Abstract
The Ru-catalysed C2–H arylation of indoles and pyrroles by using boronic acids under oxidative conditions is reported. This reaction can be applied to tryptophan derivatives and tolerates a wide range of functional groups on both coupling partners, including bromides and iodides, which can be further derivatised selectively. New indole-based ruthenacyclic complexes are described and investigated as possible intermediates in the reaction. Mechanistic studies suggest the on-cycle intermediates do not possess a para-cymene ligand and that the on-cycle metalation occurs through an electrophilic attack by the Ru centre.
Keywords: catalysis, C–H activation, heterocycles, reaction mechanisms, ruthenium
Introduction
Transition metal-catalysed C–H activation has received enormous attention in recent years as a viable synthetic strategy; it offers previously impossible transformations, new selectivities and shortened routes in the preparation of organic molecules.[1, 2] The importance of indole and pyrrole units in bioactive molecules has fuelled continued efforts to develop new approaches to their selective C–H functionalisation.[3] For instance, the 2-arylindole unit appears in several natural products,[4] potential microtubulin polymerisation inhibitors, and molecules of interest for their antifungal and antimicrobial properties.[5] To date, indole and pyrrole C–H arylation has been developed most extensively by using aryl halide[6] and hypervalent iodine[7] electrophiles. Meanwhile, the complementary method in which anionic coupling partners[8] and an external oxidant are employed has received less attention. The dehydrogenative coupling of two (hetero)arene C–H units[9] represents, in principle, the most atom economical and desirable of these approaches. However, at present, the conjunction of two selective, compatible C–H activation processes often presents regioselectivity/scope limitations, the need for a large excess of one coupling partner, harsh conditions, and/or expensive additives. In this context, organoboronates[10] are an attractive alternative for oxidative direct arylation reactions,[11–14] by offering low cost, diversity, and ease of activation, amongst other advantages.
In Ru-catalysed C–H functionalisation reactions, which have attracted considerable recent interest (Figure 1),[15, 16] the use of boronates has remained rare. Ru0-catalysed C–H arylation reactions developed by the groups of Kakiuchi,[17] and later Sames[18] and Schnürch,[19] require protected boronates. Unprotected boronic acids have been used in RuII-catalysed C–H arylation reactions with only a handful of substrates[20] and virtually no accompanying mechanistic investigation, despite a recent surge in the development of various related oxidative transformations.[21] In addition, the use of aryl halides by Ackermann and Lygin[6r] under carboxylate-assisted conditions[22] stands as the only example to date of Ru-catalysed indole or pyrrole C–H arylation.[23]
Figure 1.
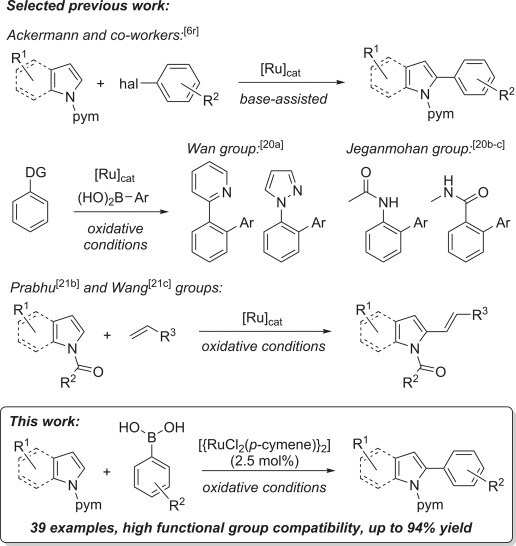
Selected Ru-catalysed C–H transformations relating to this study. Pym=2-pyrimidyl.
Herein, we report the use of unprotected, diversely functionalised arylboronic acids in the Ru-catalysed C2–H arylation of indoles and pyrroles. We also describe the synthesis of new ruthenacyclic complexes and examine their role in the reaction, which is of direct relevance to commonly proposed mechanisms.
Results and Discussion
Reaction optimisation
We undertook our investigation with indole derivative 1 a as the test-bed substrate. Sames and co-workers[18] and Ackermann and Lygin[6r, 24] pioneered the use of pyrimidine as a versatile, removable directing group[25] for catalytic C–H functionalisation. A number of groups have since described the use of this species in both the synthesis[24, 26] and derivatisation of indoles and pyrroles by using various metals.[27–32]
In our initial experiments, we isolated 2 a in 37 % yield from the coupling of 1 a and 4-tolylboronic acid with 2.5 mol % [{RuCl2(p-cymene)}2] and 1.5 equivalents of Ag2O in THF (Table 1, entry 1). Replacement of the oxidant with Cu(OAc)2 ⋅H2O (1 equiv) improved the yield to 64 % and including water raised the yield further to 86 % (Table 1, entry 3). A combination of substoichiometric amounts of Cu(OAc)2⋅H2O (0.5 equiv) and O2 proved less effective (Table 1, entry 4). As an additive, AgSbF6 proved superior to AgBF4, AgPF6, and KPF6. The addition of organic or inorganic bases, including carboxylates and carbonates (see Table 1, entry 5 and Table S5 in the Supporting Information),[33] was detrimental to the catalysis, whereas HOAc (up to 4 equiv) had no effect on the yield.[34] Replacement of the catalyst precursor with [Ru(OAc)2(p-cymene)] (5 mol %) or the more expensive [{RhCp*Cl2}2] (2.5 mol %; Cp*=1,2,3,4,5-pentamethylcyclopentadienyl) gave lower yields (Table 1, entries 9 and 10, respectively). As solvent, iPrOH proved slightly superior to the THF/H2O system (Table 1, entries 7 and 8), presumably due to the formation of alkoxyboronates in situ, giving improved nucleophile solubility and slower protodeborylation.[10] Accordingly, iPrOH was selected as the default solvent for the rest of our exploration of the reaction scope. All the arylation reactions occurred exclusively at C2. The desired reaction was not observed if the Ru catalyst, oxidant, or silver additive were excluded. Under the same conditions, arylation did not occur with indoles bearing oxygen-based directing groups, nor with a variety of other substrates commonly used in Ru-catalysed C–H arylation reactions (see the Supporting Information for further details). Thus, the pyrimidyl group seemed uniquely privileged under the conditions we explored.
Table 1.
Selected results from optimization studies[a]
| Entry | Catalyst [mol %] | Oxidant [equiv] | Additives [equiv] | Solvent | Yield[b] [%] |
|---|---|---|---|---|---|
| 1 | [{RuCl2(p-cymene)}2] (2.5) | Ag2O (1.5) | AgSbF6 (0.12) | THF | 37[c] |
| 2 | [{RuCl2(p-cymene)}2] (2.5) | Cu(OAc)2⋅H2O (1) | AgSbF6 (0.12) | THF | 64 |
| 3 | [{RuCl2(p-cymene)}2] (2.5) | Cu(OAc)2⋅H2O (1) | AgSbF6 (0.12) H2O (3.7) | THF | 86 |
| 4 | [{RuCl2(p-cymene)2}] (2.5) | Cu(OAc)2⋅H2O (0.5) O2 (balloon) | AgSbF6 (0.12) H2O (3.7) | THF | 56 |
| 5 | [{RuCl2(p-cymene)}2] (2.5) | Cu(OAc)2⋅H2O (1) | AgSbF6 (0.12) KOAc (1.0) H2O (3.7) | THF | 0 |
| 6 | [{RuCl2(p-cymene)}2] (2.5) | Cu(OAc)2⋅H2O (1) | AgOAc (0.12) H2O (3.7) | THF | 0 |
| 7 | [{RuCl2(p-cymene)}2] (2.5) | Cu(OCOCF3)2 (1) | AgSbF6 (0.12) | iPrOH | 89 |
| 8 | [{RuCl2(p-cymene)}2] (2.5) | Cu(OAc)2⋅H2O (1) | AgSbF6 (0.12) | iPrOH | 98 |
| 9 | [Ru(OAc)2(p-cymene)] (5) | Cu(OAc)2⋅H2O (1) | AgSbF6 (0.12) | THF | 53 |
| 10 | [{Cp*RhCl2}2] (2.5) | Cu(OAc)2⋅H2O (1) | AgSbF6 (0.12) H2O (3.7) | THF | 52 |
[a] 1 a (0.15 mmol), boronic acid (0.45 mmol), solvent (0.5 mL). [b] Yield was determined by 1H NMR spectroscopic analysis with respect to 1,3,5-trimethoxybenzene (0.05 mmol), which was added after the end of the reaction. [c] Yield of the isolated product.
