Abstract
Objective
Guidelines for prevention of mother-to-child transmission of HIV have developed rapidly, yet little is known about how outcomes of HIV-exposed infants have changed over time. We describe HIV-exposed infant outcomes in Kinshasa, Democratic Republic of Congo, between 2007 and 2013.
Design
Cohort study of mother–infant pairs enrolled in family-centered comprehensive HIV care.
Methods
Accounting for competing risks, we estimated the cumulative incidences of early infant diagnosis, HIV transmission, death, loss to follow-up, and combination antiretroviral therapy (cART) initiation for infants enrolled in three periods (2007–2008, 2009–2010, and 2011–2012).
Results
1707 HIV-exposed infants enrolled at a median age of 2.6 weeks. Among infants whose mothers had recently enrolled into HIV care (N = 1411), access to EID by age two months increased from 28% (95% confidence limits [CL]: 24,34%) among infants enrolled in 2007-2008 to 63% (95% CL: 59,68%) among infants enrolled in 2011–2012 (Gray's p-value <0.01). The 18-month cumulative incidence of HIV declined from 16% (95% CL: 11,22%) for infants enrolled in 2007–2008 to 11% (95% CL: 8,16%) for infants enrolled in 2011–2012 (Gray's p-value = 0.19). The 18-month cumulative incidence of death also declined, from 8% (95% CL: 5,12%) to 3% (95% CL: 2,5%) (Gray's p-value = 0.02). LTFU did not improve, with 18-month cumulative incidences of 19% (95% CL: 15,23%) for infants enrolled in 2007-2008 and 22% (95% CL: 18,26%) for infants enrolled in 2011–2012 (Gray's p-value = 0.06). Among HIV-infected infants, the 24-month cumulative incidence of cART increased from 61% (95% CL: 43,75%) to 97% (95% CL: 82,100%) (Gray's p-value < 0.01); the median age at cART decreased from 17.9 to 9.3 months. Outcomes were better for infants whose mothers enrolled before pregnancy.
Conclusions
We observed encouraging improvements, but continued efforts are needed.
Keywords: Democratic Republic of Congo, HIV-exposed infant, mother-infant pair, pediatric HIV, prevention of mother-to-child HIV transmission/vertical transmission
Introduction
Globally, an estimated 1.4 million infants are born to HIV-infected pregnant women each year [1]. The guidelines for care of HIV-exposed infants have evolved rapidly in recent years and ambitious goals for controlling the pediatric HIVepidemic have been set [2,3]. Although the incidence of pediatric HIV is declining [1], we still know little about how programmatic and clinical outcomes of HIV-exposed infants have changed over time.
A major evolution in the care of HIV-exposed infants occurred with the implementation of early infant diagnosis (EID) by virological testing, which was first recommended by the WHO in 2007 [4]. Previously, HIV infection in exposed infants could only be confirmed by serology at 18 months of age [5,6]. EID is needed to ensure timely combination antiretroviral therapy (cART) initiation. Without cART, a third of HIV-infected infants will die in the first year of life [7–9].
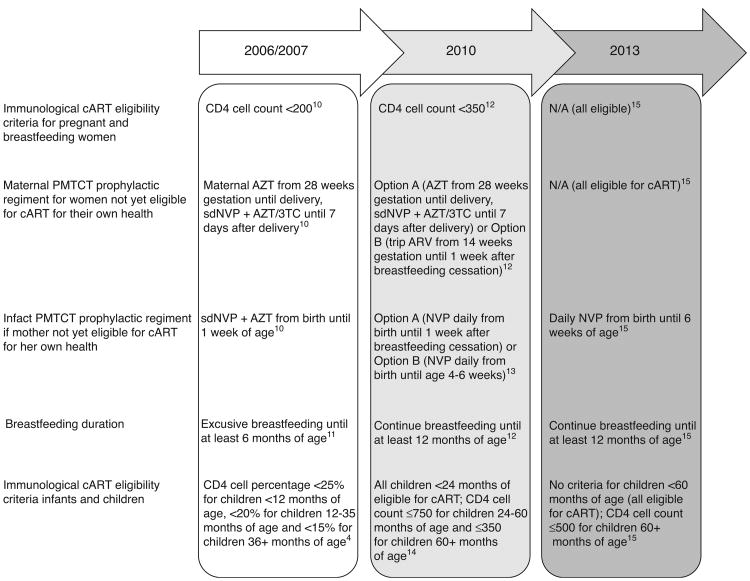
Since the scale-up of EID, other parts of the prevention of mother-to child HIV transmission (PMTCT) landscape have also evolved rapidly (Fig. 1) [4,10–15]. Mounting evidence on the importance of breastfeeding for preventing HIV-exposed infant mortality [16–18] led the WHO to increase the recommended breastfeeding period from 6 months (2006 recommendation [11]) to at least 12 months (2010 recommendation [13]). Due to the increased risk period for vertical HIV transmission through breastfeeding [19,20], the duration and complexity of antiretroviral prophylactic regimens in the 2010 guidelines also increased [12]. By 2012, the WHO endorsed lifelong cART for all pregnant women [21].
Fig. 1. Evolution of WHO guidelines for prevention of mother-to-child transmission of HIV.
3TC, lamivudine; AZT, zidovudine; cART, combination antiretroviral therapy; NVP, nevirapine; PMTCT, prevention of mother-to-child HIV transmission; sdNVP, single dose of nevirapine.
Evaluating and reporting outcomes in routine care settings is critical to demonstrate the scalability of recommended interventions for PMTCT and to assure quality care is being provided [22–24]. One study of 561 infants who received care between 2009 and 2012 in the Kilimanjaro Region of Tanzania reported 10% mother-to-child HIV transmission [25], despite the provision of prophylactic regimens that were expected to reduce vertical transmission to below 5% [12]. Another study of 311 mother–infant pairs in Malawi was able to reduce transmission to 3%, but 14% of infants died by 24 months of age [26].
