Abstract
Background
Delayed recovery in persons after mild traumatic brain injury (mTBI) is poorly understood. Community integration (CI) is endorsed by persons with neurological disorders as an important outcome. We aimed to describe CI and its associated factors in insured Ontario workers with delayed recovery following mTBI.
Methods
A cross-sectional study of insured workers in the chronic phase following mTBI was performed at a rehabilitation hospital in Ontario, Canada. Sociodemographic, occupational, injury-related, clinical, and claim-related data were collected from self-reports, medical assessments, and insurers’ referral files. Community Integration Questionnaire (CIQ) scores were compared using analysis of variance or Spearman’s correlation tests. Stepwise multivariable linear regression models were used to evaluate the associations with CI.
Results
Ninety-four workers with mTBI (45.2 ± 9.9 years old, 61.2 % male) at 197 days post-injury (interquartile range, 139–416 days) were included. The CIQ total and subscale scores were similar to those reported in more severe TBI samples. The CIQ scores were moderately to strongly correlated with various sociodemographic, claim-related, and clinical variables. In the multivariable regression analysis, several covariates accounted for 36.4 % of the CIQ variance in the final fully adjusted model.
Discussion
This study evaluated CI in workers with mTBI, and analyzed its associated variables. Analysis revealed insomnia, head or neck pain, being married or in a relationship, time since injury, and a diagnosis of possible/probable malingering were independently associated with limited CI.
Conclusions
Workers with delayed recovery from mTBI experience difficulty with CI. Insomnia is a particularly relevant covariate, explaining the greater part of its variance. To enhance participation, care should focus on clinical and non-clinical covariates.
Electronic supplementary material
The online version of this article (doi:10.1186/s12883-015-0432-z) contains supplementary material, which is available to authorized users.
Keywords: Insomnia, Traumatic brain injury, Concussion, Recovery, Community integration, Diagnostic modeling
Background
Traumatic brain injury (TBI) is a serious neurological disorder [1–3], with variable outcomes that include significant morbidity [4–7] and a decreased ability to function in society [5–9]. Moderate, severe, and penetrating TBIs are associated with the most adverse effects [9–14], although the effects of mild TBI (mTBI) have recently received increased attention, as approximately 75 % of all TBIs are due to mild or concussive events [15–18]. Many persons with mTBI recover fully, usually within days or weeks [18, 19], although 15–23 % of patients experience disabling symptoms that persist beyond 3 months [20, 21].
Many of these symptoms are not specific to TBI [22], and while the list is long, insomnia, the inability to sleep adequately at night given the opportunity [23],has been recognized as extremely important for explaining many of these symptoms in the general population, including cognitive disturbances, dizziness, fatigue, depression, and pain [24, 25].
When confronted with persistent symptoms long after the injury, most relevant parties (i.e., clinicians, insurers, and claim adjudicators) are not aware of their indicators [26]. Therefore, unnecessary clinical and diagnostic investigations may be ordered to assist the parties in their decision making. These investigations typically focus on variables that are derived from three predictive models for adverse mTBI outcomes. However, when the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study was performed at three American medical centers to externally validate these models [27], researchers reported that two clinical models [28, 29] had minimal ability to discriminate between patients with favorable and non-favorable outcomes. The third model [30], featuring education, extracranial injury, and levels of post-concussion symptoms(i.e., depression, pain) as predictors of full return to work at 6 months post injury, could not be validated because of missing data. A focus on identifying more specific outcome measures was suggested for future research [27].
Recent initiatives have emphasized the importance of patients’ perceptions when assessing neurological outcomes [31]. In this context, the most relevant outcomes include family comfort, economic and social participation [32], falling under community integration (CI) concept [33]. Therefore, post-mTBI CI may be useful for measuring injury outcomes [34].
Given the complexity of CI, we developed a model of CI for persons with TBI to investigate the following hypotheses among workers with delayed recovery from mTBI (Fig. 1): (1) CI would be poor; (2) insomnia would be negatively associated with CI; (3) previously reported clinical and claim-related variables [27–30] would be associated with CI; and (4) previously unexplored psychosocial variables (i.e., family relationship, personality traits) would be associated with CI.
Fig. 1.
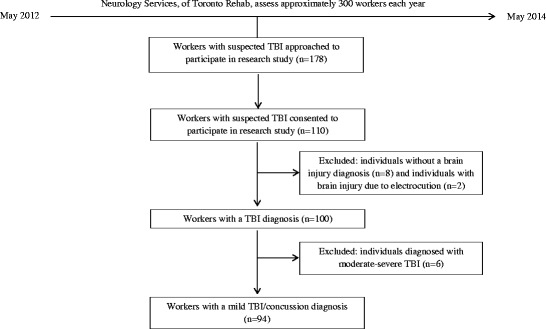
Flow chart depicting process of selection of participating individuals’ data for analysis. TBI Traumatic brain injury
Methods
This study’s design was reviewed and approved by the Research Ethics Boards at the Toronto Rehab-University Health Network (UHN) and the University of Toronto. The findings were reported in compliance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines [35].
Procedure and participants
Since 1998, the Neurology Service of the Toronto Rehab-UHN has provided exclusive expert diagnoses and treatment recommendations for Workplace Safety and Insurance Board (WSIB)-insured workers who have sustained a head trauma at work and have not returned to work within 3 months after the injury. The multidisciplinary team of specialists establishes a TBI diagnosis based on the initial loss of consciousness (LOC), Glasgow Coma Scale (GCS) score, post-traumatic amnesia (PTA), magnetic resonance imaging (MRI), and clinical assessment.
Recruitment
All participants were recruited between May 2012 and May 2014. Initial contact was made with prospective participants (n = 178) at orientation sessions, where they were informed of the ongoing study and were invited to participate. The final sample included 110 consenting (in writing) participants, including 94 participants who were later diagnosed with mTBI/concussion (Fig. 2).
Fig. 2.