Substrate scope
The reaction showed high tolerance towards a wide range of arylboronic acids, including those bearing nitro (2 h), ketone (2 i), alkenyl (2 k), silyl (2 l), sulfonyl (2 m), ferrocenyl (2 w), and halogen groups (Scheme 1). Tolerance of aryl iodides (2 f) is both rare and complementary to their frequent use as oxidants/electrophiles in C–H arylation reactions. To the best of our knowledge, this C–H arylation of indoles is the first in which the C–I functionality is preserved. Reactions with 4-trifluoromethyl- and 4-acetylphenylboronic acids gave higher yields when iPrOH was replaced with the THF/H2O solvent system. Protected boronic acids,[10, 35] such as their pinacol boronate (-Bpin), N-methyliminodiacetyl boryl (-Bmida),[36] or potassium trifluoroborate (-BF3K)[37] derivatives, were not effective under our conditions. This outcome is in line with the requirement for their prior hydrolysis and the rate acceleration observed under acidic conditions in related C–H arylation reactions.[34] Variously substituted indoles also selectively underwent the C–H arylation reaction (Scheme 2). Good yields were obtained with bromide and iodide groups on either or both coupling partners (Scheme 2; 3 e and 3 i–k). The 3-methylindole derivative 3 g was obtained in 75 % yield, despite the proximate steric bulk, whereas indoles with 6-methoxycarbonyl (3 c), 5-nitro (3 f), and 3-cyano (3 h) groups gave worse performance, hinting at the importance of the nucleophilicity of the indole unit. The protocol could further be extended to the phthaloyl-protected tryptophan derivative 1 m[38] (Scheme 3). In addition to standard analysis by NMR spectroscopy and mass spectrometry, products 2 t, 3 j, and 3 m were characterised by X-ray crystallography studies.
Scheme 1.
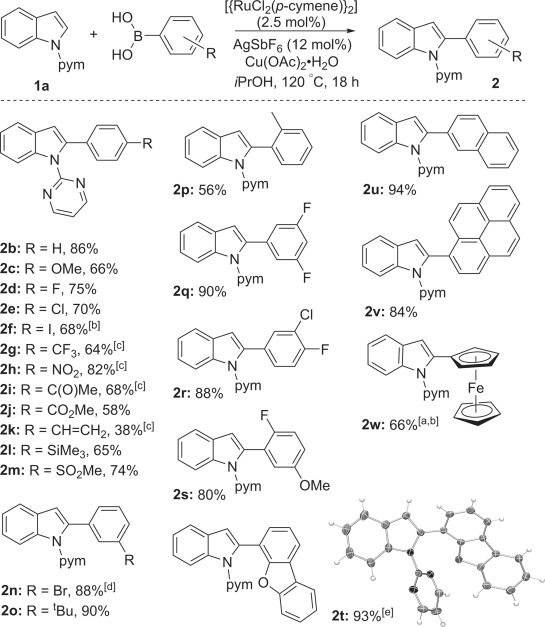
Scope of the boronic acids in the Ru-catalysed indole C2–H arylation reaction (the yields given are for the isolated products). Conditions: 1 a (0.5 mmol), boronic acid (1.5 mmol), [{RuCl2(p-cymene)}2] (2.5 mol %), AgSbF6 (12 mol %), Cu(OAc)2⋅H2O (0.5 mmol), and iPrOH (1.5 mL). [a] 2 mmol boronic acid was used. [b] t=4 h, T=100 °C. [c] Solvent system: THF (1.5 mL + 3.7 equiv water). [d] t=3 h. [e] The crystal structure ellipsoids are shown at 50 % probability.
Scheme 2.
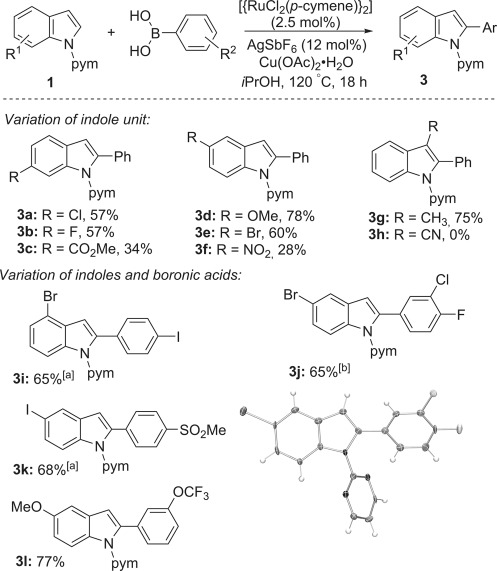
Scope of the indoles and boronic acids (the yields given are for the isolated products). Conditions as in Scheme 1, except for [a] t=6 h. [b] The crystal structure ellipsoids are shown at 50 % probability.
Scheme 3.
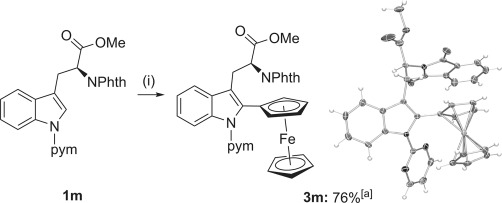
Arylation of a tryptophan derivative. Conditions: i) 1 m (0.25 mmol), ferroceneboronic acid (1.0 mmol), [{RuCl2(p-cymene)}2] (2.5 mol %), AgSbF6 (12 mol %), Cu(OAc)2⋅H2O (0.25 mmol), iPrOH (2.0 mL), 120 °C, 18 h. [a] The crystal structure ellipsoids are shown at 50 % probability. Phth=phthalate.
Pyrroles also proved amenable to the arylation reaction with various boronic acids (Scheme 4). Substrate 4 a (R1=H) gave a separable mixture of mono- (5 a) and diphenylated (5 a′) products in a combined yield of 42 %. The 2-ethylpyrrole derivatives 4 b–d afforded good-to-excellent yields, with arylation occurring exclusively at C2 for both electron-poor and electron-rich boronic acids to give products 5 b–d. 2-Methoxycarbonyl-substituted pyrrole 5 e was not formed, which is consistent with the nucleophilicity requirements of the indole substrates.
Scheme 4.
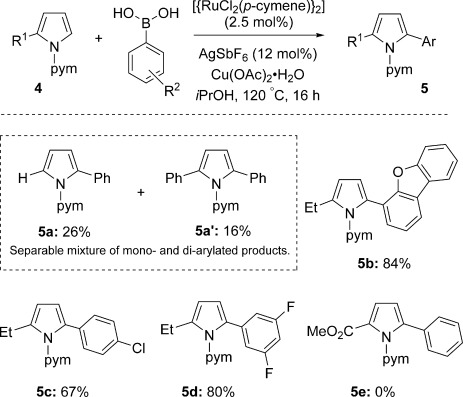
Arylation of pyrrole derivatives. Reagents: 4 (0.5 mmol), boronic acid (1.5 mmol), [{RuCl2(p-cymene)}2] (2.5 mol %), AgSbF6 (12 mol %), Cu(OAc)2⋅H2O (0.5 mmol), iPrOH (1.5 mL).