These examples from the field highlight that, despite best efforts to implement current guidelines, PMTCT programs do not always achieve intended outcomes for HIV-exposed infants. Our understanding of the impact that PMTCT programs have had on HIV-exposed infant outcomes is incomplete, in part, because guidelines often change before the impacts of previous guidelines have been assessed. The goal of this study was to describe how key clinical and programmatic outcomes of HIV-exposed infants have changed over time in Kinshasa, Democratic Republic of Congo (DRC), under evolving guidelines for care. Beginning with the implementation of HIV-exposed infant care in Kinshasa, we describe temporal trends in the timing of EID, HIV transmission, death, and loss to follow-up (LTFU), as well as cART initiation among infants identified as HIV-infected. To our knowledge, this is the first study to assess if the implementation of new guidelines has coincided with improvements in these outcomes for HIV-exposed infants over time.
Methods
Study population
The data for this study come from a family-centered HIV prevention, care, and treatment program implemented at two sites (one primary healthcare center and one pediatric hospital) in Kinshasa, with technical assistance provided by the University of North Carolina at Chapel Hill (UNC-DRC program). The two centralized sites provided comprehensive care (including PMTCT) to HIV-positive women identified through routine HIV testing at 90 maternities and their newborn infants, former patients at 32 tuberculosis clinics, and HIV-positive children, as well as HIV-positive first-line family members of these individuals. Routinely collected data from HIV-exposed infants enrolled between January 2007 and June 2012 and their mothers were linked to construct a cohort of mother–infant pairs. Mother–infant linkages were constructed using routinely assigned unique patient codes. Each individual's record contained their own patient code, as well as a list of patient codes for their family members also receiving care in the UNC-DRC program. Infants enrolled after 18 months of age and infants not linked to a mother enrolled in the UNCDRC program by the time of infant enrollment were excluded.
Routine care and clinic procedures
In accordance with the WHO guidelines, the UNC-DRC protocol included EID by virological testing over the entire study period, with initial testing at 6 weeks of age and confirmatory virological testing of all positive results performed on a second specimen. However, until the end of 2009, HIV RNA assays were the only HIV virological tests available at the national laboratory in Kinshasa and stock-outs were frequent. HIV DNA PCR testing was implemented in November 2009, by which time the availability of virological tests had stabilized. Specimens for DNA PCR testing were collected on dried blood spots, which were transported to the national laboratory on a daily basis in vehicles provided by the UNC-DRC program. Information on the turnaround time for specimen processing was not available. All HIV testing was provided free of charge.
PMTCT prophylactic regimens, cART, and breastfeeding support were provided according to current WHO guidelines. Before 2010, the only available PMTCT prophylactic regimen for mothers not yet eligible for cART (CD4+ cell count >200) and their infants was single-dose nevirapine, which was provided according to the 2006 WHO guidelines for programs with limited capacity [10]. Mothers were encouraged to exclusively breastfeed for 6 months and then wean rapidly [11]. The ‘option A’ strategy was implemented in the DRC following the release of the 2010 WHO guidelines [12]. Mothers not yet eligible for cART (CD4+ cell count >350) received zidovudine prophylaxis through pregnancy and peripartum nevirapine with a zidovudine–lamivudine ‘tail’ around delivery. Their infants received extended nevirapine until 1 week after the cessation of breastfeeding. Mothers were encouraged to continue breastfeeding for at least 12 months [13].
Clinic visits for HIV-exposed infants were scheduled to occur every 4 weeks from the first visit at 2 weeks of age through 18 weeks of age, and then every 3 months thereafter. Infants below 18 months of age were deactivated from care following a negative HIV virological or serological test result obtained more than 3 months after the cessation of breastfeeding. Infants 18 months of age or older were deactivated from care following the cessation of breastfeeding and a negative serological test. HIV-infected infants were eligible to receive lifelong care and treatment in the UNC-DRC program. In 2007, infants below 18 months of age with a CD4+ cell percentage less than 20, infants 18–24 months of age with a CD4+ cell percentage less than 15, and all infants with a WHO clinical stage 3 or 4 were eligible for cART [4]. Between 2008 and 2010, infants 12–24 months of age with a CD4+ cell percentage less than 20 and all infants less than 12 months of age were eligible to receive cART [27]. Starting in 2010, all infants below 24 months of age were eligible for cART [14].
Definitions and statistical analysis
Demographics of infants and their mothers at infant enrollment were characterized using standard descriptive statistics. Infants were considered underweight or stunted if they had a weight-for-age or height-for-age Z-score more than 2 SDs below the median value for a given age group and sex. Z-scores were derived from the WHO Child Growth Standards [28] using the WHO Anthro software (version 3.2.2, January 2011) and macro for SAS. Growth status was based on the first available weight and height values measured within a month of enrollment.
Among all HIV-exposed infants, we estimated the 18-month cumulative incidences of specimen collection for initial HIV virological testing, confirmed HIV infection, death, and LTFU. For HIV-exposed infants with confirmed HIV infection by 18 months of age, we also estimated the 24-month cumulative incidence of cART initiation. In the LTFU analysis, follow-up time was defined as days from enrollment in care, and infants without a clinic visit beyond the enrollment visit were assigned 1 day of follow-up. Infants were considered lost to follow-up on their last attended clinic visit date following 3 failed tracking attempts after a missed appointment or if more than 6 months passed since they were last seen in the clinic. In all other analyses, follow-up time was defined as days from birth. Follow-up concluded when the first of the following occurred: the event of interest, a competing event, or censoring [29].
Competing risks arise in time-to-event analyses when individuals can experience events that preclude them from ever experiencing the event of interest [29]. Our study included multiple outcomes of interest, and competing events varied by analysis. Death was treated as a competing event in the analyses of specimen collection for HIV virological testing, confirmed HIV infection, LTFU, and cART initiation. Graduation from care (i.e. deactivation from care after a confirmed negative status or transition to lifelong HIV care if positive) was treated as a competing event in the analyses of LTFU, confirmed HIV infection, and death. In the analysis of first specimen collection for HIV virological testing, a confirmatory negative diagnosis by serology was also treated as a competing event. LTFU and transfer of care to another facility were treated as censoring events. Administrative censoring occurred on the date the dataset was closed for final analysis (August 2013) or when infants aged out of the risk period of interest (19 months of age in the analyses that included all HIV-exposed infants and 25 months of age in the analysis of cART initiation among HIV-infected infants).
