Abstract
Introduction
Recent research suggests that priapism in Sickle Cell Disease (SCD) is due to dysregulation of penile erection homeostasis including alteration of nitric oxide synthase (NOS) and phosphodiesterase 5 (PDE5) activities by excessive levels of reactive oxygen species (ROS) released during hemolysis. It is unknown if sub-acute exposure to hemolysis is sufficient or if chronic reconditioning of erectile tissues is required for perturbation of homeostatic pathways and whether PDE5 inhibitor (PDE5I) treatment can restore erectile homeostasis in the sub-acute setting.
Aims
To investigate the effects of sub-acute hemolysis (3 month exposure) on priapism and NO pathway regulation.
Methods
Mice underwent bone marrow transplantation with either SCD (BM-SS) or wild type (WT) bone marrow. BM-SS mice were treated with sildenafil 100mg/kg/day. We measured intracavernous pressure (ICP) measurements with or without cavernosal nerve stimulation (CNS) following bone marrow transplantation to assess for priapism.
Main Outcome Measures
ICP and frequency of erections were assessed. Penile tissues were analyzed for NOS, PKG, PDE5, and ROS activities.
Results
BM-SS mice demonstrated a priapism phenotype. PDE5I treatment reduced the frequency of erections in BM-SS mice (1.7 ± 1.1 vs. 5.5 ± 2.8 erections/hour, p<0.05). Penile tissues from BM-SS mice demonstrated decreased NOS, PKG, PDE5 and elevated ROS activities compared to that of control mice. PDE5I treatment increased NOS (11.6 ± 1.3% vs. 7.8 ± 2.3%, p<0.05) and PDE5 (76.3 ± 9.8% vs. 52.3 ± 11.1%, p<0.05) activities and decreased ROS activity (137.8 ± 12.1% vs. 199.1 ± 11.3%, p<0.05) compared to non-PDE5I treated BM-SS mice. PKG activity was increased beyond control levels with PDE5I treatment (158.4 ± 10.3%, p<0.05).
Conclusion
Short-term hemolysis is sufficient to establish a priapism phenotype and results in loss of erectile function. PDE5I treatment ameliorates priapism, in part, due to restored NO balance with decreased ROS generation and increased PDE5 activity.
Keywords: sickle cell disease, endothelial nitric oxide synthase, reactive oxygen species, phosphodiesterase 5, priapism, PDE5 inhibitor
Introduction
Priapism is defined as a penile erection lasting longer than desired with no sexual purpose.1 It affects 30-45% of men with SCD.2 Priapism in SCD was initially attributed to sickled erythrocytes causing decreased venous flow leading to congestion of blood within the corpora cavernosa.3 Recent research suggests the etiology is more complex and involves dysregulation of erectile homeostasis including aberrant NO/cyclic guanosine monophosphate (cGMP) signaling, increased production of adenosine, and opiorphin upregulation.4–9 Early insight came from the observation that priapism is a result of chronic reduction of endothelial derived nitric oxide (NO) in endothelial nitric oxide synthase (eNOS)-KO mice which was alleviated by phosphodiesterase type 5 inhibitor (PDE5I) therapy as well as in men with sickle cell disease.10,11 This seemingly counterintuitive effect is explained, in part, by abnormally low PDE5 expression and activity levels found in sickle cell disease as a result of NO imbalance, which is corrected with PDE5I treatment.11 Of note, endogenous PDE5 expression is regulated by NO activity, which is, in turn, influenced by protein kinase G (PKG) levels.11,12 In SCD, there exists a global impairment of NO bioavailability attributed to decreased baseline NOS activity, increased NO scavenging by reactive oxygen species (ROS), and increased arginase activity.13 These deleterious effects are driven by increased ROS levels, which is hypothesized to result from intravascular hemolysis in SCD.13–16 Decreased levels of NO lead to decreased cGMP and subsequently reduced expression of PDE5. Although the pro-erectile pathways are also decreased from baseline, the surge of cGMP generated following erectogenic stimuli is unopposed leading to unregulated smooth muscle relaxation and priapism ensues. PDE5I treatment is associated with decreased ROS and increased PDE5 levels contributing to erectile homeostasis and decreased priapism.17
Aims
Similar to humans, the mouse models to date are born with SCD making it difficult to differentiate the acute effects of hemolysis and NO dysregulation from chronic hemolysis and reconditioning of erectile tissues. The aim of this report, was to use a murine bone marrow transplantation model of SCD to investigate the effects of sub-acute (3 months exposure) hemolysis on erectile function and the molecular mechanisms involved in the development of priapism.
