Abstract
Background:
Tuberculosis (TB) is a contagious disease caused by Mycobacterium tuberculosis. Development of a new vaccine for tuberculosis requires immunogenic antigens capable of inducing suitable and long-lasting T cell immunity. The emergence of multidrugs and extensively drug-resistant strains of M. tuberculosis has made it a global public health concern.
Objectives:
DNA vaccine is a straightforward method to introduce antigens to the host. In the present study, two immunodominant mycobacterial antigens (Mtb32C and HBHA) were isolated and cloned into pcDNA3.1 (+) to design and construct a new DNA vaccine. This vector is capable of producing new potent chimeric protein.
Materials and Methods:
Mtb32C (Rv0125) and heparin-binding haemagglutinin adhesion (HBHA) genes were amplified using polymerase chain reaction (PCR) of M. tuberculosis H37Rv genome and ligated into pcDNA3.1 (+). Colony-PCR and restriction enzyme analysis were used to confirm the accuracy of the cloning procedure.
Results:
In the current study, recombinant pcDNA3.1 (+) vector containing Mtb32C and HBHA genes was successfully constructed. The desired size of DNA fragment was observed using single and double digestion methods.
Conclusions:
Mtb32C and HBHA genes were successfully isolated from H37Rv genome and cloned into an eukaryotic expression vector. This vector can be considered as a vaccine to evaluate immune responses in animal models.
Keywords: Vaccines, DNA; HBHA protein; Mtb32 antigen; Mycobacterium tuberculosis
1. Background
Mycobacterium tuberculosis is a bacterial pathogen, which causes human tuberculosis disease (TB). Originally isolated by Robert Koch in 1882 from TB patients, M. tuberculosis is transmitted between people through aerosol droplets containing one to three bacilli from lungs of patients with active TB. In lung, after inhalation of infected droplets, the bacteria are taken up by alveolar macrophages. These macrophages, however, are unable to kill and degrade the bacteria. Inside resident alveolar macrophages M. tuberculosis begins to multiply indefinitely. Through lymphatic system or blood, M. tuberculosis can reach other parts of the body and causes disseminated forms such as meningitis (1-5). According to the reports of the world health organization (WHO), one third of the world’s population are asymptomatically infected by M. tuberculosis. Without proper treatment, about 5 - 10 % of initially infected persons would develop active disease during their life (6). Despite many attempts in the last 20 years, tuberculosis (TB) is still involved in global morbidity and is a leading cause of human death in developing countries.
Bacillus Calmette-Guerin (BCG) vaccine is currently the only available vaccine against TB. In many countries, this vaccine is routinely administered to infants. The BCG strain is attenuated empirically by 231 serial passages of a virulent M. bovis strain on medium containing potatoes and glycerinated bile. Worldwide, BCG is the most widely used vaccine for this disease. It is a generally safe and stable vaccine providing a long-lasting immunity. Nevertheless, there are some concerns regarding the usage of this vaccine. Among adults, BCG is not effective against pulmonary TB due to waning immunity (5, 6). Individuals with genetic deficiencies in Interleukin (IL)-12, IL-23, Interferon-gamma (IFN-γ) and signal transducer genes and those with HIV coinfection are susceptible to develop the disseminated form of this disease. Therefore, administration of BCG in those individuals should be avoided. Among healthy people, the efficacy of vaccine ranges between 0 to 80 %. This may be due to environmental factors such TB exposure intensity, nutrition, previous exposure to environmental mycobacterial strains and other reasons. These make it critical to improve the current vaccine therapies as well as designing new vaccines (7-9).
The heparin-binding haemagglutinin (HBHA) of M. tuberculosis is a surface protein, which attaches to sulfated glycoconjugates on nonphagocytic cells. Previous studies have shown that HBHA is an immunodominant antigen able to stimulate T-cells and induce the release of IFN-γ (10, 11). Mtb32A is a serine protease protein initially obtained from culture filtrate proteins (CFP) of M. tuberculosis. This protein is involved in the survival of the host cell and bacterial pathogenesis. C-terminal domain of this protein is capable of stimulating TCD8 and conferring resistance to TB (12-14).
2. Objectives
In the present study, HBHA and Mtb32C (two immunogenic antigens) were isolated from the genome of M. tuberculosis H37Rv and cloned into pcDNA 3.1 (+). Due to Immunogenicity effect of these genes, the construction can be further used to develop DNA vaccines in future studies.