The iodide group of 3 i underwent selective and efficient Heck alkenylation, highlighting the potential for derivatisation of the newly installed aryl group whilst retaining an electrophilic coupling substrate on the indole (Scheme 5).
Scheme 5.
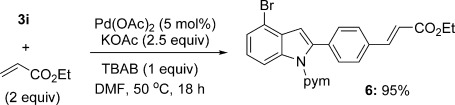
Selective derivatisation of the aryl halide functionality. TBAB=tetrabutylammonium bromide.
Mechanistic Considerations
Investigation of putative ruthenacyclic intermediates
We prepared the previously unreported complexes [7]Cl, [7]OAc, and [7-OH2]SbF6 (Scheme 6)[40] to probe the potential role of ruthenacyclic intermediates.[15a, 22, 39] In addition to standard 1H and 13C NMR spectroscopy and mass spectrometry, complex [7]Cl was characterised by means of X-Ray crystallography (see the Supporting Information for further details).
Scheme 6.
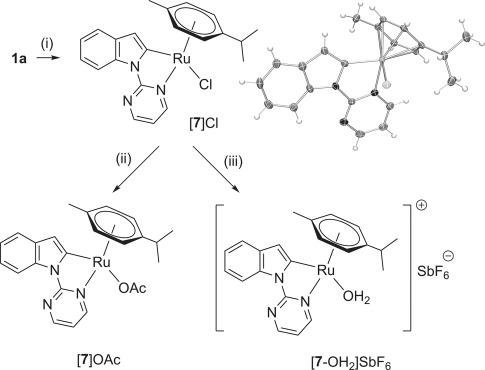
Preparation of ruthenacycles [7]Cl, [7]OAc, and [7-OH2]SbF6. Conditions: (i) [{RuCl2(p-cymene)}2] (0.5 equiv), MeOH, RT, 24 h, 78 %; ii) AgOAc (2.5 equiv), MTBE, RT, 24 h, 80 %; iii) AgSbF6 (1.1 equiv), H2O (6.9 equiv), [D8]THF, RT. ORTEP representation of complex [7]Cl. Ellipsoids are drawn at the 50 % probability level with the MeOH solvate omitted for clarity. MTBE=methyl tert-butyl ether.
Replacing [{RuCl2(p-cymene)}2] with [7]Cl, [7]OAc, or [7-OH2]SbF6 gave 86, 60, and 40 % spectroscopic yields of 2 b, respectively, under the standard reaction conditions shown in Scheme 1. Thus, species 7 are either catalytically active or are converted into catalytically active species in situ (see below).
Species [7]tol was not observed on exposure of [7]Cl, [7]OAc, or [7-OH2]SbF6 to 4-tolylboronic acid with or without Cu(OAc)2⋅H2O present (Table 2). Product 2 a formed in the absence of Cu(OAc)2⋅H2O from both [7]OAc and [7-OH2]SbF6, (15 % and trace yields, respectively), indicating that transmetalation and reductive elimination are possible for RuII species. These results complement the finding by Lan and co-workers that a RhIIICp* analogue of [7]Cl underwent transmetalation and reductive elimination with benzothiophene in the absence of an oxidant.[9g] Traces of 2 a were also observed for both [7]OAc and [7-OH2]SbF6 in the presence of Cu(OAc)2⋅H2O. An intractable mixture of indole-containing species constituted the mass balance of these reactions.
Table 2.
Transmetalation experiments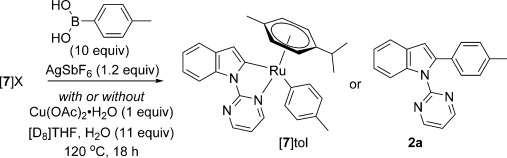
| Entry | Complex | Cu(OAc)2⋅H2O | [7]tol | 2 a |
|---|---|---|---|---|
| 1 | [7]Cl | yes | – | – |
| 2 | [7]Cl | no | – | – |
| 3 | [7]OAc | yes | – | traces |
| 4 | [7]OAc | no | –[a] | 15 % |
| 5 | [7-OH2]SbF6 | yes | – | traces |
| 6 | [7-OH2]SbF6 | no | – | traces |
[a] A symmetrical complex of the type [Ru(p-cymene)]X2 was observed by 1H NMR spectroscopic analysis at the end of the reaction.[41]
Hydrogen–deuterium exchange studies
Compound 1 a gave no H/D exchange at either C2 or C3 in the presence of D2O, catalytic [{RuCl2(p-cymene)}2], and AgSbF6 (Table 3, entry 1) at 23 °C, although the 1H NMR spectrum of the reaction mixture revealed the complete conversion of the catalyst precursor to ruthenacycle [7-OD2]SbF6. Substrate 1 a underwent significant C3–H/D and modest C2–H/D exchange when the same experiment was performed at 60 °C, again with the quantitative formation of [7-OD2]SbF6 (Table 3, entry 2). On heating to 80 °C, only traces of [7-OD2]SbF6 were observed, with the concomitant formation of uncoordinated para-cymene and 64 % deuterium incorporation at C3 (Table 3, entry 3). On heating to 100 °C, no [7-OD2]SbF6 remained and uncoordinated para-cymene was observed, but C–H/D exchange increased only incrementally (Table 3, entry 4). Finally, heating to 120 °C gave no observable Ru–cymene complexes, with 1 a showing 60 % C2–H/D exchange (Table 3, entry 5). Thus, the greatest degree of reversible C2–H activation occurred after complete loss of para-cymene from the Ru centre. When [{RuCl2(p-cymene)}2] was excluded from the reaction mixture, 15 % C2–H/D and 68 % C3–H/D exchange was observed at 120 °C. Kanai and co-workers found that simple Lewis acids, such as Sc(OTf)3, promote similar levels of C3-selective exchange for 1 a,[29f] which we attribute to the presence of AgI ions in our reaction (Table 3, entries 6 and 7). Analogous experiments with N-acetylindole or 1-(pyrimidin-2-yl)benzimidazole did not give C2–H/D exchange, which is consistent with the requirement for nucleophilicity of the heteroarene and the inability of the acetyl group to effect C2 arylation under our catalytic conditions (see Figure S1 in the Supporting Information).
Table 3.
Hydrogen–deuterium exchange studies
| Entry | [7-OD2]SbF6 | T [°] | C2–H/C2–D | C3–H/C3–D |
|---|---|---|---|---|
| 1 | yes | 23 | 100:0 | 100:0 |
| 2[a] | yes | 60 | 94:6 | 69:31 |
| 3 | traces[b] | 80 | 85:15 | 36:64 |
| 4 | no[b] | 100 | 75:25 | 32:68 |
| 5[a] | no | 120 | 40:60 | 41:59 |
| 6[c] | – | 120 | 85:15 | 32:68 |
| 7[d] | – | 120 | 100:0 | 100:0 |
[a] Average of two runs. [b] Free para-cymene observed by 1H NMR spectroscopic analysis. [c] [{RuCl2(p-cymene)}2] was excluded. [d] [{RuCl2(p-cymene)}2] and AgSbF6 were excluded.
Importance of the para-cymene ligand
Cymene-ligated Ru complexes have frequently been invoked as catalytic intermediates in C–H functionalisation reactions. For the phenylation of 1 a under our conditions, complete loss of the cymene ligand from the Ru coordination sphere could be confirmed by 1H NMR spectroscopic analysis on cooling the reaction mixture to room temperature after just 10 minutes (Scheme 7). Conversion into 2 b was 50 % at this point. The reaction mixture was reheated to 120 °C for a further 7 hours, after which the conversion into 2 b increased to 67 %, with cymene remaining uncoordinated.