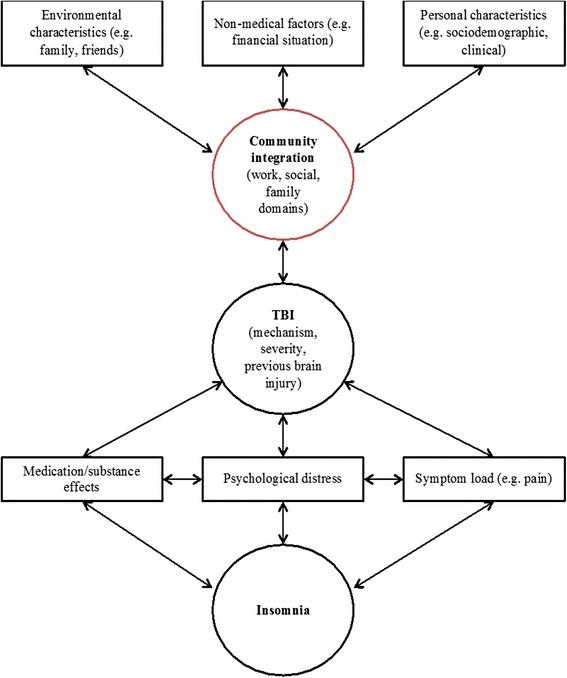
Conceptual framework of the construct of community integration in traumatic brain injury (TBI). Format adapted from Fayer & Hand [73]
To compare our sample to a consecutive sample of workers, and to indirectly assess our sample’s representativeness, we performed a retrospective chart review of consecutive individuals (n = 294) who were referred for the same services and were assessed in the same clinic during 2003. No significant differences were observed in injury severity, sex, and age. Non-significant (p >0.05) differences were observed in working status and marital status at the time of assessment. Our sample contained more people who were working part- or full-time at their assessment, and fewer people who were single, widowed, or divorced. To maintain sample homogeneity, our analyses only utilized data for participants who were diagnosed with mTBI (n = 94).
Instruments and measures
The clinical and diagnostic data included injury severity, presence of LOC, retrograde or anterograde PTA, and neuroimaging data. Clinical and claim-related variables were also collected from the participants’ medical and WSIB files, and included previous disability claims, employer-employee relations, WSIB-worker relations, and cases of malingering (DSM-IV-TR Axis IV) [36]. Occupational variables were gathered from insurer files (i.e., Employers’ Report of Injury/Illness Form 7), and included the workers’ occupation and weekly salary at the time of injury. A detailed description of the instruments that were used is presented in Additional file 1: Table S1, and the studied variables are presented in Additional file 2: Table S2.
The Community Integration Questionnaire (CIQ) [37] total score was used to measure outcome. All self-reported data were collected at the same time as the outcome assessment. The diagnostic investigations and clinical assessments were performed within a short period, during which no intervening treatments were commenced.
Statistical analysis
SAS software (version 9.3, SAS Inc., Cary, NC) was used for all data analyses. Means and standard deviations or medians and ranges were used to describe continuous data, and frequency counts were used to describe categorical data. Continuous data were tested for normality before the analysis. Spearman’s correlation coefficients (rs) were used to examine the associations between the CIQ total score and the continuous variables. A one-way analysis of variance was used to assess the associations between the CIQ total score and the categorical explanatory variables with two or more levels.
We built four regression models for the dependent variable (CIQ total score), grouping previously reported predictors and a priori hypotheses into: (1) sociodemographic, (2) clinical, (3) claim-related, and (4) injury-related models. To limit collinearity and ensure parsimonious models, Spearman’s correlation coefficients were calculated for the clinically-relevant associations between covariates. The strongest correlations were observed between depression and insomnia (rs: 0.56), and between pain and insomnia (rs: 0.38). Stepwise eliminations were performed using p-values of ≥0.05 as the limiting threshold. Sex and age were included in every model, regardless of any association. Data for two participants who were injured >10 years earlier were omitted. The final regression model was derived using variables that were significantly associated with CI in the four individual models. The final regression analysis indicated that the 92 participants provided sufficient power for this study, with approximately nine participants per independent variable [38, 39].
Results
Table 1 presents the characteristics of the 94 participants (58 men and 36 women) with a clinical diagnosis of mTBI. The mean age was 45.2 ± 9.9 years. Twenty-five workers (27 %) were single, widowed, or divorced, and 56 workers (60 %) had at least a post-secondary degree. Thirty-three workers (35 %) were in the middle or low weekly income categories at their injury.
Table 1.