3. Materials and Methods
3.1. DNA Extraction
Bacterial cells were cultured on the Lowenstein-Jensen medium (BD, USA). The colonies were extracted for DNA extraction by boiling method. In this method, some colonies were dissolved in 400 µL of specific buffer containing 400 µL Tris-Cl (Merck, Germany) 100 mM pH = 7.5, 0.05% Tween 20 (Merck, Germany) and 20 µL proteinase K (Fermentas, Germany). This solution was incubated at 55°C for three hours. To inactivate proteinase K, it was boiled for 10 minutes at 100°C. After centrifugation for 10 minutes at 5000 rpm, upper liquid contained DNA which could be used in further procedures.
3.2. Polymerase Chain Reaction for Isolation of Mtb32C and HBHA Genes
The full-length of Mtb32C and HBHA genes were amplified by polymerase chain reaction (PCR) method from genomic DNA of M. tuberculosis H37Rv genome (Pasture institute of Iran). Four specific primers were designed: 5’-GAATTCCCGCCATGGGGCAATTACATATGACGGCCGCGTCCGATAACTTC-3’ as a forward primer and 5’-GCGGCCGCCTAATCGGATCCGGCCGGGGGTCCCTCGGCCAA-3’ as a reverse primer for amplification of Mtb32C and 5’-GCGGCCGCGCTGAAAACTCGAACATTGATGAC-3’ as a forward primer and 5’-CTCGAGTAATGAgtagtagtagtagtagtaTACTTCTGGGTGACCTTCTTG-3’ as a reverse primer were used for amplification of HBHA. The underlined letters in forward and reverse primers represent EcoRI, NotI and NotI, XhoI restriction sites, respectively. Italic and bold letters represent two stop codons on HBHA reverse primer, which are followed by 18 small letters that encode six histidine amino acids. The PCR reaction mixture contained 1 μL DNA sample, 2.5 mM MgCl2 (Fermentas, Germany), 0.5 mM each dNTPs (Fermentas, Germany), 10 pmol each primers (Cinnagen, Iran) and 1 unit Taq polymerase (Cinnagen, Iran). Amplification of these genes were performed for 35 cycles (95°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute) after an initial denaturation step at 95°C for 5 minutes (performed by applied biosystems thermal cycler). PCR product was analyzed using 1.5% agarose gel by Green viewer staining (Pars Tous, Iran) and UV transillumination.
3.3. Generation of HBHA Construct
The amplified HBHA, along with pcDNA3.1 (+) (Invitrogen, USA) was digested by restriction enzymes XhoI and NotI. Digested products were purified by AccuPrep gel purification kit (Bioneer, South Korea) according to the manufacturer’s recommendations. For HBHA gene insertion into pcDNA3.1 (+) vector, ligation solution containing 1 µL T4 DNA ligase (Fermentas, Germany), 1.5 µL 10x buffer, 2.5 µL digested and purified pcDNA 3.1 (+), 9 µL digested and purified HBHA PCR product, 1 µL PEG mixed and incubated overnight at 16°C. Competent bacteria strain JM109 (Promega, USA) was transformed as described previously (15). The presence of HBHA was confirmed by colony-PCR using specific primers and restriction enzyme analysis.
3.4. Cloning of mtb32C into HBHA-pcDNA3.1 (+) Vector
HBHA-pcDNA3.1 (+) vector and amplified Mtb32C gene were digested with EcoRI and NotI restriction enzymes, respectively. Purification procedure was performed as described previously. T4 DNA ligase enzyme (Fermentas, Germany) was used to insert the digested and purified Mtb32C fragment into the digested vector. Transformation procedure was performed as described previously (15). Transformed Escherichia coli with chimeric Mtb32C-HBHA-pcDNA3.1 (+) vector could grow in the presence of Ampicillin 100 mg/mL antibiotic. Single digestion and PCR were performed on chimeric pcDNA3.1 (+) vector to confirm the ligation. The constructed vector was purified from E. coli using QIAprep Miniprep kit (Qiagen, USA).
3.5. Restriction Enzyme Analysis and Colony-PCR
To confirm the presence of Mtb32C and HBHA genes, colony-PCR using specific primers and single digestion by XhoI enzyme, was performed. For colony-PCR analysis, a master mix containing T7 and reverse primers of HBHA was made. This mixture was used as a DNA of a single bacterial colony. First, Mtb32C-HBHA was amplified by PCR and it showed a 984 bp fragment in 1% agarose gel electrophoresis. To perform restriction enzyme analysis, a mixture containing chimeric vector, 10x buffer and XhoI enzyme was prepared and incubated at 37°C for two hours. Restriction digestion products visualized on 1% agarose gel.