Scheme 7.
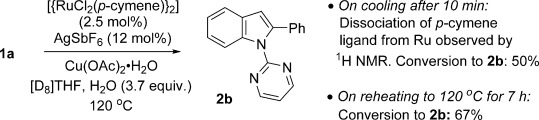
The catalytic phenylation of 1 a continued despite the loss of the para-cymene ligand from the Ru centre.
Discussion of the mechanism
We suggest that although initial ruthenation of the indole–pyrimidine unit occurs easily under our conditions, [7-OH2]SbF6 acts as a precursor rather than as a true catalytic intermediate. The inferior performance of several other transition-metal salts in our optimisation studies (see Table S2 in the Supporting Information) may indicate that the formation of such ruthenacycles at the beginning of the reaction—but prior to the catalysis—is advantageous. Facile exchange of the para-cymene ligand in ruthenacycles in the presence of excess nitrogen donors is well documented. For example, Jutand and co-workers reported that complexes of the type [Ru{κ2-C,N-(2-phenylpyridine)}(MeCN)4]OPiv (OPiv = dimethylpropanoate) formed easily in the presence of excess MeCN, but were catalytically inactive in the arylation of phenylpyridines with aryl halides.[34] We propose that under our catalytic conditions the pyrimidine groups of substrates 1 or 4 displace the para-cymene ligand of cyclometalated species 7 and on-cycle intermediates may have more than one pyrimidyl heteroarene ligand in the ruthenium coordination sphere. Thus, 1 or 4 could act as spectator ligands in the cycle prior to their own C2 ruthenation. This outcome would be consistent with the poor performance of complexes [7]Cl, [7]OAc, and [7-OH2]SbF6 in attempts to induce transmetalation and reductive elimination in the absence of unmetalated substrates 1 (Table 2). It is also in agreement with the need for a sufficiently strongly σ-donating directing group, hence why oxygen-based directing groups[42] proved ineffective.
With respect to the on-cycle C–H activation, the following observations suggest electrophilic attack by the ruthenium centre on the heteroarene: 1) the significant increase in efficiency when silver additives are used, 2) higher yields with electron-rich substrates, 3) failure of 1-(pyrimidin-2-yl)benzimidazole to undergo C–H/D exchange or C2 arylation, 4) that using Ag2O as an oxidant gives 2 a in a moderate yield (Table 1, entry 1) in the absence of carboxylates, 5) that C–H ruthenation of 1 a occurred in the absence of carboxylates during H/D exchange experiments (Table 3), suggesting the plausibility of an analogous on-cycle ruthenation reaction. In addition, KOAc had a detrimental effect on the catalysis (Table 1, entry 5) and no significant effect on the rate of C2–H/D exchange, whereas Cu(OAc)2 inhibited the rate of C2–H/D exchange (see Table S7 in the Supporting Information).
Therefore, our proposed mechanism is complementary to the carboxylate-assisted Ru-catalysed C–H functionalisation reactions[22] studied in depth by the groups of Ackermann[43] and Dixneuf,[34] in which aryl halide coupling partners were used. This mechanism also agrees with recent mechanistic proposals for related reactions in which electrophilic cationic RuII intermediates have been suggested.[44]
Conclusion
We have developed an oxidative, Ru-catalysed C2–H selective arylation reaction of indoles and pyrroles with boronic acids as the aryl source. The reaction shows high functional-group tolerance for both coupling partners, including halides, thus preserving the scope of the reaction for selective subsequent manipulation of the products. para-Cymene-ligated ruthenacycles give, at best, poor yields in attempts at transmetalation, but lose the para-cymene ligand under our catalytic conditions; therefore, these complexes are unlikely on-cyclic intermediates. Efforts to broaden this methodology to other transformations and substrates are ongoing in our laboratory.
Experimental Section
General arylation procedure
[{RuCl2(p-cymene)}2] (7.6 mg, 0.013 mmol, 2.5 mol %), substrate 1 or 4 (0.5 mmol), boronic acid (1.5 mmol), Cu(OAc)2⋅H2O (100 mg, 0.50 mmol), and AgSbF6 (21 mg, 0.06 mmol, 12 mol %) were added to a microwave vial (20 mL) equipped with a magnetic stirrer bar. The vial was evacuated and backfilled with argon. iPrOH (1.5 mL) was added by syringe, the vial sealed, and the mixture heated to 120 °C for 18 h with vigorous stirring. The reaction mixture was cooled to room temperature and loaded directly onto silica gel. The product was isolated by flash column chromatography (eluent=EtOAc/pentane).
Acknowledgments
We are grateful to the Swedish Research Council (Vetenskapsrådet) for generous funding and Dr. Emilien Demory (Uppsala University) for help with the preparation of the starting materials. We also thank Professor Lars Engman, Dr. Eszter Borbas, and Dr. Johanna Larsson for proofreading of the manuscript.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.Dyker G, editor. Handbook of C–H Transformations: Applications in Organic Synthesis. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 2a.Wencel-Delord J, Glorius F. Nat. Chem. 2013;5:369–375. doi: 10.1038/nchem.1607. For selected reviews, see. [DOI] [PubMed] [Google Scholar]
- 2b.Mercier LG, Leclerc M. Acc. Chem. Res. 2013;46:1597–1605. doi: 10.1021/ar3003305. [DOI] [PubMed] [Google Scholar]
- 2c.Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. 2012;124:9092–9142. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 2d.Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc. Chem. Res. 2012;45:826–839. doi: 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2e.Chen DYK, Youn SW. Chem. Eur. J. 2012;18:9452–9474. doi: 10.1002/chem.201201329. [DOI] [PubMed] [Google Scholar]
- 3a.Ackermann L, Vicente R, Kapdi AR. Angew. Chem. 2009;121:9976–10011. doi: 10.1002/anie.200902996. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2009;48:9792–9826. doi: 10.1002/anie.200902996. [DOI] [PubMed] [Google Scholar]
- 3b.Bandini M, Eichholzer A. Angew. Chem. 2009;121:9786–9824. [Google Scholar]