Methods
Mouse model of human sickle cell disease
Sickle cell mice (3 to 5 months of age) with knockout of all mouse hemoglobin genes and expressing exclusively human sickle cell hemoglobin (HbS) were utilized as bone marrow donors.18 This colony of “Berkeley” sickle cell mice at Johns Hopkins began with founder mice from Jackson Labs. Control bone marrow donor animals were C57BL/6 wild-type (WT) age-matched mice as they are one of the background strains for the transgenic sickle cell mice.18 Prior studies have shown hemizygous mice do not demonstrate sickle deformation nor do WT and hemizygous mice differ in regards to erectile function or penile architecture.13,19 Mice were fed Harlan Teklad 2018 before and after transplant. All animal studies were approved by the Johns Hopkins Animal Care and Use Committee.
Generating chimeric mice by bone marrow transplantation
Whole bone marrow was isolated from donor HbS femurs and tibias as previous described. Recipient C57BL/6 mice underwent whole bone marrow transplants with whole bone marrow (5 × 106 cells per recipient) injected into their femurs after myeloablative irradiation (900 cGy). Recipient mice received 4 daily intraperitoneal injections of recombinant human erythropoietin (300 U/kg, Amgen, USA) to promote erythropoiesis.13 Twelve mice received marrow from sickle cell mice (BM-SS), and 6 mice received marrow from C57BL/6 donors (Control). By 10 weeks after transplantation, 90% recipient mice survived erythroid engraftment (HbS exceeding 90% of the total hemoglobin). Animals then underwent penile erectile studies by 14 to15 weeks post-transplantation (5 months of age).
PDE5 inhibitor therapy
Oral treatment with the PDE5 inhibitor, sildenafil citrate (Pfizer, USA) was provided by mixing drug into semi-soft rodent chow (Bioserv, USAs; 4 – 6 g/d).17 For in vivo chronic studies, we used 100 mg/kg/day of sildenafil citrate in semi-soft rodent chow for 3 weeks and then performed all biochemical and physiological analyses. Control bone marrow transplant animals received chow only (Control).
Physiologic erection studies
In vivo erectile function in response to cavernous nerve stimulation (CNS) was studied in control, BM-SS, and BM-SS treated with sildenafil citrate as previously described.11,17,20 Briefly, pelvic dissection was performed to expose the MPG and penile crura for CNS and intracavernous pressure (ICP) monitoring.11,17,20 CNS was performed at a frequency of 15 Hz and pulse width of 30 milliseconds for each mouse. 1, 2, and 4 volts were applied to achieve a significant and consistent erectile response. Mice with a priapism phenotype have been shown to have increased rates of spontaneous erections.11,17 The effect of PDE5I therapy on the frequency of spontaneous erections per hour calculated pre- and post-CNS as previously described.11,17
Collection of tissue specimens
Penile specimens were obtained following a lethal dose of sodium pentobarbital (80 mg/kg intraperitoneally, Virbac, USA). Mouse penes crura were cut at the level of their attachment to the lower pubic bone. They were snap frozen in liquid nitrogen and stored at -80° C until used for molecular analysis.