Socio-demographic, injury-related, clinical, and claim-related characteristics of the study population
| Category | Variables | n (%Na) | Mean (SD)/median (Q3-Q1) (continuous variables) | CIQ score mean (SD) (binary/categorical variables)b | Rho (continuous variables)b | P-value |
|---|---|---|---|---|---|---|
| Socio-demographic | Sex | |||||
| Male | 58 (62) | NA | 13.96 (4.67) | NA | 0.086 | |
| Female | 36 (38) | 15.56 (6.01) | ||||
| Age, years | 94 (100) | 45.2 (9.94) | NA | −0.092 | 0.377 | |
| Marital status | ||||||
| Married/common law | 69 (73) | NA | 13.81 (4.63) | NA | 0.018 | |
| Single/divorced/widowed | 25 (27) | 16.68 (6.18) | ||||
| English first language | ||||||
| Yes | 77 (82) | NA | 14.82 (4.85) | NA | 0.079 | |
| No | 17 (18) | 12.47 (6.66) | ||||
| Education | ||||||
| ≤High school | 34 (36) | NA | 11.21 (3.28) | NA | 0.006 | |
| High school-college, prof. diploma | 32 (34) | 14.45 (5.78) | ||||
| University and higher | 24 (27) | 17.20 (4.89) | ||||
| Weekly income, $CAD | 94 (100) | 1056 (510) | NA | 0.189 | 0.074 | |
| Injury-related | Time since injury, days | 94 (100) | 197 (416-139) | NA | −0.166 | 0.110 |
| Mechanism of injury | ||||||
| Struck by inanimate object | ||||||
| Yes | 18 (19) | NA | 12.67 (4.33) | NA | 0.084 | |
| No | 76 (81) | 15.03 (5.32) | ||||
| Struck by another person | ||||||
| Yes | 10 (11) | 17.10 (4.89) | 0.105 | |||
| No | 84 (89) | 14.27 (5.19) | ||||
| Struck against object/structure | ||||||
| Yes | 16 (17) | 16.62 (4.81) | 0.247 | |||
| No | 78 (83) | 14.38 (5.23) | ||||
| Fall | ||||||
| Yes | 18 (19) | 13.61 (5.09) | 0.386 | |||
| No | 76 (81) | 14.80 (5.25) | ||||
| Loss of consciousness | 86 (100)a | |||||
| Yes | 29 (31) | NA | 14.17 (6.14) | NA | 0.572 | |
| No | 56 (69) | 14.86 (4.78) | ||||
| Post-traumatic amnesia | 86 (100)a | |||||
| Yes | 21 (25) | NA | 13.62 (6.00) | NA | 0.235 | |
| No | 65 (75) | 15.15 (4.81) | ||||
| Previous head trauma | 90 (100)a | |||||
| Yes | 23 (25) | NA | 15.70 (5.12) | NA | 0.299 | |
| No | 67 (75) | 14.39 (5.21) | ||||
| Trauma-related head MRI findings | 84 (100)a | |||||
| Yes | 0 | NA | NA | NA | NA | |
| No | 84 (100) | |||||
| Clinical | Comorbid conditions by self-report | |||||
| Arthritis | 93 (100)a | |||||
| Yes | 34 (37) | NA | 15.05 (5.24) | NA | 0.868 | |
| No | 59 (63) | 13.59 (5.09) | ||||
| Diabetes mellitus | ||||||
| Yes | 5 (5) | 10.40 (3.78) | 0.065 | |||
| No | 89 (95) | 14.81 (5.20) | ||||
| Heart disease | ||||||
| Yes | 6 (6) | 13.17 (8.13) | 0.057 | |||
| No | 88 (94) | 14.67 (5.01) | ||||
| Number of comorbid conditions | 94 (100) | 2.22 (1.04) | NA | −0.216 | 0.037 | |
| DSM-IV-TR disorders | ||||||
| Adjustment disorder | 88 (100)a | |||||
| Yes | 45 (51) | NA | 13.80 (5.62) | NA | 0.265 | |
| No | 43 (49) | 15.05 (4.75) | ||||
| Anxiety disorder | ||||||
| Yes | 40 (45) | 13.08 (5.49) | 0.028 | |||
| No | 48 (55) | 15.52 (4.76) | ||||
| Mood disorder | ||||||
| Yes | 37 (42) | 13.92 (6.14) | 0.456 | |||
| No | 51 (58) | 14.75 (4.57) | ||||
| Personality traits | 92 (100)a | |||||
| Cluster B | ||||||
| Yes | 15 (17) | 9.87 (3.16) | <0.0001 | |||
| No | 77 (83) | 15.50 (5.11) | ||||
| Cluster C | ||||||
| Yes | 42 (47) | 13.50 (5.66) | 0.034 | |||
| No | 50 (53) | 15.88 (4.47) | ||||
| Sleep disorder | ||||||
| Yes | 9 (10) | 17.71 (4.27) | 0.977 | |||
| No | 79 (90) | 17.79 (6.23) | ||||
| Substance-related disorder | ||||||
| Yes | 13 (15) | 12.69 (5.07) | 0.200 | |||
| No | 75 (85) | 14.71 (5.22) | ||||
| Comorbid conditions, by scales | ||||||
| Anxiety (HADS-A) | NA | 10.71 (4.74) | NA | −0.317 | 0.002 | |
| Depression (PHQ-9) | 16.77 (6.67) | −0.320 | 0.002 | |||
| Insomnia (ISI) | 17.46 (6.07) | −0.370 | <0.001 | |||
| Pain (VAS-P), current | 5.02 (2.40) | −0.344 | <0.001 | |||
| Symptom load | ||||||
| Balance issues | ||||||
| Yes | 44 (47) | NA | 14.82 (4.91) | NA | 0.673 | |
| No | 50 (53) | 14.36 (5.51) | ||||
| Bodily pain | ||||||
| Yes | 32 (34) | 13.53 (5.59) | 0.164 | |||
| No | 62 (66) | 15.11 (4.97) | ||||
| Mood disturbance | ||||||
| Yes | 62 (66) | 14.19 (4.86) | 0.327 | |||
| No | 32 (34) | 15.31 (5.84) | ||||
| Head and/or neck pain | ||||||
| Yes | 87 (93) | 14.22 (5.19) | 0.019 | |||
| No | 7 (7) | 19.00 (3.83) | ||||
| Photo-/phonophobia | ||||||
| Yes | 14 (15) | 15.07 (6.63) | 0.701 | |||
| No | 80 (85) | 14.49 (4.97) | ||||
| Claim- related | Current working status | 94 (100)a | ||||
| Working full-/part time | 40 (43) | NA | 17.78 (6.00) | NA | 0.607 | |
| On disability/laid off | 54 (57) | 16.45 (7.33) | ||||
| Previous WSIB claims | 88 (100)a | |||||
| Yes | 8 (9) | 16.12 (5.59) | 0.332 | |||
| No | 80 (91) | 14.24 (5.19) | ||||
| Probable/possible malingering, by DSM-IV-TR | ||||||
| Yes | 14 (16) | 10.93 (4.68) | 0.006 | |||
| No | 74 (84) | 15.07 (5.08) |
a N = 94 unless otherwise specified
bCommunity integration scores were compared using analysis of variance or Spearman’s correlation tests
NA Not applicable
Time since injury (TSI) distribution was greatly skewed (median, 197 days post-injury [interquartile range, 139–416 days]). The major mechanisms of injury were falls (19.1 %), being struck by (19.1 %) or against (17 %) an object, motor vehicle incidents (12.8 %), and being struck by a person (10.5 %). Among the 86 workers with available LOC and PTA data, 31 % had experienced some degree of LOC and 24.7 % had experienced PTA. Eighty-four participants underwent MRI, and none exhibited trauma-related brain changes.