3.6. Western Blot Analysis
To monitor expression of Mtb32C-HBHA recombinant protein, western blot analysis was performed. 106 Hela cells were seeded into a 6-well micro-plate and incubated overnight in Dulbecco’s modified eagle medium (DMEM) (Invitrogen, USA). When cells reached 90% confluency, they were transfected with pcDNA3.1/Mtb32C-HBHA using calcium-phosphate protocol (16). Forty-eight hours after transfection, cells were washed with PBS and then used for Western blot analysis. For Western blotting analysis, the cell extracts of transfected and non-transfected HeLa cells were prepared. PMSF (phenyl methyl sulfonyl fluoride) (Invitrogen, USA) was added to cell lysate to inactivate proteinase. After that, it was boiled for 10 minutes. The specimens were run on a 12% SDS-PAGE gel. The gel was transferred to poly vinylidine difluoride (PVDF) (BioRad, USA) membrane using dry electroblot system (BioRad, USA) and transfer buffer containing 25 mM Tris (pH 8.3), 192 mM glycine and 20% methanol at 25 volts for overnight at room temperature. The blotted membrane was blocked with 5% (w/v) BSA in TBS-T buffer (0.5M NaCl, 0.02 M Tris pH 8.5, 0.05% Tween 20) for two hours at room temperature. Membranes were incubated for two hours at room temperature with mouse anti-His Tag antibody (AbD Serotec, UK), diluted in 1:1000 TBS-T. After reactions with the primary antibody, the blots were washed three times with TBS-T and incubated with peroxidase conjugated Rabbit-anti-mouse IgG antibody (AbD Serotec, UK), at a 1:1000 dilution in TBS-T. The blots were then washed three times with TBS-T and stained by diaminobenzidine (DAB) (Sigma, Germany) (16).
4. Results
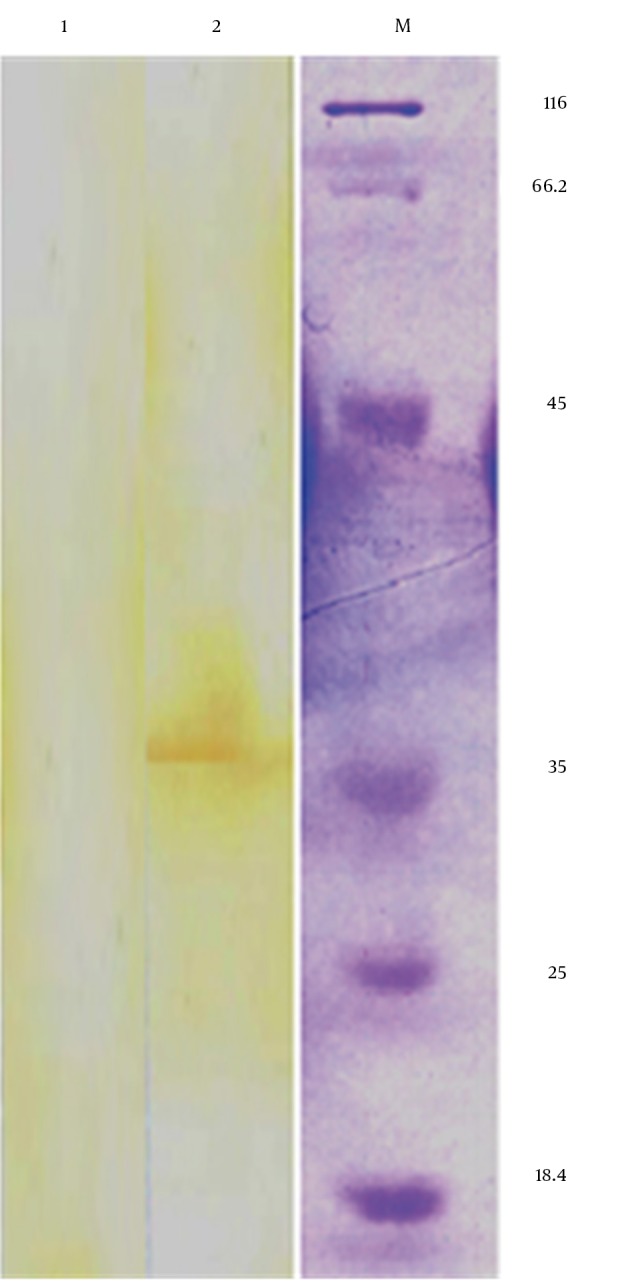
In the present study, the authors amplified Mtb32C and HBHA genes of M. tuberculosis H37Rv genome DNA by PCR. The amplified fragments showed the expected size of 600 bp for HBHA fragment and 384 bp for Mtb32C fragment (Figures 1 and 2). After digestion with restriction enzymes, DNA fragments containing Mtb32C and HBHA genes were ligated into pcDNA3.1 (+) vector (Figure 3). When cleaved at XhoI restriction sites, the recombinant vector was produced with the length of 6412 bp (Figure 4). Presence of 984 bp fragment of Mtb32C-HBHA fusion gene was confirmed using double digestion of recombinant vector (Figure 5). pcDNA3.1 (+) vector has a strong promoter used to obtain high levels of expression of recombinant protein in mammalian hosts. Western blot analysis was used to characterize Mtb32C-HBHA protein expression in transfected cells using anti-His Tag antibody. The results showed that the protein band has approximately 35.5kDa in Western blotting (Figure 6).