NOS, PKG, PDE5 enzyme activity assay
Frozen penis tissues were prepared for the various analyses per manufacturer's protocol. NOS activity in penile tissues was assessed by radiolabelled L-arginine to L-citrulline conversion as previously described (Cayman, Chemical, USA).17 Constitutive NOS activity Measurements were performed in the presence of calcium. The 2-step method was used to measure total low Km cGMP PDE5 activity (assayed in duplicate) at 1 μM substrate under linear conditions with and without added sildenafil citrate (0.1 nM-10 μM) with 0.1 mg/ml BSA and 0.1 mM EGTA per (Molecular Devices, USA).11 PKG enzyme activity was performed using ELISA following the manufacturer's protocol (CyClex, Japan).17,21,22
Reactive oxygen species (ROS) measurements
Penile tissue homogenates superoxide anion (ROS) generation production was determined by luminol-enhanced chemiluminescence (EMD Biosciences, USA) as previously described.17 Penes were homogenized in iced PBS buffer and centrifuged, and the precipitate was suspended in assay buffer to a final concentration of 100 μM luminol.
Statistical analysis
Results were analyzed using Prism 5 (GraphPad Software, USA) and expressed as mean plus or minus standard error of the mean. Multiple groups were compared using one-way analysis of variance followed by the Tukey's multiple comparison test. Statistical significance was considered as p < 0.05.
Results
Sickle cell bone marrow transplantation generates priapism phenotype
Consistent with prior studies, sickle cell mice had significantly decreased hemoglobin levels (13.5 ± 0.4 g/dL vs. 5.26 ± 0.3 g/dL, p<0.01) and elevated lactate dehydrogenase levels (125.4 ± 7.8 U/L vs. 507.6 ± 29.6 U/L, p<0.01) compared to WT, which is consistent with hemolysis. Homozygote sickle cell bone marrow transplantation recapitulated the priapism phenotype of mice with SCD (Figure 1). Without CNS, there were no significant differences in erections per hour between groups. We observed a significant increase in ICP at all voltage levels during CNS in BM-SS mice when compared to the control bone marrow transplant mice ICP (mmHg at: 1 volt − 32.3 ± 2.1 vs. 17.2 ± 0.2, p<0.05; 2 volts − 48.4 ± 3.0 vs. 18.8 ± 1.1, p<0.05; 4 volts − 57.1 ± 3.6 vs. 29.3 ± 0.4, p<0.05. Figure 1A). Consistent with our previous investigations in sickle mice, we observed a significant increase in the frequency of erections/hour pre- (3.1 ± 1.3 vs. 0.6 ± 0.6, p<0.05) and post-CNS (5.5 ± 2.8 vs. 0.7 ± 0.4, p<0.05. Figure 1B) in the BM-SS mice compared to the control bone marrow transplant mice. Taken together, the increase in ICP and frequency of erections suggest a priapism phenotype in this new sickle animal model of hemolysis-induced priapism.
Figure 1.