To assess how infant outcomes changed over time, we compared cumulative incidence estimates and 95% confidence limits between infants enrolled in three time periods (2007–2008, 2009–2010, and 2011–2012). A P-value was obtained for Gray's test for equality of the cumulative incidence function [30] using the SAS %CIF macro (version 1.0, March 2012) [31]. Since all HIV-infected women enrolled in the UNC-DRC program were eligible to receive lifelong care, the proportion of infants whose mothers were newly enrolled into care during their most recent pregnancy declined over the study period. Because outcomes among infants whose mothers were enrolled into care before their current pregnancy may be different than those whose mothers were newly enrolled, we stratified comparisons of the cumulative incidence functions by maternal enrollment status (enrolled before most recent pregnancy or newly enrolled) for all outcomes except infant cART initiation. For this outcome, the number of HIV-infected infants whose mothers were previously enrolled was too small to allow stratified analyses.
Sensitivity analyses
To assess potential selection bias induced by informative censoring due to LTFU, we assessed the extreme bounds [32,33] of the overall 18-month cumulative incidence of HIV. The upper bound was estimated assuming all infants who were lost to follow-up instead were confirmed HIV-positive on the day they were lost to follow-up. The lower bound was estimated assuming all infants who were lost to follow-up were instead confirmed HIV-negative and ‘graduated’ from care at 18 months of age.
Finally, we wanted to know if implementation of PMTCT services differed between the two sites included in the study. To assess for differences, we compared the cumulative incidence functions for each of the outcomes of interest stratified by site of care. All analyses were conducted in SAS 9.3 (SAS Institute, Inc., Cary, North Carolina, USA).
Ethics statement
Written parental informed consent for the UNC-DRC program was obtained for all infants and written informed consent was obtained for all mothers. All research was approved by the Ethics Committee of the Kinshasa School of Public Health and the University of North Carolina at Chapel Hill Institutional Review Board.
Results
Study population characteristics
Among the 1908 HIV-exposed infants enrolled during the study period, 1707 were linked to a mother receiving care in the UNC-DRC program and included in the analysis. At enrollment, infants were a median of 2.6 weeks of age [interquartile range (IQR) 2.1–6.4] and almost all (90.6%) were breastfeeding (Table 1). Three hundred and thirty-six (20.3%) infants were underweight and 381 (23.1%) were stunted at enrollment, with little change over time. The proportion of infants who failed to receive a PMTCT prophylactic regimen declined from 29.7% for infants enrolled in 2007–2008 to 7.7% for infants enrolled in 2011–2012.
Table 1. Characteristics of HIV-exposed infants and their mothers at infant enrollment into care, by calendar period, Kinshasa, Democratic Republic of Congo.
| Year of infant enrollment into care | ||||
|---|---|---|---|---|
|
|
||||
| 2007–2012 (N = 1707) | 2007–2008 (N = 335) | 2009–2010 (N = 730) | 2011–2012 (N = 642) | |
| Infants | ||||
| Median age in weeks (IQR) | 2.6 (2.1–6.4) | 2.9 (2.1–9.7) | 2.4 (2.1–6.0) | 2.6 (2.1–6.0) |
| Sex [N (%)] | ||||
| Female | 872 (51.1) | 175 (52.2) | 375 (51.4) | 322 (50.2) |
| Male | 835 (48.9) | 160 (47.8) | 355 (48.6) | 320 (49.8) |
| PMTCT regimen [N (%)] | ||||
| No | 243 (16.7) | 76 (29.7) | 119 (20.9) | 48 (7.7) |
| Yes | 1210 (83.3) | 180 (70.3) | 451 (79.1) | 579 (92.3) |
| Breastfeeding [N (%)] | ||||
| No | 123 (9.4) | 29 (13.1) | 58 (13.1) | 36 (5.6) |
| Yes | 1182 (90.6) | 193 (86.9) | 384 (86.9) | 605 (94.4) |
| Cotrimoxazole prophylaxis [N (%)] | ||||
| No | 86 (5.0) | 19 (5.7) | 28 (3.8) | 39 (6.1) |
| Yes | 1621 (95.0) | 316 (94.3) | 702 (96.2) | 603 (93.9) |
| Underweighta [N (%)] | ||||
| No | 1317 (79.7) | 248 (79.2) | 564 (79.1) | 505 (80.5) |
| Yes | 336 (20.3) | 65 (20.8) | 149 (20.9) | 122 (19.5) |
| Stuntedb [N (%)] | ||||
| No | 1268 (76.9) | 233 (75.6) | 545 (76.5) | 490 (77.9) |
| Yes | 381 (23.1) | 75 (24.4) | 167 (23.5) | 139 (22.1) |
| Mothers | ||||
| Median age in years (IQR) | 31 (27–35) | 31 (28–35) | 31 (27–34) | 31 (27–35) |
| Median days enrolled (IQR) | 94 (23–168) | 75 (32–129) | 89 (0–154) | 117 (23–531) |
| Newly enrolled during most recent pregnancy [N (%)] | ||||
| No | 296 (17.3) | 20 (6.0) | 94 (12.9) | 182 (28.3) |
| Yes | 1411 (82.7) | 315 (94.0) | 636 (87.1) | 460 (71.7) |
| PMTCT regimen [N (%)]c | ||||
| No | 376 (22.0) | 76 (22.7) | 176 (24.1) | 124 (19.3) |
| Yes | 1331 (78.0) | 259 (77.3) | 554 (75.9) | 518 (80.7) |
| cART initiated [N (%)]d | ||||
| No | 1232 (72.2) | 279 (83.3) | 563 (77.1) | 390 (60.7) |
| Yes | 475 (27.8) | 56 (16.7) | 167 (22.9) | 252 (39.3) |
cART, combination antiretroviral therapy; IQR, interquartile range; PMTCT, prevention of mother-to-child HIV transmission. Covariate totals may not add up to the column totals due to missing data.
Underweight defined as weight-for-age Z-score −2 or less.