Figure 1. Agarose Gel Electrophoresis of Mtb32C PCR Product.
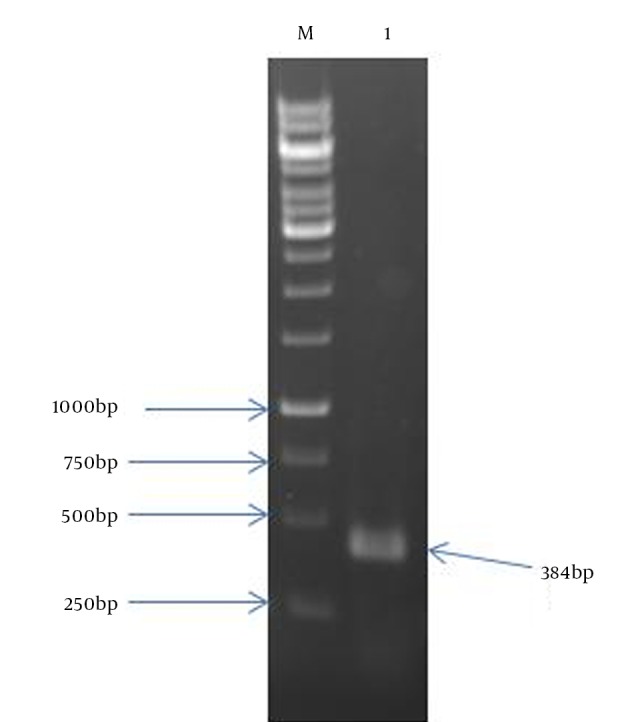
Lane 1: 384 bp Mtb32C PCR product; Lane M: 1 kb DNA size marker.
Figure 2. Agarose Gel Electrophoresis of HBHA PCR Product.
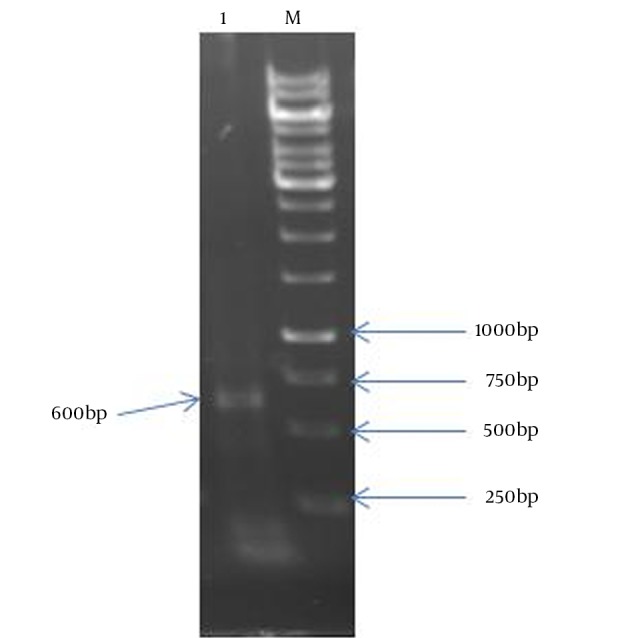
Lane 1: a 600 bp fragment of HBHA; Lane M: 1 kb DNA size marker.
Figure 3. Colony-PCR Results Using T7 Primer and Reverse Primer of HBHA.