- Angew. Chem. Int. Ed. 2009;48:9608–9644. doi: 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- 3c.Beck EM, Gaunt MJ. Top. Curr. Chem. 2010;292:85–121. doi: 10.1007/128_2009_15. [DOI] [PubMed] [Google Scholar]
- 3d.Broggini G, Beccalli EM, Fasana A, Gazzola S. Beilstein J. Org. Chem. 2012;8:1730–1746. doi: 10.3762/bjoc.8.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3e.Lebrasseur N, Larrosa I. In: Advances in Heterocyclic Chemistry, Vol. 105. Alan K, editor. Academic Press; 2012. pp. 309–351. Chapter 4. [Google Scholar]
- 4.Ishikura M, Yamada K. Nat. Prod. Rep. 2009;26:803–852. doi: 10.1039/b820693g. [DOI] [PubMed] [Google Scholar]
- 5a.Flynn BL, Hamel E, Jung MK. J. Med. Chem. 2002;45:2670–2673. doi: 10.1021/jm020077t. [DOI] [PubMed] [Google Scholar]
- 5b.Gastpar R, Goldbrunner M, Marko D, von Angerer E. J. Med. Chem. 1998;41:4965–4972. doi: 10.1021/jm980228l. [DOI] [PubMed] [Google Scholar]
- 5c.Kaushik N, Kaushik N, Attri P, Kumar N, Kim C, Verma A, Choi E. Molecules. 2013;18:6620–6662. doi: 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Feng J, Lu G, Lv M, Cai C. J. Organomet. Chem. 2014;761:28–31. For Pd-catalysed examples, see. [Google Scholar]
- 6b.Preciado S, Mendive-Tapia L, Albericio F, Lavilla R. J. Org. Chem. 2013;78:8129–8135. doi: 10.1021/jo400961x. [DOI] [PubMed] [Google Scholar]
- 6c.Islam S, Larrosa I. Chem. Eur. J. 2013;19:15093–15096. doi: 10.1002/chem.201302838. [DOI] [PubMed] [Google Scholar]
- 6d.Wu K-J, Dai L-X, You S-L. Org. Lett. 2012;14:3772–3775. doi: 10.1021/ol301663h. [DOI] [PubMed] [Google Scholar]
- 6e.Sun L-L, Liao Z-Y, Tang R-Y, Deng C-L, Zhang X-G. J. Org. Chem. 2012;77:2850–2856. doi: 10.1021/jo3000404. [DOI] [PubMed] [Google Scholar]
- 6f.Wang L, Yi W-b, Cai C. Chem. Commun. 2011;47:806–808. doi: 10.1039/c0cc01666g. [DOI] [PubMed] [Google Scholar]
- 6g.Nadres ET, Lazareva A, Daugulis O. J. Org. Chem. 2011;76:471–483. doi: 10.1021/jo1018969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6h.Joucla L, Batail N, Djakovitch L. Adv. Synth. Catal. 2010;352:2929–2936. [Google Scholar]
- 6i.Roger J, Doucet H. Adv. Synth. Catal. 2009;351:1977–1990. [Google Scholar]
- 6j.Lebrasseur N, Larrosa I. J. Am. Chem. Soc. 2008;130:2926–2927. doi: 10.1021/ja710731a. [DOI] [PubMed] [Google Scholar]
- 6k.Wang X, Gribkov DV, Sames D. J. Org. Chem. 2007;72:1476–1479. doi: 10.1021/jo061979v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6l.Touré BB, Lane BS, Sames D. Org. Lett. 2006;8:1979–1982. doi: 10.1021/ol053021c. [DOI] [PubMed] [Google Scholar]
- 6m.Lane BS, Brown MA, Sames D. J. Am. Chem. Soc. 2005;127:8050–8057. doi: 10.1021/ja043273t. [DOI] [PubMed] [Google Scholar]
- 6n.Lane BS, Sames D. Org. Lett. 2004;6:2897–2900. doi: 10.1021/ol0490072. for a Rh-catalysed example, see. [DOI] [PubMed] [Google Scholar]
- 6o.Wang X, Lane BS, Sames D. J. Am. Chem. Soc. 2005;127:4996–4997. doi: 10.1021/ja050279p. for Ru-catalysed examples, see. [DOI] [PubMed] [Google Scholar]
- 6p.Adrio LA, Gimeno J, Vicent C. Chem. Commun. 2013;48:8320–8322. doi: 10.1039/c3cc43452d. [DOI] [PubMed] [Google Scholar]
- 6q.Li B, Bheeter CB, Darcel C, Dixneuf PH. ACS Catal. 2011;1:1221–1224. [Google Scholar]
- 6r.Ackermann L, Lygin AV. Org. Lett. 2011;13:3332–3335. doi: 10.1021/ol2010648. for a Cu-catalysed example, see. [DOI] [PubMed] [Google Scholar]
- 6s.Do H-Q, Daugulis O. Chem. Commun. 2009:6433–6435. doi: 10.1039/b912890e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Wagner AM, Sanford MS. Org. Lett. 2011;13:288–291. doi: 10.1021/ol102734g. [DOI] [PubMed] [Google Scholar]
- 7b.Phipps RJ, Grimster NP, Gaunt MJ. J. Am. Chem. Soc. 2008;130:8172–8174. doi: 10.1021/ja801767s. [DOI] [PubMed] [Google Scholar]
- 7c.Deprez NR, Kalyani D, Krause A, Sanford MS. J. Am. Chem. Soc. 2006;128:4972–4973. doi: 10.1021/ja060809x. [DOI] [PubMed] [Google Scholar]
- 8a.Lu M-Z, Lu P, Xu Y-H, Loh T-P. Org. Lett. 2014;16:2614–2617. doi: 10.1021/ol500754h. For examples using silanes, see. [DOI] [PubMed] [Google Scholar]
- 8b.Liang Z, Yao B, Zhang Y. Org. Lett. 2010;12:3185–3187. doi: 10.1021/ol101147b. for a decarbonylative approach, see. [DOI] [PubMed] [Google Scholar]
- 8c.Zhang L, Xue X, Xu C, Pan Y, Zhang G, Xu L, Li H, Shi Z. ChemCatChem. 2014;6:3069–3074. for desulfitative approaches, see. [Google Scholar]
- 8d.Miao T, Li P, Wang G-W, Wang L. Chem. Asian J. 2013;8:3185–3190. doi: 10.1002/asia.201300913. [DOI] [PubMed] [Google Scholar]
- 8e.Wu M, Luo J, Xiao F, Zhang S, Deng G-J, Luo H-A. Adv. Synth. Catal. 2012;354:335–340. [Google Scholar]
- 9a.Girard SA, Knauber T, Li C-J. Angew. Chem. 2014;126:76–103. doi: 10.1002/anie.201304268. For selected reviews, see. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]
- 9b.Yu D-G, Li B-J, Shi Z-J. Tetrahedron. 2012;68:5130–5136. [Google Scholar]
- 9c.Wencel-Delord J, Nimphius C, Patureau FW, Glorius F. Angew. Chem. 2012;124:2290–2294. doi: 10.1002/anie.201107842. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:2247–2251. doi: 10.1002/anie.201107842. [DOI] [PubMed] [Google Scholar]
- 9d.Zhao D, You J, Hu C. Chem. Eur. J. 2011;17:5466–5492. doi: 10.1002/chem.201003039. [DOI] [PubMed] [Google Scholar]
- 9e.Yeung CS, Dong VM. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]
- 9f.Cho SH, Kim JY, Kwak J, Chang S. Chem. Soc. Rev. 2011;40:5068–5083. doi: 10.1039/c1cs15082k. for Rh-catalysed examples with indoles, see. [DOI] [PubMed] [Google Scholar]
- 9g.Qin X, Liu H, Qin D, Wu Q, You J, Zhao D, Guo Q, Huang X, Lan J. Chem. Sci. 2013;4:1964–1969. for Pd-catalysed examples with indoles, see. [Google Scholar]
- 9h.Pintori DG, Greaney MF. J. Am. Chem. Soc. 2011;133:1209–1211. doi: 10.1021/ja1090854. [DOI] [PubMed] [Google Scholar]
- 9i.Gong X, Song G, Zhang H, Li X. Org. Lett. 2011;13:1766–1769. doi: 10.1021/ol200306y. [DOI] [PubMed] [Google Scholar]