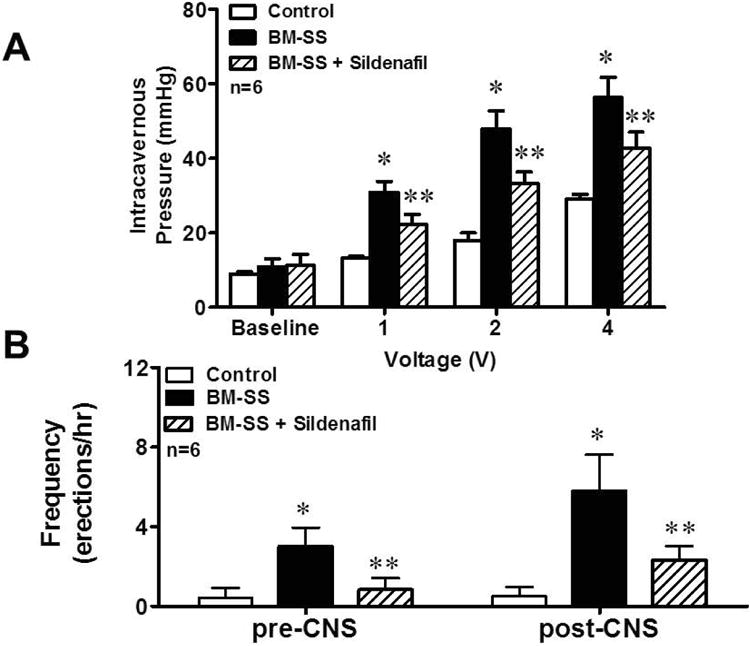
BM-SS mice display a priapism phenotype, which is ameliorated by sildenafil treatment. (A) Intracavernous pressure (ICP) measurements at baseline and after increasing cavernosal nerve stimulation (CNS) of control, BM-SS and BM-SS mice treated with sildenafil. Untreated BM-SS animals had significantly elevated (*P<0.05) ICPs compared to control animals. Sildenafil treatment of BM-SS animals significantly (**P<0.05) reduced ICP compared to untreated BM-SS mice. (B) BM-SS mice have increased frequency of erections, which is improved with sildenafil treatment. Both pre- and post-CNS, BM-SS mice have increased frequencies of erections compared to control animals (*P<0.05), which are significantly reduced (**P<0.05) with sildenafil treatment. N=6 in each group.
Improvement of priapism with PDE5 inhibition
To explore whether the functional effects of PDE5I therapy are replicated in the current animal model of hemolysis-induced priapism, we treated BM-SS mice with daily dosing of the PDE5I sildenafil citrated for 3 weeks. We found that daily sildenafil administration to BM-SS mice decreased the frequency of erections per hour pre- (1.2 ± 0.4, p<0.05 compared to non-treated BM-SS) and post-CNS (1.7 ± 1.1, p<0.05 compared to non-treated BM-SS. Figure 1B). PDE5I treatment also decreased the peak ICP to CNS at all voltages tested (mmHg compared to non-treated BM-SS at: 1 volt − 22.8 ± 1.9, p<0.05; 2 volts − 35.3 ± 2.3, p<0.05; 4 volts − 40.8 ± 3.3, p<0.05. Figure 1B). These data suggest that the priapism phenotype was at least partially corrected with PDE5I therapy and coincide with our previous findings in sickle mice.17
Sickle cell bone marrow transplanted mice have increased levels of ROS, NO imbalance, and PDE5 dysregulation which are ameliorated with PDE5I treatment
In the current series of experiments, we evaluated the possible mechanisms whereby BM-SS mice developed a priapism phenotype. NO signal transduction and PDE5 activity was assessed in the BM-SS penes to determine if the priapism phenotype was due to NO imbalance and PDE5 dysregulation (Figure 2). Activities of constitutive NOS (7.8 ± 2.3 vs. 15.7 ± 3.2 pmol*mg protein-1*hr-1, p<0.05), PKG (74.8 ± 8.9% of control mice activity, p<0.05) and PDE5 (52.3 ± 11.1% of control mice activity, p<0.05) were significantly decreased in the BM-SS penes compared with control mouse penes (Figure 2A – 2C). These results are consistent with our underlying hypothesis that aberrant NO signaling from the vascular endothelium leads to reduction in downstream mediators of NO and PDE5 dysregulation. We evaluated markers of oxidative stress in the penes of BM-SS mice to determine if increased production of ROS contributes to the development of NO imbalance and thus priapism. BM-SS mice penes had significantly greater ROS generation than that of control mice as measured by luminol production (199.1 ± 11.3% of control tissue ROS generation, p<0.05. Figure 3).
Figure 2.