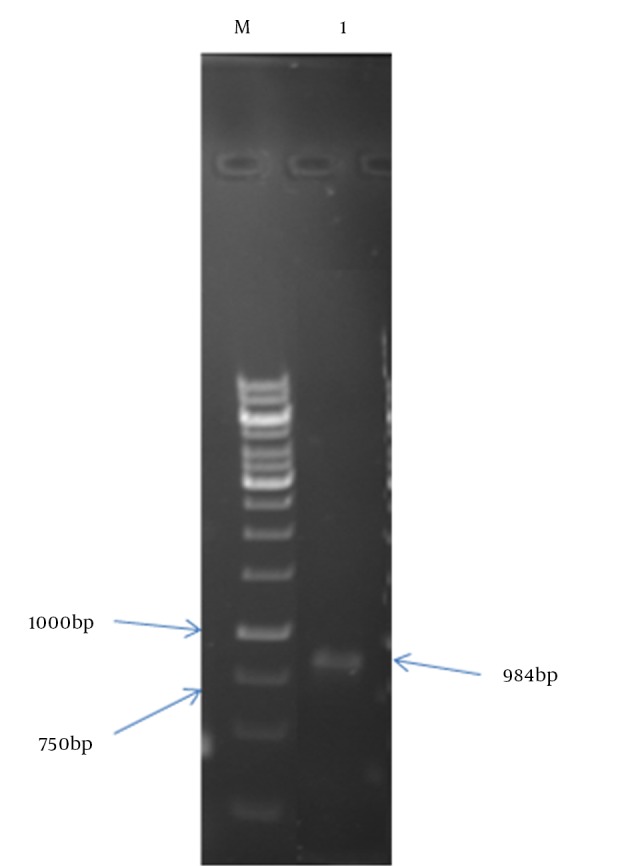
Lane M: 1 kb size marker; Lane 1: PCR product.
Figure 4. Analysis of Positive Clone With Restriction Enzyme XhoI.
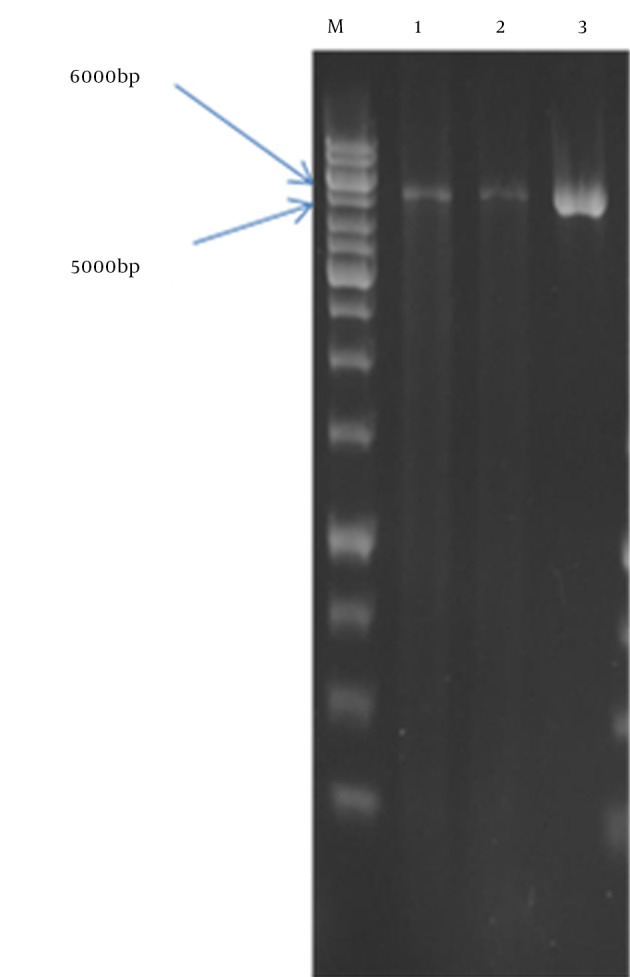
Lanes 1 and 2: recombinant vector linearized by XhoI; Lane3: pCDNA3.1 (+) vector linearized by XhoI; M: 1 kb DNA size marker.
Figure 5. Double Digestion of Recombinant Vector by XhoI and EcoRI Restriction Enzyme Leading to Ejection of Mtb32C-HBHA Fusion Gene.