Stunted defined as height-for-age Z-score −2 or less.
Includes any maternal regimen (described in more detail in the methods section), including maternal cART if initiated before delivery.
Includes maternal cART initiated before infant enrollment.
At infant enrollment, mothers were a median of 31.1 years of age (IQR 27.0–34.7) and had been enrolled in the UNC-DRC program for a median of 94 days (IQR 23–168) (Table 1). Overall, 1331 (78.0%) of mothers received a PMTCT prophylactic regimen or cART during their pregnancy and 475 (27.8%) had initiated cART by the time of infant enrollment.
Outcomes of HIV-exposed infants
The 18-month cumulative incidence of having a specimen collected for initial virological HIV testing increased over the study period, with 99% [95% confidence limit (CL): 99, 100%] of the infants enrolled in 2011–2012 having a specimen collected by 18 months of age (Table 2). Among infants whose mothers were newly enrolled into care, the cumulative incidence of specimen collection by 2 months of age was 28% (95% CL: 24, 34%) for infants enrolled in 2007–2008, 49% (95% confidence limits 45, 53%) for infants enrolled in 2009–2010, and 63% (95% confidence limits 59, 68%) for infants enrolled in 2011–2012.
Table 2. Follow-up of HlV-exposed infants in Kinshasa, Democratic Republic of Congo, by calendar period at infant enrollment into care and enrollment status of the mother (newly enrolled during pregnancy or previously enrolled).
| Overall | Newly Enrolled | Previously Enrolled | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 2007–2008 | 2009–2010 | 2011–2012 | 2007–2008 | 2009–2010 | 2011–2012 | 2007–2008 | 2009–2010 | 2011–2012 | |
| All HlV-exposed infants (N) | 335 | 730 | 642 | 315 | 636 | 460 | 20 | 94 | 182 |
| First specimen collection for virological HIV test | |||||||||
| Person-months of follow-up | 1435 | 1943 | 694 | 1265 | 1692 | 483 | 170 | 251 | 211 |
| N | 220 | 604 | 612 | 212 | 522 | 435 | 8 | 82 | 177 |
| 18-month cumulative incidence (95% CL) | 0.73 (0.68, 0.78) | 0.89 (0.86, 0.91) | 0.99 (0.98, 1.00) | 0.76 (0.70, 0.80) | 0.88 (0.85, 0.91) | 0.99 (0.98, 1.00) | 0.40 (0.19, 0.60) | 0.89 (0.80, 0.94) | 0.99 (0.96, 1.00) |
| Median weeks from enrollment to collection (IQR)a | 12.3 (4.9–63.1) | 4.9 (3.7–27.9) | 4.0 (1.3–4.9) | 9.9 (4.7–50.0) | 4.9 (3.7–32.7) | 4.0 (0.1–5.0) | .c (30.7-.)c | 4.7 (3.9–8.6) | 4.3 (3.7–4.9) |
| Median age in weeks at collection (IQR)b | 30.3 (8.1–.)c | 8.3 (6.7–39.3) | 7.0 (6.6–10.4) | 25.4 (8.0–71.4) | 9.0 (6.9–39.4) | 7.3 (6.6–12.3) | .c (39.3-.)c | 7.3 (6.6–12.0) | 6.9 (6.4–7.6) |
| Final outcomes | |||||||||
| Person-months of follow-up | 3425 | 6446 | 8378 | 3227 | 5459 | 5614 | 198 | 987 | 2764 |
| Confirmed HIV infection (N) | 39 | 67 | 36 | 38 | 62 | 35 | 1 | 5 | 1 |
| 18-month cumulative incidence (95% CL) | 0.15 (0.11, 0.21) | 0.12 (0.09, 0.15) | 0.08 (0.06, 0.11) | 0.16 (0.11, 0.22) | 0.13 (0.10, 0.16) | 0.11 (0.08, 0.16) | 0.05 (0.00, 0.21) | 0.06 (0.02, 0.12) | 0.01 (0.00, 0.03) |
| Death (N) | 23 | 33 | 17 | 22 | 28 | 12 | 1 | 5 | 5 |
| 18-month cumulative incidence (95% CL) | 0.08 (0.05, 0.11) | 0.05 (0.04, 0.07) | 0.03 (0.02, 0.05) | 0.08 (0.05, 0.12) | 0.05 (0.03, 0.07) | 0.03 (0.02, 0.05) | 0.05 (0.00, 0.21) | 0.06 (0.02, 0.12) | 0.03 (0.01, 0.07) |
| LTFU (N) | 59 | 103 | 111 | 59 | 97 | 96 | 0 | 9 | 15 |
| 18-month cumulative incidence (95% CL) | 0.18 (0.14, 0.22) | 0.15 (0.13, 0.18) | 0.18 (0.15, 0.21) | 0.19 (0.15, 0.23) | 0.16 (0.13, 0.19) | 0.22 (0.18, 0.26) | 0.00 (0.00, 0.00) | 0.1 (0.05, 0.17) | 0.09 (0.05, 0.14) |
| HIV-infected infants (N) | 39 | 67 | 36 | ||||||
| Person-months of follow-up | 364 | 387 | 151 | ||||||
| Died before cART (N) | 6 | 8 | 1 | ||||||
| LTFU before cART (N) | 5 | 3 | 1 | ||||||
| cART (N) | 21 | 56 | 34 | ||||||
| 24-month cumulative incidence (95% CL) | 0.61 (0.43, 0.75) | 0.88 (0.77, 0.94) | 0.97 (0.82, 1.00) | ||||||
| Median months from enrollment to cART (IQR)a | 12.1 (5.0-.)c | 4.9 (3.0–11.8) | 3.8 (1.2–6.3) | ||||||
| Median age in months at cART (IQR)b | 17.9 (10.8-.)c | 9.2 (5.1–17.2) | 9.3 (5.5–13.5) | ||||||
cART, combination antiretroviral therapy; CL, confidence limits; IQR, interquartile range; LTFU, loss to follow-up
Estimated from the cumulative incidence function using time since enrollment as the time scale.
Estimated from the cumulative incidence function using age as the time scale.
