–726. doi: 10.1016/s0022-2836(02)00681-2. [DOI] [PubMed] [Google Scholar]
- 8.Ono H, Inoue K, Abe-Yoshizumi R, Kandori H. J. Phys. Chem. B. 2014;118:4784–4792. doi: 10.1021/jp500756f. [DOI] [PubMed] [Google Scholar]
- 9.Balashov SP, Imasheva ES, Dioumaev AK, Wang JM, Jung K-H, Lanyi JK. Biochemistry. 2014;53:7549–7561. doi: 10.1021/bi501064n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gushchin I, et al. Nat. Struct. Mol. Biol. 2015;22:390–395. doi: 10.1038/nsmb.3002. [DOI] [PubMed] [Google Scholar]
- 11.Murata T, Yamato I, Kakinuma Y, Leslie AG, Walker JE. Science. 2005;308:654–659. doi: 10.1126/science.1110064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