- 9j.Potavathri S, Pereira KC, Gorelsky SI, Pike A, LeBris AP, DeBoef B. J. Am. Chem. Soc. 2010;132:14676–14681. doi: 10.1021/ja107159b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9k.Potavathri S, Dumas AS, Dwight TA, Naumiec GR, Hammann JM, DeBoef B. Tetrahedron Lett. 2008;49:4050–4053. doi: 10.1016/j.tetlet.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9l.Stuart DR, Villemure E, Fagnou K. J. Am. Chem. Soc. 2007;129:12072–12073. doi: 10.1021/ja0745862. [DOI] [PubMed] [Google Scholar]
- 9m.Stuart DR, Fagnou K. Science. 2007;316:1172–1175. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]
- 9n.Itahara T. J. Org. Chem. 1985;50:5272–5275. [Google Scholar]
- 9o.Itahara T. J. Chem. Soc. Chem. Commun. 1981:254–255. [Google Scholar]
- 9p.Campbell AN, Meyer EB, Stahl SS. Chem. Commun. 2011;47:10257–10259. doi: 10.1039/c1cc13632a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9q.Sun C-L, Li B-J, Shi Z-J. Chem. Commun. 2010;46:677–685. doi: 10.1039/b908581e. for a Cu-catalysed example with indoles, see. [DOI] [PubMed] [Google Scholar]
- 9r.Nishino M, Hirano K, Satoh T, Miura M. Angew. Chem. 2012;124:7099–7103. doi: 10.1002/anie.201201491. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:6993–6997. doi: 10.1002/anie.201201491. [DOI] [PubMed] [Google Scholar]
- 10.Hall DG. Structure, Properties, and Preparation of Boronic Acid Derivatives, Vol. 1. Weinheim: Wiley-VCH; 2011. pp. 1–133. [Google Scholar]
- 11a.Kirchberg S, Fröhlich R, Studer A. Angew. Chem. 2009;121:4299–4302. doi: 10.1002/anie.200901072. For Pd-catalysed examples using indole substrates, see. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2009;48:4235–4238. doi: 10.1002/anie.200901072. [DOI] [PubMed] [Google Scholar]
- 11b.Nimje RY, Leskinen MV, Pihko PM. Angew. Chem. 2013;125:4918–4922. doi: 10.1002/anie.201300833. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2013;52:4818–4822. doi: 10.1002/anie.201300833. [DOI] [PubMed] [Google Scholar]
- 11c.Yang S-D, Sun C-L, Fang Z, Li B-J, Li Y-Z, Shi Z-J. Angew. Chem. 2008;120:1495–1498. [Google Scholar]
- Angew. Chem. Int. Ed. 2008;47:1473–1476. doi: 10.1002/anie.200704619. [DOI] [PubMed] [Google Scholar]
- 11d.Zhang L, Li P, Liu C, Yang J, Wang M, Wang L. Catal. Sci. Technol. 2014;4:1979–1988. [Google Scholar]
- 11e.Yang L, Luo L, Zhang S, Su X, Lan J, Chen C-T, You J. Chem. Commun. 2010;46:3938–3940. doi: 10.1039/c0cc00112k. [DOI] [PubMed] [Google Scholar]
- 11f.Huang Y, Ma T, Huang P, Wu D, Lin Z, Cao R. ChemCatChem. 2013;5:1877–1883. [Google Scholar]
- 11g.Ruiz-Rodríguez J, Albericio F, Lavilla R. Chem. Eur. J. 2010;16:1124–1127. doi: 10.1002/chem.200902676. [DOI] [PubMed] [Google Scholar]
- 11h.Zhao J, Zhang Y, Cheng K. J. Org. Chem. 2008;73:7428–7431. doi: 10.1021/jo801371w. [DOI] [PubMed] [Google Scholar]
- 11i.Kannaboina P, Anilkumar K, Aravinda S, Vishwakarma RA, Das P. Org. Lett. 2013;15:5718–5721. doi: 10.1021/ol4027478. [DOI] [PubMed] [Google Scholar]
- 12a.Kirchberg S, Tani S, Ueda K, Yamaguchi J, Studer A, Itami K. Angew. Chem. 2011;123:2435–2439. doi: 10.1002/anie.201007060. For other Pd-catalysed examples, see. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:2387–2391. doi: 10.1002/anie.201007060. [DOI] [PubMed] [Google Scholar]
- 12b.Koley M, Dastbaravardeh N, Schnürch M, Mihovilovic MD. ChemCatChem. 2012;4:1345–1352. doi: 10.1002/cctc.201200155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12c.Guan J, Wu G-J, Han F-S. Chem. Eur. J. 2014;20:3301–3305. doi: 10.1002/chem.201303056. [DOI] [PubMed] [Google Scholar]
- 12d.Joncour R, Susperregui N, Pinaud N, Miqueu K, Fouquet E, Sotiropoulos J-M, Felpin F-X. Chem. Eur. J. 2013;19:9291–9296. doi: 10.1002/chem.201300858. [DOI] [PubMed] [Google Scholar]
- 12e.Ranjit S, Liu X. Chem. Eur. J. 2011;17:1105–1108. doi: 10.1002/chem.201002787. [DOI] [PubMed] [Google Scholar]
- 12f.Engle KM, Thuy-Boun PS, Dang M, Yu J-Q. J. Am. Chem. Soc. 2011;133:18183–18193. doi: 10.1021/ja203978r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12g.Meng X, Kim S. J. Org. Chem. 2013;78:11247–11254. doi: 10.1021/jo401716p. [DOI] [PubMed] [Google Scholar]
- 12h.Chen X, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:12634–12635. doi: 10.1021/ja0646747. [DOI] [PubMed] [Google Scholar]
- 12i.Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]
- 12j.Nishikata T, Abela AR, Huang S, Lipshutz BH. J. Am. Chem. Soc. 2010;132:4978–4979. doi: 10.1021/ja910973a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12k.Thuy-Boun PS, Villa G, Dang D, Richardson P, Su S, Yu J-Q. J. Am. Chem. Soc. 2013;135:17508–17513. doi: 10.1021/ja409014v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12l.Wang D-H, Wasa M, Giri R, Yu J-Q. J. Am. Chem. Soc. 2008;130:7190–7191. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- 12m.Chan KSL, Wasa M, Chu L, Laforteza BN, Miura M, Yu J-Q. Nat. Chem. 2014;6:146–150. doi: 10.1038/nchem.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12n.Wei Y, Kan J, Wang M, Su W, Hong M. Org. Lett. 2009;11:3346–3349. doi: 10.1021/ol901200g. [DOI] [PubMed] [Google Scholar]
- 12o.Feng J, Lu G, Lv M, Cai C. Synlett. 2013;24:2153–2159. [Google Scholar]
- 12p.Chu J-H, Tsai S-L, Wu M-J. Synthesis. 2009;2009:3757–3764. [Google Scholar]
- 12q.Chu J-H, Wu C-C, Chang D-H, Lee Y-M, Wu M-J. Organometallics. 2013;32:272–282. [Google Scholar]
- 13.Zheng J, Zhang Y, Cui S. Org. Lett. 2014;16:3560–3563. doi: 10.1021/ol5014312. For a Rh-catalysed example with indoles, see. [DOI] [PubMed] [Google Scholar]
- 14a.Ban I, Sudo T, Taniguchi T, Itami K. Org. Lett. 2008;10:3607–3609. doi: 10.1021/ol8013717. For Cu-catalysed examples, see. [DOI] [PubMed] [Google Scholar]
- 14b.Baslé O, Li C-J. Org. Lett. 2008;10:3661–3663. doi: 10.1021/ol8012588. [DOI] [PubMed] [Google Scholar]
- 14c.Shang M, Sun S-Z, Dai H-X, Yu J-Q. Org. Lett. 2014;16:5666. doi: 10.1021/ol5027377. [DOI] [PubMed] [Google Scholar]
- 15a.Arockiam PB, Bruneau C, Dixneuf PH. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]
- 15b.Liu PM, Frost CG. Org. Lett. 2013;15:5862–5865. doi: 10.1021/ol402936c. [DOI] [PubMed] [Google Scholar]