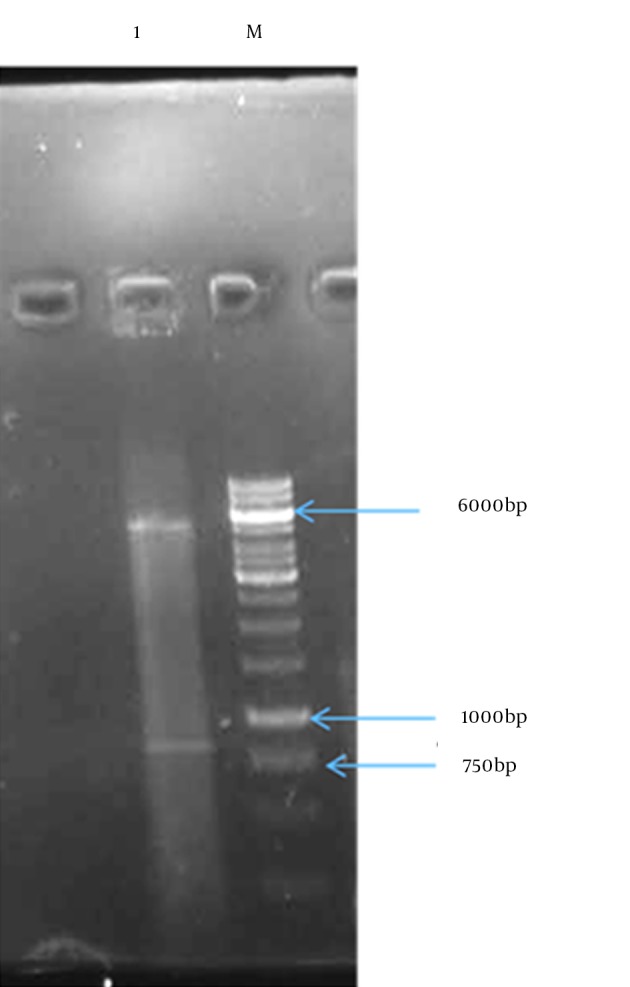
Lane1: vector and Mtb32C-HBHA fusion gene; M: 1 kb DNA size marker.
Figure 6. Detection of Mtb 32C-HBHA Protein in Transfected Cells by Western Blot Analysis Showing a Protein Band With a Size of Approximately 35.5 kDa (lane 2) in Transfected Cells But not in Non-transfected Cells (lane 1).
5. Discussion
Tuberculosis is one of the most important diseases responsible for an annual death of 2 - 3 million people and is a fundamental problem still to be addressed (17). Attempts to reduce the spread of mycobacterial infections have faced variety of challenges including inefficiency of BCG vaccine in preventing diseases and the emergence of multidrug resistant strains. Immune memory slowly declines after BCG vaccination and repeated BCG administration cannot increase the protection and may be harmful. Therefore, development of new and effective vaccines is critical (7, 18). One of the main objectives in research about tuberculosis is to identify antigens and examine their ability to stimulate cellular and/or humoral immunity to use them in diagnostic methods or vaccine design (19, 20). Tuberculosis is an intracellular pathogen. Therefore, cell-mediated immunity, especially IFN-γ-secreting TCD4 and TCD8, has an important role in the control of TB (21, 22). Previous studies showed that mice with a disrupted IFN-γ gene or mutated IFN-γ receptor have high susceptibility to mycobacterial infections (11, 23).
HBHA is an adhesion, which reacts with non-phagocytic cells in lung and plays a key role in extra-pulmonary dissemination (18, 24). HBHA is an important virulence factor, which participates in extrapulmonary dissemination. Disruption of the HBHA gene in M. tuberculosis impairs bacterial dissemination from the lungs to the spleen (25). Recently, a designed vaccine based on HBHA is close to phase I of clinical trial (26, 27). Several studies have shown that immunization of mice with purified HBHA along with different adjuvants motivates T cell CD4, T cell CD8 and consequent cytokine production. In the absence of adjuvant initial immunization with BCG and using HBHA as a booster vaccine has led to an increase in IFN-γ, IL-12, TGF-β cytokines compared to when BCG is injected alone. These cytokines have important role in generation, preservation and expansion of effector-memory T cell and increase the lethal activity of macrophages against TB leading to significant reduction of bacterial load in the lungs (18, 28).