Most participants (57 %) were receiving disability benefits at the time of assessment, and the rest were working full- or part-time. The most common pre-morbid occupational categories were skilled or factory workers and machine operators or assemblers (44 %); elementary occupations (35 %); managerial, professional, associate professional, or technician positions (14 %); and clerical support, service, or sales workers (7 %). Forty-five workers (47.9 %) worked shifts at their injury; 38 (84 %) worked rotating shifts; and 7 (16 %) worked night shifts.
Substantial proportions of the workers had one or more DSM-IV TR diagnoses (Table 1), including anxiety (45.5 %), mood (42.1 %), somatoform (29 %), and substance-related (14.8 %) disorders. Nine workers (10.2 %) were diagnosed with a sleep disorder, including 8 with a sleep-related breathing disorder. The most common post-morbid symptoms that affected functioning were head and neck pain (92.6 %), cognitive (71.3 %), mood- (66 %), sleep- (63 %),and balance-related (47 %) disturbances, and bodily pain (34 %).
The CIQ was normally distributed and its internal consistency was appropriate(Cronbach’s alpha, α = 0.70)(Figs. 3 and 4).
Fig. 3.
Fit diagnostics for community integration (a-h)
Fig. 4.
Residuals by regressor for community integration
Bivariate analyses
Single, widowed, or divorced workers had significantly higher CIQ total scores (p <0.001), compared to workers who were married or in relationships. Workers whose first language was English also had significantly higher CIQ scores (p = 0.006). In addition, participants who were employed as managers, professionals, technicians, or associate professionals had significantly higher CIQ scores (p = 0.014), compared to participants who worked in clerical support or service work, sales, and elementary occupations. Significant differences were observed in the CIQ scores for workers who did and did not report head and neck pain (p = 0.005). Workers with Axis IV-TR diagnoses of possible/probable malingering (p <0.001), cluster B disorders (p = 0.004), cluster C disorders (p = 0.009), mood disorders (p = 0.049), and cognitive disorders (p = 0.004) had significantly lower CIQ scores. The CIQ scores were also negatively correlated with pain (p <0.001), anxiety (p = 0.009), depression (p <0.001), and insomnia (p <0.001).
Multivariable regression analyses
Four preliminary multivariate linear regression analyses were performed to evaluate associations with CI. All models were age- and sex-adjusted (Fig. 5).
Fig. 5.
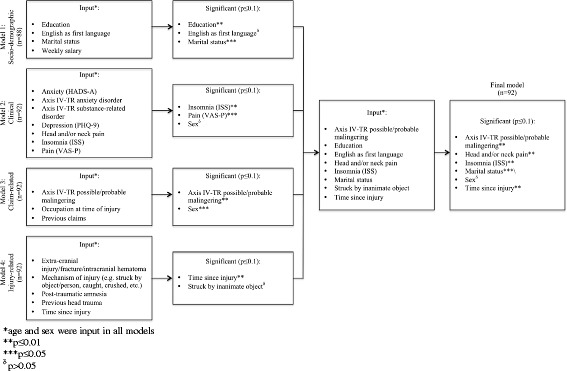
Depiction of multivariate regression analysis for community integration
The final regression model included education, marital status, English as the first language, TSI, being struck by inanimate objects, insomnia, head and/or neck pain, and Axis IV-TR malingering as independent variables. After the stepwise selection, the final model explained 36.6 % of the CI variance, and contained five significant variables: insomnia (β = –0.250, p <0.001), Axis IV-TR malingering (β = –4.923, p <0.001), TSI (β = –0.002, p = 0.025), head and/or neck pain (β = –4.186, p = 0.015), and marital status (β = –2.087, p = 0.048) (Table 2 and Fig. 5).
Table 2.
Summary of the stepwise multiple regression analysis for the final model, sex and age adjusted
| From model | Variable | β Coefficient | SE | P value | Partial R2 | Model R2 |
|---|---|---|---|---|---|---|
| #1 Socio-demographic | Education | 0.380 | 0.323 | 0.242 | 0.011 | 0.011 |
| English as first language | 1.304 | 1.179 | 0.272 | 0.018 | 0.029 | |
| Marital status | −2.087 | 1.041 | 0.048 | 0.043 | 0.072 | |
| #2 Clinical | Insomnia | −0.250 | 0.076 | <0.001 | 0.159 | 0.231 |
| Head and/or neck pain | −4.186 | 1.679 | 0.015 | 0.039 | 0.270 | |
| #3 Claim-related | Axis-IV-TR malingering | −4.923 | 1.302 | <0.001 | 0.069 | 0.339 |
| #4 Injury- related | Time since injury | −0.002 | 0.001 | 0.025 | 0.075 | 0.414 |
| Struck by inanimate object | 1.965 | 1.059 | 0.076 | 0.014 | 0.428 |
Discussion
In this study of 94 workers with mTBI, we found that insomnia, head or neck pain, being married or in a relationship, TSI, and a diagnosis of possible/probable malingering were independently associated with limited CI.
Unfortunately, there are no normative data for CIQ scores in mTBI. However, the CIQ total and subscale scores in the present study were similar to the mean scores at 1 year post-injury in participants with more severe TBI, as reported by Sanders et al. (GCS: 8.43 ± 3.8) [40] and Seale et al. (GCS: 6.5 ± 3.7) [41]. As in previous studies [42, 43], the highest scores were observed in the social integration domain and the lowest scores were observed in the productive activities domain. However, our participants had slightly higher scores in the home integration domain. One possible explanation for this discrepancy is that our study evaluated workers with persistent symptoms, and the majority of participants were receiving disability benefits at their time of assessment. Thus, our data suggests that disability status is strongly associated with impaired productive activity, although it had a lesser effect on social and family integration in our study.
Our findings also highlight the associations between insomnia, pain, and depression, in agreement with earlier reports [42–49]. However, insomnia has been viewed as a symptom that occurs in the context of another disorder (i.e., depression [42–45], pain [46–48], or brain injury [49]), rather than as an independent disorder within mTBI. In many depressed individuals, however, insomnia signals the onset of depression and may prevent recovery, even after adequate treatment [50, 51]. Similarly, the stress created by insomnia may aggravate or serve as a catalyst for the involvement of the hypothalamic orexinergic or hypocretinergic system in pain and headaches [52]. This may be relevant for our participants, as the majority continued to experience pain and depressive symptoms, despite being diagnosed with and treated for both at their assessment. Therefore, insomnia and its causes should be addressed separately from depression and pain in mTBI, in order to maximize treatment outcomes, especially regarding CI.