The cumulative incidence in this period was less than 0.5 (median) or 0.75 (upper quartile range) by the end of follow-up.
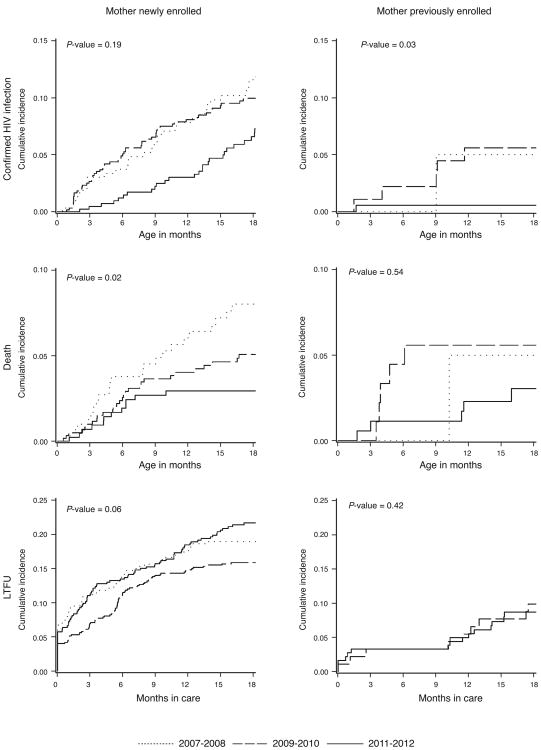
Among the 1411 infants with newly enrolled mothers, 135 infants were confirmed HIV-positive, 62 died, and 252 were lost to follow-up over 14 300 person-months of follow-up (Table 2). The 18-month cumulative incidence of HIV declined from 16% (95% CL: 11, 22%) for infants enrolled in 2007–2008 to 11% (95% CL: 8, 16%) for infants enrolled in 2011–2012 and death declined from 8% (95% CL: 5, 12%) to 3% (95% CL: 2, 5%) (Table 2 and Fig. 2). The 18-month cumulative incidence of LTFU did not improve over calendar time, with 18-month cumulative incidences ranging from 16% (95% CL: 13, 19%) to 22% (95% CL: 18, 26%) (Fig. 3).
Fig. 2. Eighteen-month cumulative incidence functions of confirmed HIV infection, death, and loss to follow-up among HIV-exposed infants in Kinshasa, Democratic Republic of Congo.
Cumulative incidence functions are plotted by calendar period at infant enrollment into care and enrollment status of the mother (newly enrolled during pregnancy or previously enrolled). P-values are for Gray's test for equality of the cumulative incidence functions. LTFU, loss to follow-up.
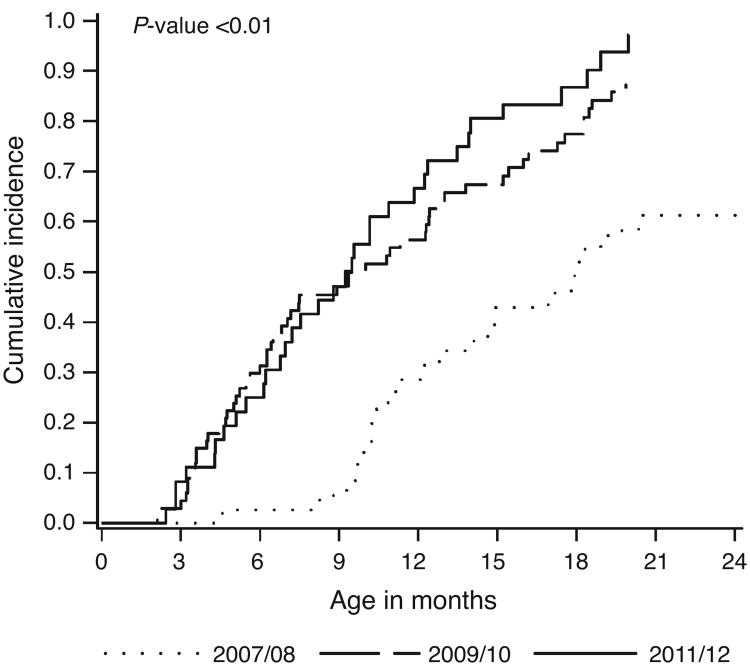
Fig. 3. Twenty-four-month cumulative incidence function of combination antiretroviral therapy initiation among HIV-infected infants in Kinshasa, Democratic Republic of Congo.
Cumulative incidence functions are plotted by calendar period at infant enrollment into care. The P-value is for Gray's test for equality of the cumulative incidence functions.
Among the 296 infants whose mothers enrolled before their current pregnancy, 7 infants were confirmed HIV-positive, 11 died, and 24 were lost to follow-up over 3949 person-months of follow-up (Table 2). The 18-month cumulative incidence of HIV declined from 5% (95% CL: 0, 21%) for infants enrolled in 2007–2008 to 1% (95% CL: 0, 3%) for infants enrolled in 2011–2012 (Table 2 and Fig. 2). Death also declined over calendar time, with 18-month cumulative incidences of 5% (95% CL: 0, 21%) for infants enrolled in 2007–2008 and 3% (95% CL: 1, 7%) for infants enrolled in 2011–2012. LTFU increased from 0% (95% CL: 0, 0%) to 9% (95% CL: 5, 14%).
Combination antiretroviral therapy initiation among HIV-infected infants
Among the 142 infants with confirmed HIV infection by 18 months of age, the median age at enrollment into care was 6.4 weeks (IQR: 2.3–32.0 weeks) and the median age at specimen collection for initial virological HIV testing was 17.3 weeks (IQR: 7.3–46.0 weeks). The 24-month cumulative incidence of starting cART increased over time, from 61% (95% CL: 43, 75%) for infants enrolled in 2007–2008 to 97% (95% CL: 82, 100%) for infants enrolled in 2011–2012 (Table 2 and Fig. 2). The median time between enrollment and cART initiation declined from 12.1 to 3.8 months, and the median age at cART initiation decreased from 17.9 to 9.3 months.