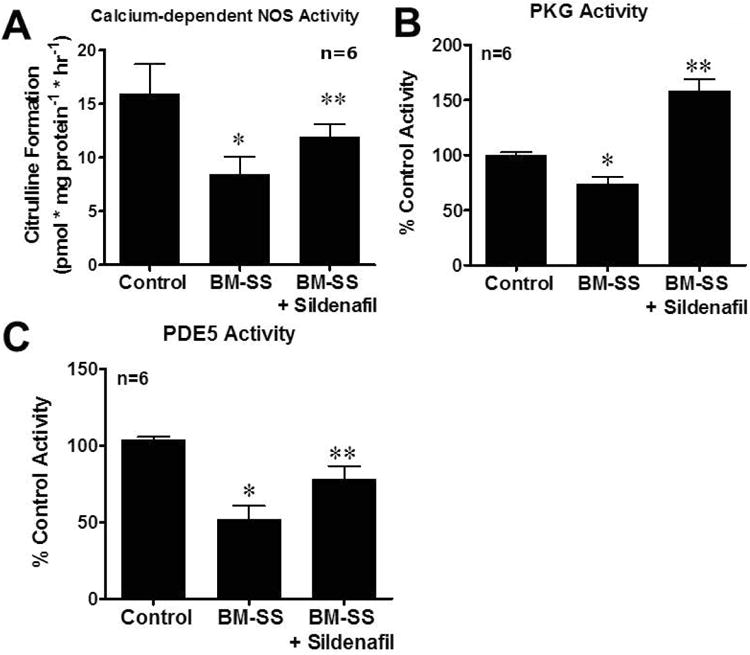
(A) Constitutive (endothelial) nitric oxide synthase (NOS), (B) protein kinase G (PKG) activity, and (C) phosphodiesterase type 5 (PDE5) activity, in control, BM-SS, and BM-SS mice treated with sildenafil. BM-SS mice penes had a significant (*P<0.05) reduction in constitutive (endothelial) NOS, PDE5, and PKG activities compared to control animals. After chronic PDE5 inhibitor therapy with sildenafil, constitutive NOS and PDE5 activities were similar to values from control mice penes. PKG activity was significantly increased (**P<0.05) to values greater than both control and SS BMT mice penes. N=6 in each group.
Figure 3.
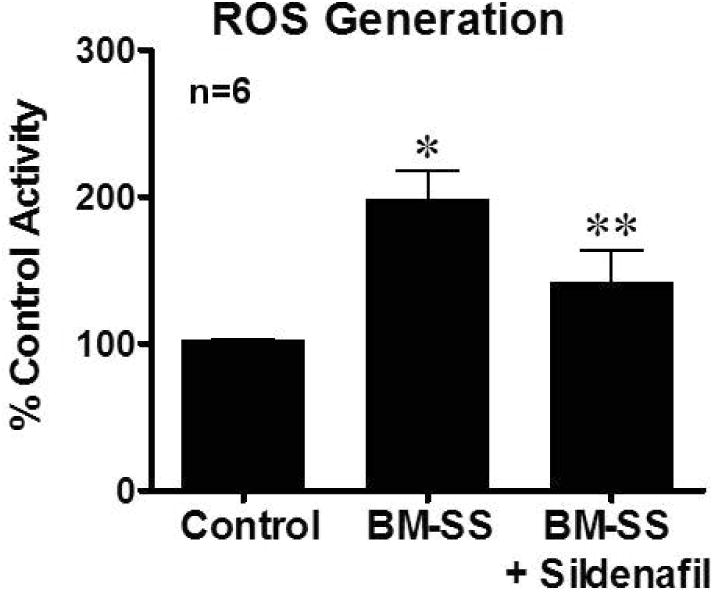
BM-SS mouse penes have significantly increased (*P<0.05) levels of reactive oxygen species (ROS) activity compared to control animals. This was significantly reduced (**P<0.05) in BM-SS penes with sildenafil treatment. N=6 in each group.