We also found that a diagnosis of possible/probable malingering was associated with a poorer CI. Although the presence of malingering or symptom exaggeration does not preclude the existence of symptoms or disorders, it does make the quantification of these issues impossible [53, 54]. Therefore, future research should be designed identify the determinants of a malingering diagnosis among injured workers. According to the DSM-IV, a diagnosis of malingering is appropriate when ≥2 of 4 criteria are met [36, 55]: (1) presentation of symptoms in a medico-legal context, (2) discordance between the individual’s stated disability and objective data, (3) uncooperative behavior during evaluation, and (4) presentation of antisocial personality disorder. However, our participants met the first two criteria, as they were referred by the WSIB for evaluation and mTBI is a clinical diagnosis that cannot be confirmed with objective data (i.e., none of our participants had positive MRI findings, and there is currently no sensitive and specific imaging technique to diagnose mTBI) [55, 56]. In addition, a worker with certain personality traits may find being questioned about their disability or injury to be unnerving, which may satisfy the third criterion independent of any malingering. Furthermore, recommendations have been published to implement neuropsychological testing for possible malingering in persons with TBI [57]. This raises the issue of language proficiency [49, 58], as these evaluations would be performed using tests that were developed in English and have not been validated in other languages. Therefore, English proficiency is an important variable to consider in future studies regarding performance validity.
We also found that a longer TSI was associated with a poorer CI after mTBI. Although this association was not evaluated longitudinally, our results may indicate that other relevant factors (e.g., psychological, medical, or other) can develop after the mTBI and strengthen over time, thereby impeding CI [59]. Similarly, psychological and psychosocial factors that present in the early post-injury period can influence outcomes across the entire recovery timeline [60]. Unfortunately, the cross-sectional nature of our study does not allow us to provide insight regarding this topic. Nonetheless, our results support the notion that patient assessments should begin as early as possible, which can establish baseline findings for CI and time-dependent outcomes.
Unexpectedly, being married was not related to a better CI, and our results indicate that marriage had a negative effect on CI. In contrast, earlier studies have reported that married people with a disability have higher levels of life satisfaction [61, 62], fewer handicaps [63], and longer life expectancy [64], compared to their unattached counterparts. However, our results should be interpreted with caution, as one-third of our participants were single, divorced, or widowed at the time of assessment, and we did not assess whether or not this status changed after their injury. Nevertheless, our findings suggest that workers who are married or in relationships may rely on their partners for assistance in performing their family and societal duties, which would hinder their independent CI. This observation highlights uncertainty regarding the role of marriage and/or partner support in the context of compromised CI, and longitudinal analysis of relationship status and patient outcomes (both personal and familial) may provide insight regarding their interdependence and independence.
The strengths of our study include a well-characterized sample of insured workers with a confirmed mTBI diagnosis. In addition, this is the first study to simultaneously address the prevalence of various sociodemographic, clinical, claim-related, and TBI-related variables, and their subsequent impact on CI. Furthermore, we utilized the TRIPOD checklist, which is a valuable reference for good reporting of multivariable prediction models. Therefore, the present study provides foundational data for a comprehensive longitudinal study that can evaluate the risk factors for prolonged recovery and reduced post-mTBI CI.
Our study has several limitations. First, our model for estimating CI was complex, which reflects the large number of covariates that were associated with CI and were not included in our final model. Therefore, a study with a large sample, standardized data collection and calculations will be needed to validate our model. Second, generalization of our findings may be limited, as a high prevalence of clinically relevant disorders was observed in our sample, and each of these disorders have been associated with poor post-injury outcomes. Furthermore, our study only aimed to evaluate workers who had a prolonged recovery after their injury, as they experience the greatest effects of mTBI and are the most difficult to rehabilitate. Third, the R2 value of 0.366 for our final regression analysis indicates that that only 36. 6 % of the variance in the mean CI can be explained by the above named variables and insomnia explains most of the variance (i.e., 15.9 %). This finding may be due to the omission of information regarding various critical CI areas, such as psychological sense of community, satisfaction with community, and perception of safety [65–67]. However, given that our results support the notion of CI as a time-dependent construct, and our data consisted of various time series, the R2 value for insomnia provides solid support of it as a covariate of CI, bearing in mind the fact that we are looking for meaningful associations in the context of delayed recovery from mTBI in the presence of many a priori defined relationships and a relatively small sample size.
Finally, this study highlighted the factors that were associated with CI in a population of workers with mTBI, although the longitudinal relationships between these factors and poor post-injury outcomes remain to be determined. Therefore, further research regarding this topic may facilitate the development of interventions that improve the CI of injured workers with mTBI.
Conclusions
Community integration is increasingly being recognized as a highly relevant outcome in outpatient populations, and is currently listed among the criteria that are used to assess the participation domain of the International Classification of Functioning, Disability, and Health [68] after TBI. Our results suggest that the CI may differ across various clinical populations, based on the presence or absence of insomnia and head or neck pain. Therefore, specialists who assess workers with mTBI should be particularly sensitive to these complaints, and should thoroughly investigate the etiology of these symptoms. In addition, we found that marital status may hinder CI, and that CI was related to an Axis IV-TR diagnosis of malingering. Thus, efforts to increase post-injury CI should be guided by a comprehensive understanding of the diverse factors that contribute to outcomes beyond the persistent post-concussive symptoms.