Sensitivity analyses
Our sensitivity analysis suggested that our estimates of the 18-month cumulative incidence of HIV may be susceptible to bias due to LTFU. Among all infants, the 18-month cumulative incidence of HIV was 11% (95% CL: 9, 13%). Assuming all infants who were lost to follow-up were instead confirmed HIV-positive, the estimate was 26% (95% CL: 24, 28%). Assuming all infants who were lost to follow-up were HIV-negative and ‘graduated’ from care at 18 months of age, the estimate was 9% (95% CL: 8, 11%).
There appeared to be some differences in infant outcomes between the two sites included in the analysis. The only differences that were statistically significant (i.e. Gray's P-value < 0.05) were specimen collection for HIV virological testing and confirmed HIV infection. At the pediatric hospital, the 18-month cumulative incidence of specimen collection was 82% (95% confidence limits 78, 85%) and the 18-month cumulative incidence of HIV was 12% (95% CI: 8, 16%). At the primary care center, the 18-month cumulative incidence of specimen collection was 94% (95% CI: 93, 96%) and the 18-month cumulative incidence of confirmed HIV was 11% (95% CI: 9, 14%).
Discussion
Our program's experience in Kinshasa shows that improvements over time can be achieved in a routine care setting under evolving guidelines for care. Among infants whose mothers were newly enrolled during their most recent pregnancy, we observed declines in both vertical HIV transmission and infant death. The 18-month cumulative incidence of HIV was 30% lower among infants enrolled in 2011–2012 than among infants enrolled in 2007–2008. The 18-month cumulative incidence of death declined by 60%. Among HIV-infected infants, we also observed an increase in the proportion initiating cART, with over 95% of infants enrolled in 2011–2012 initiating cART by 24 months of age.
Despite these achievements, our results, also reveal areas for improvement. Among infants in the 2011–2012 cohort, whose mothers were newly enrolled, the proportion with confirmed HIV infection by 18 months of age was still over 10% and our sensitivity analyses suggested that this may be an underestimate if those who were lost to follow-up were at a higher risk of HIV than those who were retained in care. Effective interventions can reduce transmission to below 5% in breastfeeding populations [34]. In our study, about 20% of mothers had not received a PMTCT prophylactic regimen or cART by delivery. Furthermore, most HIV-infected infants enrolled in 2011–2012 still did not have their positive status confirmed until after 9 months of age, and 21% of all infants died or were lost to follow-up before their HIV status could be determined. The proportion of infants presenting to care in poor health also remained high throughout the study period, with about 20% under-weight and 20% stunted overall.
Early infant diagnosis by virological testing did not become consistently available in Kinshasa until about halfway through the study period. An important limitation to our study is that the UNC-DRC program did not routinely collect information on how much time passed between specimen collection for virological testing and when results became available for clinical decision-making. Although the 18-month cumulative incidence of specimen collection increased to almost 100% over the study period, many infants failed to have a specimen collected by the 4–6-week target [15]. By 2 months of age, about 40% of infants with newly enrolled mothers still had not had a specimen collected. This figure leaves ample room for improvement, but is substantially higher than that reported for the country as a whole; a recent Joint United Nations Programme on HIV and AIDS report estimated that only 3% of HIV-exposed infants in the DRC received a virological test for HIV by 2 months of age [1].
Diagnosing HIV in infected infants as early as possible is crucial because up to a third may die in the first year of life without cART [7–9,14]. We observed that the median months from enrollment in care to cART initiation decreased from 12 months among infants enrolled in 2007–2008 to 4–5 months for infants enrolled in later periods, likely due to the increased availability of EID. However, half of HIV-infected infants enrolled in 2011– 2012 had not initiated cART by 9 months of age and over a quarter had not initiated cART by 12 months of age. There are likely several reasons why infants continue to experience delayed cART initiation despite the availability of EID. In our setting, one reason may have been that HIV-infected infants enrolled in care later than the population of all HIV-exposed infants. Although median age at enrollment was only 3 weeks more, over a quarter of HIV-infected infants enrolled in care after 32 weeks of age.
The number of studies describing obstacles to EID has increased [35–38], but a recent meta-analysis found that only one study traced HIV-infected infants from enrollment in PMTCT care to cART initiation [39]. The study, which included 202 HIV-infected infants in Malawi, observed that although the delay in cART initiation declined over time, the overall proportion of HIV-infected infants initiating cART did not improve [38]. It is crucial that PMTCT programs routinely record individual-level information at each step of the test-and-treat cascade and future research should assess where and why bottlenecks to the provision of early cART exist. Initiating the EID process at birth, particularly for high-risk infants, may also decrease delays in cART initiation [40].
Research has shown that infant nevirapine, a component of the PMTCT options A, B, and B+, can select non-nucleoside reverse transcriptase inhibitor mutations in the majority of infants who become infected with HIV despite prophylaxis [41]. Diagnosing HIV in infected infants early reduces exposure to nevirapine prophylaxis and thus the potential for HIV drug resistance. Given existing challenges to EID implementation in many settings, future studies should assess how HIV drug resistance and other treatment outcomes of HIV-infected infants change with the implementation of new PMTCT guidelines.
Unlike other outcomes assessed in this study, LTFU did not improve over calendar time and even appeared to increase among infants whose mothers enrolled in HIV care before their current pregnancy (although the increase was not statistically significant). The new consolidated WHO guidelines [15], which recommend interventions to improve program retention, may reduce obstacles to continued engagement in care. The UNC-DRC program increased its efforts to track patients who missed appointments over the study period, but it does not appear that these efforts led to a reduction in the proportion of infants who were lost to follow-up. Research suggests that infants are less likely to be retained in care if their caregivers perceive them as healthy and more likely to be retained in care if they are identified as HIV-infected [42,43]. Thus, it is possible that the implementation of EID and the communication of initial negative test results, as well as the implementation of more effective prophylactic regimens, also affected the proportion of infants who were lost to follow-up. In addition, increased tracking efforts likely led to infants being classified as lost to follow-up more quickly in later periods than in earlier periods, potentially leading to some degree of bias with regards to ascertaining infant mortality or HIV infection status.