The effect of daily PDE5I therapy on all molecular indices contributing to the priapism phenotype was assessed. Oral PDE5I treatment significantly (p<0.05) increased constitutive NOS activity in BM-SS animals (11.6 ± 1.3, p<0.05 compared to non-treated BM-SS mice) but did not return them to levels of control mice (Figure 2A). BM-SS mice had decreased levels of PKG activity compared to control animals (WT mean optical density/protein ranged 0.19 – 0.31), however, PDE5I treatment significantly increased PKG activity in BM-SS mice to 158.4 ± 10.3% of control levels (p<0.05, Figure 2B). Perturbation of NOS activity and PKG levels were associated with a significant decrease in PDE5 activity level in BM-SS mice compared to control mice. PDE5 activity levels were significantly increased with PDE5I treatment (76.3 ± 9.8% of control activity, p<0.05 compared to non-treated BM-SS mice), however not completely corrected to control levels (Figure 2C). Finally, PDE5I treatment significantly reduced levels of ROS in BM-SS mice (137.8 ± 12.1% of control activity, p<0.05 compared to non-treated BM-SS mice. Figure 3).
Discussion
Priapism in SCD is attributed to molecular dysregulation of erection homeostasis. In SCD patients and animal models, elevated ROS generation due to hemolysis leads to reduced NO bioavailability.13,17,23 Our laboratory has previously demonstrated reduction in constitutive (endothelial) NOS activity results in decreased activities of downstream signaling molecules, in particular cGMP and PKG, which causes diminished PDE5 expression and activity levels.11,17 The rapid increase in cGMP levels following erectogenic stimuli is unopposed due to reduced PDE5 regulation resulting in priapism. Prior studies utilizing mice with germ cell mutations and SCD at birth have served to elucidate the complex process of priapism in SCD. It is unknown however, what the relative significance of short-term sub-acute hemolysis and ROS generation is versus chronic exposure of erectile tissues to elevated ROS in the development of priapism. This study utilizes a bone marrow transplantation model of SCD to investigate the effects of short-term sub-acute hemolysis.13 We found similar changes in the NO signal transduction pathway and PDE5 dysregulation seen in other animal models of SCD. The bone marrow transplantation model of SCD has the advantage of determining when the mice develop SCD and it allows the mice to develop without exposure to SCD. Although it requires a significant amount of time to allow the donor bone marrow to successfully graft within the irradiated wiltd-type recipients, the model is otherwise easy to generate and reliable. We propose that this new animal model provides a basis to study the effects sub-acute hemolysis on erection homeostasis and development of priapism.
Acute hemolysis, and subsequent development of hemolytic anemia, has been shown to be important in several SCD specific pathobiologies associated with decreased NO bioavailability.13 This is due, in part, to decompartmentalization of erythrocyte hemoglobin and arginase resulting in NO sequestration, elevated ROS, arginine degradation, and eNOS uncoupling.13,15,17,24,25 ROS, which are both a product and driver in SCD pathophysiology, are generated from several sources including hemolysis, ischemia-reperfusion injury, inflammatory cell activation, and NOS uncoupling.15,23,26–28 Similar to mice born with SCD, elevated ROS in BM-SS mice is associated with NO imbalance, in particular a reduction in constitutive (endothelial) NOS activity. This reduction and subsequent decrease in PKG activity results in diminished PDE5 activity levels due to the positive regulation of PDE5 by the NO pathway.11,12
PDE5I treatment has been shown to decrease ROS and increase PDE5 expression and activity levels in sickle mice with resolution of priapism in both experimental animal models and clinically in men with SCD11,29. Although PDE5I treatment improves priapism, it does so without complete normalization of the NO pathway. In our BM-SS model treated with PDE5I, NOS and PDE5 activities were partially restored to age-matched control penile levels. In contrast, PKG activity increased to greater than that of control animals. It is possible that within the context of decreased PDE5 activity, reduction of ROS and improvement of NOS facilitates exaggerated PKG levels in the time points used in this study. It is also plausible that PDE5I therapy increases NO biosynthesis and downstream PKG phosphorylation resulting in the significant change in PKG activity. Further studies are warranted to delineate the exact mechanisms. PDE5I treatment has been shown to increase NOS dimerization in SCD mice reducing oxidative and nitrosative stress.17 However, ROS levels did not completely normalize with PDE5I therapy in BM-SS mice. This is likely due to ongoing hemolysis and subsequent ROS generation, which are not alleviated with PDE5I alone and underscore the difficulty of treating this condition.