- 15c.Mehta VP, García-López J-A, Greaney MF. Angew. Chem. Int. Ed. 2014;53:1529–1533. doi: 10.1002/anie.201309114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Hofmann N, Ackermann L. J. Am. Chem. Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. For Ru-catalysed C–H functionalisation with unusual meta selectivity, see. [DOI] [PubMed] [Google Scholar]
- 16b.Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. J. Am. Chem. Soc. 2011;133:19298–19301. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]
- 16c.Reynolds WR, Liu PM, Kociok-Kohn G, Frost CG. Synlett. 2013;24:2687–2690. [Google Scholar]
- 17a.Kakiuchi F, Kan S, Igi K, Chatani N, Murai S. J. Am. Chem. Soc. 2003;125:1698–1699. doi: 10.1021/ja029273f. [DOI] [PubMed] [Google Scholar]
- 17b.Kakiuchi F, Matsuura Y, Kan S, Chatani N. J. Am. Chem. Soc. 2005;127:5936–5945. doi: 10.1021/ja043334n. [DOI] [PubMed] [Google Scholar]
- 18.Pastine SJ, Gribkov DV, Sames D. J. Am. Chem. Soc. 2006;128:14220–14221. doi: 10.1021/ja064481j. [DOI] [PubMed] [Google Scholar]
- 19a.Schwarz M, Dastbaravardeh N, Kirchner K, Schnürch M, Mihovilovic M. Monatsh. Chem. 2013;144:539–552. doi: 10.1007/s00706-013-0947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19b.Dastbaravardeh N, Schnürch M, Mihovilovic MD. Org. Lett. 2012;14:1930–1933. doi: 10.1021/ol300627p. [DOI] [PubMed] [Google Scholar]
- 19c.Dastbaravardeh N, Kirchner K, Schnürch M, Mihovilovic MD. J. Org. Chem. 2013;78:658–672. doi: 10.1021/jo302547q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Li H, Wei W, Xu Y, Zhang C, Wan X. Chem. Commun. 2011;47:1497–1499. doi: 10.1039/c0cc04322b. [DOI] [PubMed] [Google Scholar]
- 20b.Chinnagolla RK, Jeganmohan M. Org. Lett. 2012;14:5246–5249. doi: 10.1021/ol3024067. [DOI] [PubMed] [Google Scholar]
- 20c.Chinnagolla RK, Jeganmohan M. Chem. Commun. 2014;50:2442–2444. doi: 10.1039/c3cc49398a. [DOI] [PubMed] [Google Scholar]
- 20d.Li H, Xu Y, Shi E, Wei W, Suo X, Wan X. Chem. Commun. 2011;47:7880–7882. doi: 10.1039/c1cc12843d. [DOI] [PubMed] [Google Scholar]
- 21a.Ackermann L, Wang L, Lygin AV. Chem. Sci. 2012;3:177–180. For selected recent examples, see. [Google Scholar]
- 21b.Lanke V, Prabhu KR. Org. Lett. 2013;15:2818–2821. doi: 10.1021/ol4011486. [DOI] [PubMed] [Google Scholar]
- 21c.Li B, Ma J, Xie W, Song H, Xu S, Wang B. J. Org. Chem. 2013;78:9345–9353. doi: 10.1021/jo401579m. [DOI] [PubMed] [Google Scholar]
- 21d.Zhang L-Q, Yang S, Huang X, You J, Song F. Chem. Commun. 2013;49:8830–8832. doi: 10.1039/c3cc44787a. [DOI] [PubMed] [Google Scholar]
- 21e.Ueyama T, Mochida S, Fukutani T, Hirano K, Satoh T, Miura M. Org. Lett. 2011;13:706–708. doi: 10.1021/ol102942w. [DOI] [PubMed] [Google Scholar]
- 21f.Lanke V, Ramaiah Prabhu K. Org. Lett. 2013;15:6262–6265. doi: 10.1021/ol4031149. [DOI] [PubMed] [Google Scholar]
- 21g.Reddy MC, Jeganmohan M. Org. Lett. 2014;16:4866–4869. doi: 10.1021/ol502375p. [DOI] [PubMed] [Google Scholar]
- 21h.Louillat M-L, Biafora A, Legros F, Patureau FW. Angew. Chem. 2014;126:3573–3577. doi: 10.1002/anie.201308601. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2014;53:3505–3509. doi: 10.1002/anie.201308601. [DOI] [PubMed] [Google Scholar]
- 21i.Singh KS, Dixneuf PH. Organometallics. 2012;31:7320–7323. [Google Scholar]
- 21j.Li J, Kornhaaß C, Ackermann L. Chem. Commun. 2012;48:11343–11345. doi: 10.1039/c2cc36196e. [DOI] [PubMed] [Google Scholar]
- 21k.Biafora A, Patureau FW. Synlett. 2014;25:2525–2530. [Google Scholar]
- 22.Ackermann L. Chem. Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. For a review of the role of carboxylates in C–H functionalisation, see. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Snieckus V. Adv. Synth. Catal. 2014;356:1527–1532. A single, low-yielding Ru0-catalysed example has recently been reported. [Google Scholar]
- 24.Ackermann L, Lygin AV. Org. Lett. 2012;14:764–767. doi: 10.1021/ol203309y. [DOI] [PubMed] [Google Scholar]
- 25a.Rousseau G, Breit B. Angew. Chem. 2011;123:2498–2543. doi: 10.1002/anie.201006139. For reviews on removable directing groups, see. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:2450–2494. doi: 10.1002/anie.201006139. [DOI] [PubMed] [Google Scholar]
- 25b.Wang C, Huang Y. Synlett. 2013;24:145–149. [Google Scholar]
- 26a.Chen J, Pang Q, Sun Y, Li X. J. Org. Chem. 2011;76:3523–3526. doi: 10.1021/jo1025546. [DOI] [PubMed] [Google Scholar]
- 26b.Song W, Ackermann L. Chem. Commun. 2013;49:6638–6640. doi: 10.1039/c3cc43915a. [DOI] [PubMed] [Google Scholar]
- 27a.Kou X, Zhao M, Qiao X, Zhu Y, Tong X, Shen Z. Chem. Eur. J. 2013;19:16880–16886. doi: 10.1002/chem.201303637. Cu-catalysed examples. [DOI] [PubMed] [Google Scholar]
- 27b.Nishino M, Hirano K, Satoh T, Miura M. Angew. Chem. 2012;124:7099–7103. doi: 10.1002/anie.201201491. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:6993–6997. doi: 10.1002/anie.201201491. [DOI] [PubMed] [Google Scholar]
- 27c.Odani R, Nishino M, Hirano K, Satoh T, Miura M. Heterocycles. 2014;88:595–602. [Google Scholar]
- 27d.Pan C, Jin H, Xu P, Liu X, Cheng Y, Zhu C. J. Org. Chem. 2013;78:9494–9498. doi: 10.1021/jo4014904. [DOI] [PubMed] [Google Scholar]
- 27e.Xu H, Qiao X, Yang S, Shen Z. J. Org. Chem. 2014;79:4414–4422. doi: 10.1021/jo5003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Pan C, Jin H, Liu X, Cheng Y, Zhu C. Chem. Commun. 2013;49:2933–2935. doi: 10.1039/c3cc40709h. Pd-catalysed examples. [DOI] [PubMed] [Google Scholar]
- 28b.Wang Z, Song F, Zhao Y, Huang Y, Yang L, Zhao D, Lan J, You J. Chem. Eur. J. 2012;18:16616–16620. doi: 10.1002/chem.201203004. [DOI] [PubMed] [Google Scholar]
- 28c.Xu S, Huang X, Hong X, Xu B. Org. Lett. 2012;14:4614–4617. doi: 10.1021/ol302070t. [DOI] [PubMed] [Google Scholar]
- 28d.Yan X-B, Shen Y-W, Chen D-Q, Gao P, Li Y-X, Song X-R, Liu X-Y, Liang Y-M. Tetrahedron. 2014;70:7490–7495. [Google Scholar]
- 28e.Zhou W, Li P, Zhang Y, Wang L. Adv. Synth. Catal. 2013;355:2343–2352. [Google Scholar]