The main effects of the vaccine are stimulation of TCD4 cells, production of IFN-γ and increase in germicidal activity of macrophages. Consequently, those antigens of M. tuberculosis capable of stimulating stronger T cells, would be a better candidate for vaccine development. Studies have shown that IFN-γ has a key role in protection against tuberculosis. In a study conducted in 2011, it was found that HBHA recognized by T lymphocytes leads to induction of IFN-γ production to high-level. T CD4 and TCD8 cells both contribute to the production of IFN-γ. These cells are important in controlling infection by M. tuberculosis. Unlike those who are merely infected, patients infected with active form of tuberculosis are not able to produce IFN gamma when exposed to HBHA. In healthy people with positive PPD, high levels of IFN gamma are produced in response to HBHA. This reflects the immunity against tuberculosis. Therefore, HBHA is a suitable vaccine candidate and is a good marker to differentiate individuals with active tuberculosis from those infected with M. tuberculosis (26, 27). In a study by Guerrero, primary vaccinations with BCG followed by HBHA antigen as a booster led to production of effector-memory T cell against M. tuberculosis and reduction in load of bacteria in the lungs and spleen in animal model (26, 27). These studies suggest that HBHA is a proper antigen to design new vaccines against TB.
Filtered culture medium of M. tuberculosis contained immunogenic components such as serine proteases family. Members of this family like mtb32b and mtb32a with molecular weight of approximately 32 kDa have more than 66 % homology together. Mtb32a, unlike mtb32b, can enhance the activity of phagocytic cells. Gene encoding mtb32a is found in complex tuberculosis, but is absent in non-pathogenic and environmental species. This gene is contributed to bacterial metabolism and cell survival. C-terminal domain of mtb32a (Mtb32C) can strongly motivate TCD8 cells, which produce cytokines and is a vaccine candidate (12, 29-31).
HBHA and Mtb32C activate cellular immune response. These activated cells release IFN-γ, which is a potential cytokine in the activation of macrophages and granuloma formation. Granuloma can limit the growth and dissemination of M. tuberculosis. Designing new vaccine based on these potent antigens can be used as booster vaccine to raise the efficacy of BCG vaccine. In the present study, HBHA and Mtb32C (two immunogenic antigens) were isolated from M. tuberculosis H37Rv genome and were fused together. Definitively this fusion and chimeric gene in this construct can produce new antigen that would contain different antigenically and immunological characteristics. These specifications remain to be determined and this study is the first report of the construction of this new DNA vaccine.
Although in this study, Mtb32C-HBHA DNA vaccine was constructed; further studies are encouraged to take into account their responses in animal models, or their efficiency. In addition, more studies should be performed to determine the epitope conformation of this new antigen and check adhesion activity of HBHA in chimeric protein. Many efforts are under way to develop new vaccines against TB and to replace the existing vaccines with better alternatives. Based on the result of the current study, this construct can be used to develop new DNA vaccines against TB. Immunological responses can be evaluated in animal models in further studies.
Acknowledgments
The current study was extracted from a thesis presented for obtaining PhD degree from Mashhad university of medical sciences, Mashhad, Iran (Thesis No. 4541).
Footnotes
Authors’ Contributions:Roghayeh Teimourpour: Assistance with performing laboratory tests; Ali Sadeghian: obtaining funding for the study, conception and design of the study, guarantor of integrity of the entire study; Zahra Meshkat: conception and design of the study, guarantor of integrity of the entire study; Majid Esmaelizad: assistance with performing laboratory tests; Mojtaba Sankian: assistance with performing laboratory tests; Ahmad-Reza Jabbari: assistance with preparing the manuscript.
Funding/Support:This study was financially supported by the research council of Mashhad university of medical sciences, Mashhad, Iran (Grant No. 910016).