This novel animal model of SCD hemolysis induced priapism serves to elucidate the effects of sub-acute hemolysis on changes in NO production, ROS generation, and vascular reactivity.13–15 We demonstrate that mice develop priapism shortly following bone marrow transplantation suggesting chronic reconditioning of tissue is not necessary for priapism in this model. This is consistent with observations that pulmonary tissues from SCD mice that developed pulmonary hypertension have relatively normal lung histology.13,30 However, it is unclear how the tissues may be altered during the 3 months following transplantation and prior to experimentation. Mice transfused with sensitized blood from other mice develop alloimmune hemolysis within 3 days and demonstrate enhanced vasoconstriction and blunted vasodilation similar to SCD animals but sometimes to a lesser extent.13,31 Additionally, the majority of men with SCD do not develop priapism until their second decade of life suggesting chronic exposure to the SCD environment has an effect.2 Additional studies are needed to determine how penile tissues are altered in this model.
Although this study did not investigate the effects of hemolysis lasting less than 3 months, it suggests that hemolysis has an early impact on erectile function. Further studies, including transplanting WT marrow into SCD animals may serve to elucidate what role tissue remodeling plays and whether these mice will remain at risk for priapism despite resolution of hemolysis. We evaluated whole penis lysate. We may gain additional information by assessing the effects of short-term hemolysis specifically on isolated corporal tissues. Additionally, constitutive NOS, which includes eNOS and nNOS was measured. Mechanistic insight may be gained by analyzing how these two populations of NOS are individually affected following SCD bone marrow transplantation. It is also unknown how the major pelvic ganglion is altered in the setting of short-term SCD, which may have an important role in the development of priapism.
In the current animal model, we did not see a complete resolution of the priapism phenotype with PDE5I therapy, which is likely due to the inability to completely normalize activity levels of NOS, PDE5 and ROS. Importantly, following PDE5I therapy we observed improvement in erection homeostasis with increased activity levels of NOS, PKG, and PDE5 levels, which was associated with improvement in priapic episodes. It is unclear whether these findings may be due to the increased relative activity of PDE5 compared to other constituents of the NO pathway so that even partial restoration of PDE5 regulation is sufficient for improved erectile homeostasis.4
In summary, our data support the hypothesis that short-term hemolysis and concomitant elevation of ROS are sufficient to cause changes in the NO signal transduction pathway causing NO imbalance and PDE5 dysregulation with subsequent development of a priapism phenotype in this mouse model.
Acknowledgments
This study was supported in part by grant funding from the National Institutes of Health (K08DK090370). J.L.H. was funded Urology Care Foundation Research Fellowship and T.J.B the Urological Care Foundation Rising Star. N.A.S. was funded by a Koch Foundation Grant.