Most of the LTFU we observed occurred in the first 6 months after infants enrolled, with about 5% never returning for a follow-up visit. A recent meta-analysis that assessed the magnitude of LTFU of HIV-exposed infants along the PMTCT cascade also reported high LTFU early in follow-up, with 4–75% LTFU by 3 months of age [44]. Interventions to improve retention should be implemented at the first contact. Point-of-care services may also improve retention [45,46].
We observed that infants whose mothers were newly enrolled into care generally had worse outcomes than infants whose mothers who were already enrolled in care when they became pregnant. Retaining mothers in care beyond the pregnancy and postnatal period may improve outcomes among subsequent HIV-exposed infants. As cART has been associated with reduced LTFU among HIV-infected adults [47,48], increasing access to lifelong cART for pregnant women could facilitate retaining mothers in care.
It is important to note that the infants included in this study and their mothers received PMTCTand cART services at centralized sites providing comprehensive HIV care. PMTCT services may not be delivered as effectively at sites in Kinshasa providing decentralized services [49]. The sites in this study provided routine care services, were integrated into the existing healthcare system, and were supervised by the government. In addition, they served a patient population that was likely representative of other patient populations in Kinshasa. However, technical assistance of the caliber provided by the UNC-DRC program is not routinely available in most settings. Quality assurance was an explicit program priority and may have contributed to the improvements we observed [50].
We believe our program's results are more or less representative of what can be achieved by other programs that provide technical assistance to clinics delivering PMTCT services. However, specific implementation challenges will likely vary between settings. Even within our own program, we observed differences in some infant outcomes that suggested there were differences between the two sites in how effectively EID and other PMTCT services were implemented. It is important that programs assess outcomes disaggregated by clinic in order to assure quality improvement activities are appropriately targeted, even if aggregated program results are ultimately disseminated.
Another major evolution in the care of HIV-exposed infants is occurring now with the worldwide scale-up of the 2013 WHO guidelines that endorse lifelong cART for all pregnant women (option B+) [15]. Although PMTCT programs should continue to adopt new guidelines as they become available, they should also pay close attention to the quality of care provided and routinely monitor intended program outcomes. We observed encouraging improvements in the outcomes of HIV-exposed infants in Kinshasa, but there remains abundant progress to be made. Modifiable barriers to delivering interventions in routine care settings need to be identified and addressed, and we should continue to evaluate our progress over time.
Acknowledgments
L.F. conceptualized and designed the study, and conducted analyses under the guidance of A.E., S.R.C, A.V.R., B.H.C., and F.B. L.F., A.E., J.L.C., V.O., J.L., A.V.R., and F.B. were collaborators on the parent project (the UNC-DRC program) that supplied data for the analysis and oversaw data collection and quality. J.L.C., V.O., and J.L. directed implementation of clinical protocols. L.F. drafted the initial manuscript and revisions were made by all co-authors. All co-authors approved the final draft. We are grateful for the patient care, program administration and coordination, and data entry contributions provided by the entire UNC-DRC program staff. The UNC-DRC program was funded by the Centers for Disease Control and Prevention President's Emergency Plan for AIDS Relief, with additional support from the Elizabeth Glaser Pediatric AIDS Foundation, the Belgian Development Cooperation, the William J. Clinton Foundation, the United Nations Children's Fund, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
L.F. was supported by the National Institutes of Health training grant (# 2T32AI070114-06 and # 5T32AI070114-07). For the remaining authors none were declared.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. UNAIDS; 2013. [Google Scholar]
- 2.UNAIDS. Countdown to zero: global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive (2011 – 2015) UNAIDS; 2011. [Google Scholar]
- 3.World Health Organization and UNICEF. Countdown to 2015: maternal, newborn and child survival: building a future for women and children: the 2012 report. World Health Organization; 2012. [Google Scholar]
- 4.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach (2006) World Health Organization; 2007. [PubMed] [Google Scholar]
- 5.Stevens W, Sherman G, Downing R, Parsons LM, Ou CY, Crowley S, et al. Role of the laboratory in ensuring global access to ARV treatment for HIV-infected children: consensus statement on the performance of laboratory assays for early infant diagnosis. Open AIDS J. 2008;2:17–25. doi: 10.2174/1874613600802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chantry CJ, Cooper ER, Pelton SI, Zorilla C, Hillyer GV, Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J. 1995;14:382–387. doi: 10.1097/00006454-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20:207–215. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22:1333–1343. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 9.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access: recommendations for a public health approach (2006 version) World Health Organization; 2006. [PubMed] [Google Scholar]
- 11.World Health Organization. HIV and infant feeding: Update based on the technical consultation held on behalf of the Inter-agency Task Team (IATT) on prevention of HIV infection in pregnant women, mothers and their infants (Geneva, 25 – 27 October 2006) World Health Organization; 2007. [Google Scholar]
- 12.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach (2010 Version) World Health Organization; 2010. [PubMed] [Google Scholar]
- 13.World Health Organization. Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence (2010) World Health Organization; 2010. [PubMed] [Google Scholar]
- 14.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach (2010 revision) World Health Organization; 2010. [PubMed] [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach (June 2013) World Health Organization; 2013. [PubMed] [Google Scholar]