Acknowledgements
We gratefully acknowledge Mr. Kevin McCurley for his help with the data management, Mr. Chen Xiong for his help with the re-abstraction of the data, Ms. Lee Vernich (Dalla Lana School of Public Health) for her help with the statistical analysis. The first author was supported by 2012/2013 Toronto Rehabilitation Institute Scholarship, the Ontario Graduate Scholarship 2012/2013 and the 2013/2015 Frederick Banting and Charles Best Doctoral Research Award from the Canadian Institutes of Health Research (CIHR) and the Work Disability Prevention CIHR Strategic Training Program. AC was supported by the CIHR Chair in Gender, Work and Health (CGW-126580). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CIQ
Community integration questionnaire
- GCS
Glasgow coma scale
- HADS-A
Hospital anxiety and depression scale
- LOC
Loss of consciousness
- MRI
Magnetic resonance imaging
- mTBI
Mild traumatic brain injury
- MVI
Motor vehicle injury
- PHQ-9
Patient health questionnaire-9
- PTA
Post-traumatic amnesia
- P-VAS
Pain visual analogue scale
- RTW
Return to work
- SD
Standard deviation
- TSI
Time since injury
- TBI
Traumatic brain injury
- TRIPOD
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
- UHN
University health network
- WSID
Workplace safety and insurance board
Additional files
Standardized measured used, with examples of uses in TBI populations and their evaluated applicability [69–72]. (DOCX 45 kb)
Categories and types of variables collected. (DOCX 16 kb)
Footnotes
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
TM: developed study concept and design, was responsible for acquisition of data, statistical analysis (with the help of LV, acknowledged), interpretation of data and drafting of the manuscript. CMS: participated in the design of the study, interpretation of data and general supervision of the first author. SM: provided administrative, technical, proofreading, and material support. DJC: participated in the design of the study, interpretation of data and general supervision of the first author. AC: supervised TM in the development of the study design, statistical analysis and interpretation of data. All authors provided critical revision of the manuscript for important intellectual content and approved the final manuscript.
Authors’ information
Not applicable.
Availability of data and materials
Not applicable.
Contributor Information
Tatyana Mollayeva, Email: tatyana.mollayeva@utoronto.ca.
Colin M. Shapiro, Email: colinshapiro@rogers.com
Shirin Mollayeva, Email: shirin.mollayeva@utoronto.ca.
J. David Cassidy, Email: dcassidy@health.sdu.dk.
Angela Colantonio, Email: angela.colantonio@utoronto.ca.
References
- 1.World Health Organization . Traumatic brain injury: neurological disorders. Public health challenges. Geneva: WHO Press; 2006. [Google Scholar]
- 2.Hyder A, Wunderlich C, Puvanachandra P, Gururaj G, Kobusingye O. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–53. [PubMed] [Google Scholar]
- 3.World Health Organization . Projection of Mortality and Burden of Disease to 2030. Death by Income Group. Geneva: WHO Press; 2002. [Google Scholar]
- 4.Liao CC, Chou YC, Yeh CC, Hu CJ, Chiu WT, Chen TL. Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin Proc. 2014;89:163–72. doi: 10.1016/j.mayocp.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Catroppa C, Crossley L, Hearps SJ, Yeates KO, Beauchamp M, Rogers K, et al. Social and behavioral outcomes: pre-injury to 6 months following childhood traumatic brain injury. J Neurotrauma. 2015;32:109–15. doi: 10.1089/neu.2013.3276. [DOI] [PubMed] [Google Scholar]
- 6.Corrigan JD, Cuthbert JP, Harrison-Felix C, Whiteneck GG, Bell JM, Miller AC, et al. US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil. 2014;29:E1–9. doi: 10.1097/HTR.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 7.Lewis FD, Horn GJ. Traumatic brain injury: analysis of functional deficits and posthospital rehabilitation outcomes. J Spec Oper Med. 2013;13:56–61. doi: 10.55460/ATYP-5WSB. [DOI] [PubMed] [Google Scholar]
- 8.Hart T, Sherer M, Temkin N, Whyte J, Dikmen S, Heinemann AW, et al. Participant-proxy agreement on objective and subjective aspects of societal participation following traumatic brain injury. J Head Trauma Rehabil. 2010;25:339–48. doi: 10.1097/HTR.0b013e3181c7e60b. [DOI] [PubMed] [Google Scholar]
- 9.Williams MW, Rapport LJ, Millis SR, Hanks RA. Psychosocial outcomes after traumatic brain injury: life satisfaction, community integration, and distress. Rehabil Psychol. 2011;59:298–305. doi: 10.1037/a0037164. [DOI] [PubMed] [Google Scholar]
- 10.Turgeon AF, Lauzier F, Simard JF, Scales DC, Burns KE, Moore L, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183:1581–8. doi: 10.1503/cmaj.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grauwmeijer E, Heijenbrok-Kal MH, Ribbers GM. Health-related quality of life 3 years after moderate to severe traumatic brain injury: a prospective cohort study. Arch Phys Med Rehabil. 2014;95:1268–76. doi: 10.1016/j.apmr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Forslund MV, Roe C, Sigurdardottir S, Andelic N. Predicting health-related quality of life 2 years after moderate-to-severe traumatic brain injury. Acta Neurol Scand. 2013;128:220–7. doi: 10.1111/ane.12130. [DOI] [PubMed] [Google Scholar]
- 13.Wertheimer JC, Hanks RA, Hasenau DL. Comparing functional status and community integration in severe penetrating and motor vehicle-related brain injuries. Arch Phys Med Rehabil. 2008;89:1983–90. doi: 10.1016/j.apmr.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Sugerman DE, Xu L, Pearson WS, Faul M. Patients with severe traumatic brain injury transferred to a Level I or II trauma center: United States, 2007 to 2009. J Trauma Acute Care Surg. 2012;73:1491–9. doi: 10.1097/TA.0b013e3182782675. [DOI] [PubMed] [Google Scholar]