Footnotes
Conflict of Interest: None
Disclosures: Nothing to disclose
Bibliography
- 1.Broderick Ga, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7(1 Pt 2):476–500. doi: 10.1111/j.1743-6109.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 2.Adeyoju AB, Olujohungbe ABK, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications - an international multicentre study. [Accessed July 4, 2014];BJU Int. 2002 90(9):898–902. doi: 10.1046/j.1464-410x.2002.03022.x. http://www.ncbi.nlm.nih.gov/pubmed/12460353. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AL. Priapism pathophysiology: clues to prevention. Int J Impot Res. 2003;15(Suppl 5):S80–S85. doi: 10.1038/sj.ijir.3901077. [DOI] [PubMed] [Google Scholar]
- 4.Bivalacqua TJ, Musicki B, Kutlu O, Burnett AL. New insights into the pathophysiology of sickle cell disease-associated priapism. J Sex Med. 2012;9(1):79–87. doi: 10.1111/j.1743-6109.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanika ND, Tar M, Tong Y, Kuppam DSR, Melman A, Davies KP. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol. 2009;297(4):C916–C927. doi: 10.1152/ajpcell.00656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mi T, Abbasi S, Zhang H, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118(4):1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claudino MA, Franco-Penteado CF, Corat MAF, et al. Increased cavernosal relaxations in sickle cell mice priapism are associated with alterations in the NO-cGMP signaling pathway. J Sex Med. 2009;6(8):2187–2196. doi: 10.1111/j.1743-6109.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 8.Fu S, Tar MT, Melman A, Davies KP. Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells. FASEB J. 2014;28(8):3633–3644. doi: 10.1096/fj.13-248708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning C, Wen J, Zhang Y, et al. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1α mediated reduction of PDE5 gene expression. FASEB J. 2014;28(6):2725–2735. doi: 10.1096/fj.13-247833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67(5):1043–1048. doi: 10.1016/j.urology.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102(5):1661–1666. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CS, Chow S, Lau a, Tu R, Lue TF. Identification and regulation of human PDE5A gene promoter. Biochem Biophys Res Commun. 2001;280(3):684–692. doi: 10.1006/bbrc.2000.4220. [DOI] [PubMed] [Google Scholar]
- 13.Hsu LL, Champion HC, Campbell-Lee Sa, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109(7):3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106(9):3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm799. [DOI] [PubMed] [Google Scholar]
- 16.Liao JC. Blood feud: keeping hemoglobin from nixing NO. Nat Med. 2002;8(12):1350–1351. doi: 10.1038/nm1202-1350. [DOI] [PubMed] [Google Scholar]
- 17.Bivalacqua TJ, Musicki B, Hsu LL, Berkowitz DE, Champion HC, Burnett AL. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS One. 2013;8(7):e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pászty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. [Accessed September 19, 2014];Science. 1997 278(5339):876–878. doi: 10.1126/science.278.5339.876. http://www.ncbi.nlm.nih.gov/pubmed/9346488. [DOI] [PubMed] [Google Scholar]
- 19.Bivalacqua TJ, Musicki B, Hsu LL, Gladwin MT, Burnett AL, Champion HC. Establishment of a Transgenic Sickle-Cell Mouse Model to Study the Pathophysiology of Priapism. J Sex Med. 2009;6(9):2494–2504. doi: 10.1111/j.1743-6109.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannan JL, Albersen M, Kutlu O, et al. Inhibition of rho-kinase improves erectile function, increases nitric oxide signaling and decreases penile apoptosis in a rat model of cavernous nerve injury. J Urol. 2013;189(3):1155–1161. doi: 10.1016/j.juro.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51(6):1732–1740. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musicki B, Champion HC, Hsu LL, Bivalacqua TJ, Burnett AL. Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis. J Sex Med. 2011;8(2):419–426. doi: 10.1111/j.1743-6109.2010.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aslan M, Ryan TM, Townes TM, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278(6):4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 24.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 25.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19(8):989–991. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- 27.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslan M, Ryan TM, Adler B, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98(26):15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnett AL, Anele UA, Trueheart IN, Strouse JJ, Casella JF. Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. Am J Med. 2014;127(7):664–668. doi: 10.1016/j.amjmed.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107(4):1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell-Lee SA, Liu J, Velliquette RW, et al. The production of red blood cell alloantibodies in mice transfused with blood from transgenic Fyb-expressing mice. Transfusion. 2006;46(10):1682–1688. doi: 10.1111/j.1537-2995.2006.00966.x. [DOI] [PubMed] [Google Scholar]