References
- 1.Sato K, Tomioka H, Shimizu T, Gonda T, Ota F, Sano C. Type II alveolar cells play roles in macrophage-mediated host innate resistance to pulmonary mycobacterial infections by producing proinflammatory cytokines. J Infect Dis. 2002;185(8):1139–47. doi: 10.1086/340040. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Dolin R, Bennett JE. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. London: Elsevier; 2009. [Google Scholar]
- 3.Gengenbacher M, Kaufmann SH. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev. 2012;36(3):514–32. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinb). 2010;90(6):361–6. doi: 10.1016/j.tube.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich G, Viret JF, Hess J. Mycobacterium bovis BCG-based vaccines against tuberculosis: novel developments. Vaccine. 2003;21(7-8):667–70. doi: 10.1016/s0264-410x(02)00577-7. [DOI] [PubMed] [Google Scholar]
- 6.Dara M, Dadu A, Kremer K, Zaleskis R, Kluge HH. Epidemiology of tuberculosis in WHO European Region and public health response. Eur Spine J. 2013;22 Suppl 4:549–55. doi: 10.1007/s00586-012-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31 Suppl 3:S64–7. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 8.Delogu G, Fadda G. The quest for a new vaccine against tuberculosis. J Infect Dev Ctries. 2009;3(1):5–15. doi: 10.3855/jidc.99. [DOI] [PubMed] [Google Scholar]
- 9.Orme IM. Current progress in tuberculosis vaccine development. Vaccine. 2005;23(17-18):2105–8. doi: 10.1016/j.vaccine.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412(6843):190–4. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 11.Brighenti S, Andersson J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis. 2012;205 Suppl 2:S316–24. doi: 10.1093/infdis/jis043. [DOI] [PubMed] [Google Scholar]
- 12.Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172(12):7618–28. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-Guimaraes ML, Pessolani MC. Comparative genomics of mycobacterial proteases. Microb Pathog. 2007;43(5-6):173–8. doi: 10.1016/j.micpath.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro-Guimaraes ML, Tempone AJ, Amaral JJ, Nery JA, Gomes Antunes SL, Pessolani MC. Expression analysis of proteases of Mycobacterium leprae in human skin lesions. Microb Pathog. 2007;43(5-6):249–54. doi: 10.1016/j.micpath.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a Laboratory Manual. 2 ed. Vol. 2. New York: Cold Spring Harbor; 2001. [Google Scholar]
- 16.Jordan M, Wurm F. Transfection of adherent and suspended cells by calcium phosphate. Methods. 2004;33(2):136–43. doi: 10.1016/j.ymeth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Global tuberculosis report 2013. 2013
- 18.Shin AR, Lee KS, Lee JS, Kim SY, Song CH, Jung SB, et al. Mycobacterium tuberculosis HBHA protein reacts strongly with the serum immunoglobulin M of tuberculosis patients. Clin Vaccine Immunol. 2006;13(8):869–75. doi: 10.1128/CVI.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8(5):e21556. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parida SK, Kaufmann SH. Novel tuberculosis vaccines on the horizon. Curr Opin Immunol. 2010;22(3):374–84. doi: 10.1016/j.coi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, van Dissel JT. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet. 2002;32(1):97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 22.van de Vosse E, Hoeve MA, Ottenhoff TH. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004;4(12):739–49. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- 23.Masungi C, Temmerman S, Van Vooren JP, Drowart A, Pethe K, Menozzi FD, et al. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J Infect Dis. 2002;185(4):513–20. doi: 10.1086/338833. [DOI] [PubMed] [Google Scholar]
- 24.Pethe K, Aumercier M, Fort E, Gatot C, Locht C, Menozzi FD. Characterization of the heparin-binding site of the mycobacterial heparin-binding hemagglutinin adhesin. J Biol Chem. 2000;275(19):14273–80. doi: 10.1074/jbc.275.19.14273. [DOI] [PubMed] [Google Scholar]
- 25.Vidal Pessolani MC, Marques MA, Reddy VM, Locht C, Menozzi FD. Systemic dissemination in tuberculosis and leprosy: do mycobacterial adhesins play a role? Microbes Infect. 2003;5(7):677–84. doi: 10.1016/s1286-4579(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero GG, Debrie AS, Locht C. Boosting with mycobacterial heparin-binding haemagglutinin enhances protection of Mycobacterium bovis BCG-vaccinated newborn mice against M. tuberculosis. Vaccine. 2010;28(27):4340–7. doi: 10.1016/j.vaccine.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 27.Rouanet C, Debrie AS, Lecher S, Locht C. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microbes Infect. 2009;11(13):995–1001. doi: 10.1016/j.micinf.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero GG, Locht C. Recombinant HBHA boosting effect on BCG-induced immunity against Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:730702. doi: 10.1155/2011/730702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin SM, Izzo AA, Dow SW, Skeiky YA, Reed SG, Alderson MR, et al. Tracking antigen-specific CD8 T lymphocytes in the lungs of mice vaccinated with the Mtb72F polyprotein. Infect Immun. 2005;73(9):5809–16. doi: 10.1128/IAI.73.9.5809-5816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skeiky YA, Lodes MJ, Guderian JA, Mohamath R, Bement T, Alderson MR, et al. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect Immun. 1999;67(8):3998–4007. doi: 10.1128/iai.67.8.3998-4007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun. 2004;72(11):6622–32. doi: 10.1128/IAI.72.11.6622-6632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