- 15.Zelnick LR, Morrison LJ, Devlin SM, Bulger EM, Brasel KJ, Sheehan K, et al. Addressing the challenges of obtaining functional outcomes in traumatic brain injury research: missing data patterns, timing of follow-up, and three prognostic models. Resuscitation Outcomes Consortium Investigators. J Neurotrauma. 2014;31:1029–38. doi: 10.1089/neu.2013.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Injury Prevention and Control. Centers for Disease Control and Prevention (CDC) Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. [Atlanta: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 17.Mild Traumatic Brain Injury Committee. A. C. o. R. M. Head Injury Interdisciplinary Special Interest Group Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–7. doi: 10.1097/00001199-199309000-00010. [DOI] [Google Scholar]
- 18.Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83:217–23. doi: 10.1136/jnnp-2011-300767. [DOI] [PubMed] [Google Scholar]
- 19.Merrick D, Stålnacke BM. Five years post whiplash injury: symptoms and psychological factors in recovered versus non-recovered. BMC Res Notes. 2010;3:190. doi: 10.1186/1756-0500-3-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maroon JC, Mathyssek C, Bost J. Cerebral concussion: a historical perspective. Prog Neurol Surg. 2014;28:1–13. doi: 10.1159/000358746. [DOI] [PubMed] [Google Scholar]
- 21.Kraus JF, Hsu P, Schafer K, Afifi AA. Sustained outcomes following mild traumatic brain injury: results of a five-emergency department longitudinal study. Brain Inj. 2014;28:1248–56. doi: 10.3109/02699052.2014.916420. [DOI] [PubMed] [Google Scholar]
- 22.Carroll LJ, Cassidy JD, Peloso PM, Garritty C, Giles-Smith L, et al. WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ. Insomnia. JAMA. 2013;309:706–16. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paunio T, Korhonen T, Hublin C, Partinen M, Koskenvuo K, Koskenvuo M, et al. Poor sleep predicts symptoms of depression and disability retirement due to depression. J Affect Disord. 2014;172C:381–389. doi: 10.1016/j.jad.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Sivertsen B, Lallukka T, Salo P, Pallesen S, Hysing M, Krokstad S, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23(2):124–32. doi: 10.1111/jsr.12102. [DOI] [PubMed] [Google Scholar]
- 26.Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak. 2006;6:38. doi: 10.1186/1472-6947-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingsma HF, Yue JK, Maas AI, Steyerberg EW, Manley GT, TRACK-TBI Investigators et al. Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the track-TBI pilot study. J Neurotrauma. 2015;32:83–94. doi: 10.1089/neu.2014.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs B, Beems T, Stulemeijer M, van Vugt AB, van der Vliet TM, Borm GF, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma. 2010;27:655–68. doi: 10.1089/neu.2009.1059. [DOI] [PubMed] [Google Scholar]
- 29.MRC CRASH Trial Collaborators. Perel P, Arango M, Clayton T, Edwards P, Komolafe E, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–9. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stulemeijer M, van der Werf S, Borm GF, Vos PE. Early prediction of favourable recovery 6 months after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:936–42. doi: 10.1136/jnnp.2007.131250. [DOI] [PubMed] [Google Scholar]
- 31.Quatrano LA, Cruz TH. Future of outcome measurement: impact on research in medical rehabilitation and neurologic populations. Arch Phys Med Rehabil. 2011;92(Suppl 10):S7–11. doi: 10.1016/j.apmr.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Parker G, Bernard S, Gridley K, Aspinal F, Light K. Rapid systematic review of international evidence on integrated models of care for people with long-term neurological conditions: technical report. SPRU Working Paper No SDO 2400. York: Social Policy Research Unit, University of York; 2010. [Google Scholar]
- 33.von Bonsdorff MB, Seitsamo J, Ilmarinen J, Nygård CH, von Bonsdorff ME, Rantanen T. Work ability in midlife as a predictor of mortality and disability in later life: a 28-year prospective follow-up study. CMAJ. 2011;183(4):E235–42. doi: 10.1503/cmaj.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willer B, Rosental M, Kreutzer JS, Gordon WA, Rempel R. Assessment of community integration following rehabilitation for traumatic brain injury. J Head Trauma Rehabil. 1993;8:75–87. doi: 10.1097/00001199-199308020-00009. [DOI] [Google Scholar]
- 35.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68:134–43. doi: 10.1016/j.jclinepi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association . Diagnostic and statistical manual of mental disorder. 4. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 37.Lequerica AH, Chiaravalloti ND, Sander AM, Pappadis MR, Arango-Lasprilla JC, Hart T, et al. The Community Integration Questionnaire: factor structure across racial/ethnic groups in persons with traumatic brain injury. J Head Trauma Rehabil. 2013;28:E14–22. doi: 10.1097/HTR.0b013e31826e3ca8. [DOI] [PubMed] [Google Scholar]
- 38.Norman GR, Streiner DL. Biostatistics: the bare essentials. Hamilton: B.C. Decker; 2000. [Google Scholar]
- 39.Dupont DW. Statistical modelling for biomedical researchers. A simple introduction to the analysis of complex data. 2. New York: Cambridge University Press; 2009. [Google Scholar]
- 40.Sander AM, Fuchs KL, High WM, Hall KM, Kreutzer JS, Rosental M. The Community Integration Questionnaire revisited: an assessment of factor structure and validity. Arch Phys Med Rehabil. 1999;80:1303–8. doi: 10.1016/S0003-9993(99)90034-5. [DOI] [PubMed] [Google Scholar]
- 41.Seale GS, Caroselli JS, High WM, Jr, Becker CL, Neese LE, Scheibel R. Use of the Community Integration Questionnaire (CIQ) to characterize changes in functioning for individuals with traumatic brain injury who participated in a post-acute rehabilitation programme. Brain Inj. 2002;16:955–67. doi: 10.1080/02699050210155258. [DOI] [PubMed] [Google Scholar]