- 29a.Ding Z, Yoshikai N. Beilstein J. Org. Chem. 2012;8:1536–1542. doi: 10.3762/bjoc.8.174. Co-catalysed examples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29b.Ding Z, Yoshikai N. Angew. Chem. 2012;124:4776–4779. [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:4698–4701. doi: 10.1002/anie.201200019. [DOI] [PubMed] [Google Scholar]
- 29c.Punji B, Song W, Shevchenko GA, Ackermann L. Chem. Eur. J. 2013;19:10605–10610. doi: 10.1002/chem.201301409. [DOI] [PubMed] [Google Scholar]
- 29d.Song W, Ackermann L. Angew. Chem. 2012;124:8376–8379. [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:8251–8254. doi: 10.1002/anie.201202466. [DOI] [PubMed] [Google Scholar]
- 29e.Sun B, Yoshino T, Matsunaga S, Kanai M. Adv. Synth. Catal. 2014;356:1491–1495. [Google Scholar]
- 29f.Yoshino T, Ikemoto H, Matsunaga S, Kanai M. Chem. Eur. J. 2013;19:9142–9146. doi: 10.1002/chem.201301505. [DOI] [PubMed] [Google Scholar]
- 30a.Gong B, Shi J, Wang X, Yan Y, Li Q, Meng Y, Xu HE, Yi W. Adv. Synth. Catal. 2014;356:137–143. Rh-catalysed examples. [Google Scholar]
- 30b.Hong X, Wang H, Qian G, Tan Q, Xu B. J. Org. Chem. 2014;79:3228–3237. doi: 10.1021/jo500087g. [DOI] [PubMed] [Google Scholar]
- 30c.Parthasarathy K, Azcargorta AR, Cheng Y, Bolm C. Org. Lett. 2014;16:2538–2541. doi: 10.1021/ol500918t. [DOI] [PubMed] [Google Scholar]
- 30d.Reddy VP, Qiu R, Iwasaki T, Kambe N. Org. Lett. 2013;15:1290–1293. doi: 10.1021/ol400230y. [DOI] [PubMed] [Google Scholar]
- 30e.Ryu J, Shin K, Park SH, Kim JY, Chang S. Angew. Chem. 2012;124:10042–10046. [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:9904–9908. doi: 10.1002/anie.201205723. [DOI] [PubMed] [Google Scholar]
- 30f.Shi J, Zhao G, Wang X, Xu HE, Yi W. Org. Biomol. Chem. 2014;12:6831–6836. doi: 10.1039/c4ob00637b. [DOI] [PubMed] [Google Scholar]
- 30g.Shi J, Zhou B, Yang Y, Li Y. Org. Biomol. Chem. 2012;10:8953–8955. doi: 10.1039/c2ob26767e. [DOI] [PubMed] [Google Scholar]
- 30h.Shi Z, Boultadakis-Arapinis M, Glorius F. Chem. Commun. 2013;49:6489–6491. doi: 10.1039/c3cc43903h. [DOI] [PubMed] [Google Scholar]
- 30i.Wang H, Schroeder N, Glorius F. Angew. Chem. 2013;125:5495–5499. doi: 10.1002/anie.201301165. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2013;52:5386–5389. doi: 10.1002/anie.201301165. [DOI] [PubMed] [Google Scholar]
- 30j.Xie F, Qi Z, Yu S, Li X. J. Am. Chem. Soc. 2014;136:4780–4787. doi: 10.1021/ja501910e. [DOI] [PubMed] [Google Scholar]
- 30k.Yu S, Li X. Org. Lett. 2014;16:1200–1203. doi: 10.1021/ol5000764. [DOI] [PubMed] [Google Scholar]
- 30l.Nonoyama M, Nakajima K. Polyhedron. 1998;18:533–543. [Google Scholar]
- 30m.Qi J, Huang L, Wang Z, Jiang H. Org. Biomol. Chem. 2013;11:8009–8013. doi: 10.1039/c3ob41590b. see also refs. [8a, 8c, 9g] [DOI] [PubMed] [Google Scholar]
- 31.Pospech J, Tlili A, Spannenberg A, Neumann H, Beller M. Chem. Eur. J. 2014;20:3135–3141. doi: 10.1002/chem.201304314. For a Ru-catalysed example, see. See also refs. [6r] and [30m] [DOI] [PubMed] [Google Scholar]
- 32.Liu H-H, Wang Y, Deng G, Yang L. Adv. Synth. Catal. 2013;355:3369–3374. For a metal-free functionalisation, see. [Google Scholar]
- 33.Özdemir I, Demir S, Çetinkaya B, Gourlaouen C, Maseras F, Bruneau C, Dixneuf PH. J. Am. Chem. Soc. 2008;130:1156–1157. doi: 10.1021/ja710276x. [DOI] [PubMed] [Google Scholar]
- 34a.Fabre I, von Wolff N, Le Duc G, Ferrer Flegeau E, Bruneau C, Dixneuf PH, Jutand A. Chem. Eur. J. 2013;19:7595–7604. doi: 10.1002/chem.201203813. For studies on the effect of HOAc in related C–H arylation reactions with aryl halides, see. [DOI] [PubMed] [Google Scholar]
- 34b.Ferrer Flegeau E, Bruneau C, Dixneuf PH, Jutand A. J. Am. Chem. Soc. 2011;133:10161–10170. doi: 10.1021/ja201462n. [DOI] [PubMed] [Google Scholar]
- 35.Lennox AJ, Lloyd-Jones GC. Chem. Soc. Rev. 2014;43:412–443. doi: 10.1039/c3cs60197h. [DOI] [PubMed] [Google Scholar]
- 36a.Dick GR, Knapp DM, Gillis EP, Burke MD. Org. Lett. 2010;12:2314–2317. doi: 10.1021/ol100671v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36b.Gillis EP, Burke MD. J. Am. Chem. Soc. 2007;129:6716–6717. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]
- 36c.Gillis EP, Burke MD. J. Am. Chem. Soc. 2008;130:14084–14085. doi: 10.1021/ja8063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36d.Gillis EP, Burke MD. Aldrichimica Acta. 2009;42:17–27. [PMC free article] [PubMed] [Google Scholar]
- 36e.Knapp DM, Gillis EP, Burke MD. J. Am. Chem. Soc. 2009;131:6961–6963. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36f.Lee SJ, Gray KC, Paek JS, Burke MD. J. Am. Chem. Soc. 2008;130:466–468. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36g.Woerly EM, Roy J, Burke MD. Nat. Chem. 2014;6:484–491. doi: 10.1038/nchem.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molander GA, Ellis N. Acc. Chem. Res. 2007;40:275–286. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]
- 38.Isidro-Llobet A, Álvarez M, Albericio F. Chem. Rev. 2009;109:2455–2504. doi: 10.1021/cr800323s. The N-Boc-protected analogue of 1 m did not give the desired arylated product; for a review of amino acid protecting groups, see. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Roisnel T, Darcel C, Dixneuf PH. Dalton Trans. 2012;41:10934–10937. doi: 10.1039/c2dt31401k. [DOI] [PubMed] [Google Scholar]
- 40. For recently reported Rh and Pd analogues, see refs. [9g] and [30l], respectively; the synthesis of an indolyl ruthenacycle with an oxygen-based directing group has recently been reported as unsuccessful, see ref. [21c]
- 41.Tocher DA, Gould RO, Stephenson TA, Bennett MA, Ennett JP, Matheson TW, Sawyer L, Shah VK. J. Chem. Soc. Dalton Trans. 1983:1571–1581. [Google Scholar]
- 42.De Sarkar S, Liu W, Kozhushkov SI, Ackermann L. Adv. Synth. Catal. 2014;356:1461–1479. [Google Scholar]
- 43.Ackermann L, Vicente Rn, Potukuchi HK, Pirovano V. Org. Lett. 2010;12:5032–5035. doi: 10.1021/ol102187e. [DOI] [PubMed] [Google Scholar]
- 44.Liu W, Ackermann L. Chem. Commun. 2014;50:1878–1881. doi: 10.1039/c3cc49502g. For two recent representative examples, see ref. [21i] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