- 16.Alvarez-Uria G, Midde M, Pakam R, Bachu L, Naik PK. Effect of formula feeding and breastfeeding on child growth, infant mortality, and HIV transmission in children born to HIV-infected pregnant women who received triple antiretroviral therapy in a resource-limited setting: data from an HIV cohort study. ISRN Pediatr. 2012;2012:763591. doi: 10.5402/2012/763591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young SL, Mbuya MN, Chantry CJ, Geubbels EP, Israel-Ballard K, Cohan D, et al. Current knowledge and future research on infant feeding in the context of HIV: basic, clinical, behavioral, and programmatic perspectives. Adv Nutr. 2011;2:225–243. doi: 10.3945/an.110.000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coovadia H, Kindra G. Breastfeeding to prevent HIV transmission in infants: balancing pros and cons. Curr Opin Infect Dis. 2008;21:11–15. doi: 10.1097/QCO.0b013e3282f40689. [DOI] [PubMed] [Google Scholar]
- 19.Read JS. Human milk, breastfeeding, and transmission of human immunodeficiency virus type 1 in the United States. American Academy of Pediatrics Committee on Pediatric AIDS. Pediatrics. 2003;112:1196–1205. doi: 10.1542/peds.112.5.1196. [DOI] [PubMed] [Google Scholar]
- 20.WHO/UNAIDS/UNICEF/UNFPA. HIV transmission through breastfeeding: a review of available evidence: 2007 update. World Health Organization; 2008. [Google Scholar]
- 21.World Health Organization. Programmatic update: Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: executive summary. World Health Organization; 2012. [Google Scholar]
- 22.Stringer EM, Chi BH, Chintu N, Creek TL, Ekouevi DK, Coetzee D, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Heal Organ. 2008;86:57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi BH, Stringer JSA, Moodley D. Antiretroviral drug regimens to prevent mother-to-child transmission of HIV: a review of scientific, program, and policy advances for sub-Saharan Africa. Curr HIV/AIDS Rep. 2013;10:124–133. doi: 10.1007/s11904-013-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi BH, Adler MR, Bolu O, Mbori-ngacha D, Ekouevi DK, Gieselman A, et al. Progress, challenges, and new opportunities for the prevention of mother-to-Child transmission of HIV under the US President's Emergency Plan for AIDS Relief. J Acquir Immune Defic Syndr. 2012;60(Suppl):78–87. doi: 10.1097/QAI.0b013e31825f3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwendo EM, Mtuy TB, Renju J, Rutherford GW, Nondi J, Sichalwe AW, et al. Effectiveness of prevention of mother-to-child HIV transmission programmes in Kilimanjaro region, northern Tanzania. Trop Med Int Heal. 2014;19:267–274. doi: 10.1111/tmi.12255. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano M, Andreotti M, Liotta G, Jere H, Sagno JB, Maulidi M, et al. Maternal antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Malawi: maternal and infant outcomes two years after delivery. PLoS One. 2013;8:e68950. doi: 10.1371/journal.pone.0068950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Report of the WHO technical reference group, paediatric HIV/ART care guideline group meeting. World Health Organization; 2008. [Google Scholar]
- 28.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva: World Health Organization; 2006. [Google Scholar]
- 29.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 31.Lin G, So Y, Johnston G. Proceedings of the SAS Global Forum 2012 Conference. Cary, NC: SAS Institute Inc.; 2012. Analyzing survival data with competing risks using SAS® software. [Google Scholar]
- 32.Cole S, Hudgends M. Survival analysis in infectious disease research: describing events in time. 2010;24:2423–2431. doi: 10.1097/QAD.0b013e32833dd0ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cain LE, Cole SR, Chmiel JS, Margolick JB, Rinaldo CR, Detels R. Effect of highly active antiretroviral therapy on multiple AIDS-defining illnesses among male HIV seroconverters. Am J Epidemiol. 2006;163:310–315. doi: 10.1093/aje/kwj045. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. PMTCT Strategic Vision 2010 – 2015: preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals: moving towards the elimination of paediatric HIV. World Health Organization; 2010. [Google Scholar]
- 35.Donahue MC, Dube Q, Dow A, Umar E, Van Rie A. They have already thrown away their chicken': barriers affecting participation by HIV-infected women in care and treatment programs for their infants in Blantyre. Malawi AIDS Care. 2012;24:1233–1239. doi: 10.1080/09540121.2012.656570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58:115–119. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 37.Cook RE, Ciampa PJ, Sidat M, Blevins M, Burlison J, Davidson MA, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e104–e109. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun M, Kabue MM, McCollum ED, Ahmed S, Kim M, Aertker L, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wettstein C, Mugglin C, Egger M, Blaser N, Salazar L, Estill J, et al. Missed opportunities to prevent mother-to-child-transmission in sub-Saharan Africa: Systematic review and meta-analysis. AIDS. 2012;26:2361–2373. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32:1080–1085. doi: 10.1097/INF.0b013e318290622e. [DOI] [PubMed] [Google Scholar]
- 41.Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis. 2013;207(Suppl 2):93–100. doi: 10.1093/infdis/jit110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wachira J, Middlestadt SE, Vreeman R, Braitstein P. Factors underlying taking a child to HIV care: implications for reducing loss to follow-up among HIV-infected and -exposed children. SAHARA J. 2012;9:20–29. doi: 10.1080/17290376.2012.665255. [DOI] [PubMed] [Google Scholar]
- 43.Braitstein P, Katshcke A, Shen C, Sang E, Nyandiko W, Ochieng VO, et al. Retention of HIV-infected and exposed children in a comprehensive HIV clinical care program in Western Kenya. Trop Med Int Heal. 2010;15:833–841. doi: 10.1111/j.1365-3156.2010.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sibanda E, Weller I, Hakim J, Cowan F. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27:2787–2797. doi: 10.1097/QAD.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 46.Faal M, Naidoo N, Glencross DK, Venter WDF, Sa FCP, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr. 2011;58:54–59. doi: 10.1097/QAI.0b013e3182303921. [DOI] [PubMed] [Google Scholar]
- 47.Krebs DW, Chi BH, Mulenga Y, Morris M, Cantrell RA, Mulenga L, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–317. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 48.McGuire M, Munyenyembe T, Szumilin E, Heinzelmann A, Le Paih M, Bouithy N, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Heal. 2010;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 49.Edmonds A, Thompson D, Okito V, Feinstein L, Kawende B, Behets F. PMTCT decentralization does not assure optimal service delivery: revelations from successful individual-level tracking of HIV-infected mothers and their infants [Abstract THAE0103] 19th International AIDS Conference. 2012 [Google Scholar]
- 50.Feinstein L, Chalachala J, Thompson D, Edmonds A, Okitolonda V, Mukalakala G, et al. Structured monitoring and evaluation activities can improve patient care: encouraging results in Kinshasa, Democratic Republic of Congo [Abstract PE134] 19th International AIDS Conference. 2012 [Google Scholar]