- 42.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. The diagnosis and management of insomnia in clinical practice: a practical evidence-based approach. CMAJ. 2000;162(2):216–20. [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton EL. Psychiatric disorders and sleep issues. Med Clin North Am. 2014;98:1123–43. doi: 10.1016/j.mcna.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Mason EC, Harvey AG. Insomnia before and after treatment for anxiety and depression. J Affect Disord. 2014;168:415–21. doi: 10.1016/j.jad.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Soehner AM, Kaplan KA, Harvey AG. Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J Affect Disord. 2014;167:93–7. doi: 10.1016/j.jad.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung S, Bohnen NI, Albin RL, Frey KA, Müller ML, Chervin RD. Insomnia and sleepiness in Parkinson disease: associations with symptoms and comorbidities. J Clin Sleep Med. 2013;9:1131–7. doi: 10.5664/jcsm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alföldi P, Wiklund T, Gerdle B. Comorbid insomnia in patients with chronic pain: a study based on the Swedish quality registry for pain rehabilitation (SQRP) Disabil Rehabil. 2014;36:1661–9. doi: 10.3109/09638288.2013.864712. [DOI] [PubMed] [Google Scholar]
- 48.Emery PC, Wilson KG, Kowal J. Major depressive disorder and sleep disturbance in patients with chronic pain. Pain Res Manag. 2014;19:35–41. doi: 10.1155/2014/480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouellet MC, Savard J, Morin CM. Insomnia following traumatic brain injury: a review. Neurorehabil Neural Repair. 2004;18:187–98. doi: 10.1177/1545968304271405. [DOI] [PubMed] [Google Scholar]
- 50.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36:83–90. doi: 10.5665/sleep.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holland P, Goadsby PJ. The hypothalamic orexinergic system: pain and primary headaches. Headache. 2007;47(6):951–62. doi: 10.1111/j.1526-4610.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 53.Belanger H, Barwick F, Kip K, Kretzmer T, Vanderploeg R. Postconcussive symptom complaints and potentially malleable positive predictors. Clin Neuropsychol. 2013;27:343–55. doi: 10.1080/13854046.2013.774438. [DOI] [PubMed] [Google Scholar]
- 54.LoPiccolo CJ, Goodkin K, Baldewicz TT. Current issues in the diagnosis and management of malingering. Ann Med. 1999;31:166–74. doi: 10.3109/07853899909115975. [DOI] [PubMed] [Google Scholar]
- 55.Fox WC, Park MS, Belverud S, Klugh A, Rivet D, Tomlin JM. Contemporary imaging of mild TBI: the journey toward diffusion tensor imaging to assess neuronal damage. Neurol Res. 2013;35:223–32. doi: 10.1179/1743132813Y.0000000162. [DOI] [PubMed] [Google Scholar]
- 56.Schrader H, Mickeviciene D, Gleizniene R, Jakstiene S, Surkiene D, Stovner LJ, Obelieniene D. Magnetic resonance imaging after most common form of concussion. BMC Med Imaging. 2009;9:11. doi: 10.1186/1471-2342-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bordini EJ, Chaknis MM, Ekman-Turner RM, Perna RB. Advances and issues in the diagnostic differential of malingering versus brain injury. NeuroRehabilitation. 2002;17:93–104. [PubMed] [Google Scholar]
- 58.Rosselli M, Ardila A, Santisi MN, Arecco Mdel R, Salvatierra J, Conde A, et al. Stroop effect in Spanish-English bilinguals. J Int Neuropsychol Soc. 2002;8:819–27. doi: 10.1017/S1355617702860106. [DOI] [PubMed] [Google Scholar]
- 59.Lishman W. Physiogenesis and psychogenesis in the post concussional syndrome. Br J Psychiatry. 1988;153:460–9. doi: 10.1192/bjp.153.4.460. [DOI] [PubMed] [Google Scholar]
- 60.Silverberg N, Iverson G. Etiology of the post-concussion syndrome: physiogenesis and psychogenesis revisited. NeuroRehabilitation. 2011;29:317–29. doi: 10.3233/NRE-2011-0708. [DOI] [PubMed] [Google Scholar]
- 61.Ross CE. Reconceptualizing marital status as a continuum of social attachment. J Marriage Fam. 1995;57:129–40. doi: 10.2307/353822. [DOI] [Google Scholar]
- 62.Soons JPM, Liefbroer AC, Kalmijn M. The long-term consequences of relationship formation for subjective well-being. J Marriage Fam. 2009;71:1254–70. doi: 10.1111/j.1741-3737.2009.00667.x. [DOI] [Google Scholar]
- 63.Kreuter M, Sullivan M, Dahllof A, Siosteen A. Partner relationships, functioning, mood and global quality of life in persons with spinal cord injury and traumatic brain injury. Spinal Cord. 1998;36:252–61. doi: 10.1038/sj.sc.3100592. [DOI] [PubMed] [Google Scholar]
- 64.Chang FH, Wang YH, Jang Y, Wang CW. Factors associated with quality of life among people with spinal cord injury: application of the International Classification of Functioning, Disability and Health model. Arch Phys Med Rehabil. 2012;93:2264–70. doi: 10.1016/j.apmr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Rogers S, Howard G. Patient and community engagement: the approach at university health network. Healthc Q. 2014;17:70–3. doi: 10.12927/hcq.2014.24023. [DOI] [PubMed] [Google Scholar]
- 66.Kirmayer LJ, Narasiah L, Munoz M, Rashid M, Ryder AG, Guzder J, et al. Common mental health problems in immigrants and refugees: general approach in primary care. CMAJ. 2011;183(12):E959–67. doi: 10.1503/cmaj.090292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMillan DW, Chavis DM. Sense of community: a definition and theory. J Community Psychol. 1986;14:6–23. doi: 10.1002/1520-6629(198601)14:1<6::AID-JCOP2290140103>3.0.CO;2-I. [DOI] [Google Scholar]
- 68.World Health Organization . Towards a common language for functioning, disability and health: ICF. 2002. [Google Scholar]
- 69.Fann JR, Bombardier CH, Dikmen S, Esselman P, Warms CA, Pelzer E, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:501–11. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Dahm J, Wong D, Ponsford J. Validity of the Depression Anxiety Stress Scales in assessing depression and anxiety following traumatic brain injury. J Affect Disord. 2013;151:392–6. doi: 10.1016/j.jad.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Gagnon C, Bélanger L, Ivers H, Morin CM. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701–10. doi: 10.3122/jabfm.2013.06.130064. [DOI] [PubMed] [Google Scholar]
- 72.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–38. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 73.Fayers PM, Hand DJ. Factor analysis, causal indicators, and quality of life. Qual Life Res. 1997;6:139–50. doi: 10.1023/a:1026490117121. [DOI] [PubMed] [Google Scholar]


