Abstract
The nervous system must balance excitatory and inhibitory input to constrain network activity levels within a proper dynamic range. This is a demanding requirement during development when networks form and throughout adulthood as networks respond to constantly changing environments. Defects in the ability to sustain a proper balance of excitatory and inhibitory activity are characteristic of numerous neurological disorders such as schizophrenia, Alzheimer’s disease, and autism. A variety of homeostatic mechanisms appear to be critical for balancing excitatory and inhibitory activity in a network. These are operative at the level of individual neurons, regulating their excitability by adjusting the numbers and types of ion channels, and at the level of synaptic connections, determining the relative numbers of excitatory versus inhibitory connections a neuron receives. Nicotinic cholinergic signaling is well positioned to contribute at both levels because it appears early in development, extends across much of the nervous system, and modulates transmission at many kinds of synapses. Further, it is known to influence the ratio of excitatory-to-inhibitory synapses formed on neurons during development. GABAergic inhibitory neurons are likely to be key for maintaining network homeostasis (limiting excitatory output), and nicotinic signaling is known to prominently regulate the activity of several GABAergic neuronal subtypes. But how nicotinic signaling achieves this and how networks may compensate for the loss of such input are important questions remaining unanswered. These issues are reviewed.
Keywords: Nicotinic, homeostasis, compensation, neural network, E/I ratio, circuits
Graphical Abstract
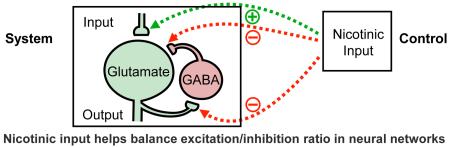
Introduction
A striking feature of neural networks is their ability to maintain input-output relationships, adjusting to accommodate in ways that sustain functional behavioral responses. Key here is the homeostatic nature of circuits, adjusting the excitatory-to-inhibitory balance (E/I ratio) across networks and within individual neurons comprising the circuits. Fundamental to this process is the threshold set within individual neurons for firing action potentials and the contribution of their output to network activity. A second level of regulation involves the number and ratio of excitatory versus inhibitory synapses a neuron receives. Deficiencies in the E/I ratio for networks in the brain overall emerge as a central feature in a number of neurological disorders, underscoring the importance of homeostatic regulation. Examples include epilepsy, schizophrenia, autism, and Rett syndrome (1-5).
Nicotinic cholinergic signaling is initiated early in development and extends across much of the central nervous system. It involves the neurotransmitter acetylcholine (ACh) activating a variety of ligand-gated ion channels termed nicotinic acetylcholine receptors (nAChRs). Because nAChRs are widely distributed and can elevate intracellular calcium levels locally, they can exert numerous modulatory functions in the nervous system. These include regulation of presynaptic transmitter release at a variety of synapses, as well as promotion of synaptic plasticity through a number of postsynaptic actions. The importance of nicotinic signaling during development is reflected in the fact that early exposure to nicotine is known to cause long-lasting behavioral changes seen in the adult (6-20). The contributions of nicotinic signaling to homeostatic regulation and maintenance of the E/I ratio are only beginning to be understood. This review will first summarize the nature of E/I control, and then consider how nicotinic signaling influences fundamental features of the nervous system relevant for E/I balance, including its actions on interneurons. It concludes with a consideration of mechanisms employed by the nervous system to compensate for long-lasting alterations in nicotinic signaling.
Maintaining the excitatory-to-inhibitory balance in neural networks
Throughout the brain, excitatory and inhibitory synaptic inputs are tightly regulated with respect to number and function, balancing their effects against each other (21, 22). Network activity appears to be maintained within a given dynamic range (rather than at an exact point) by compensatory alterations, or ‘synaptic homeostasis’ (23-25). This prevents runaway signaling while providing stable long-term effectiveness and integrity. The balance between excitation and inhibition in networks results from the coordinated and highly regulated activities of recurrent excitatory and inhibitory connections and governs numerous brain processes including, for example, integration of sensory information in the cortex (26-30).
Activity patterns within a network are shaped by the firing properties of individual neurons and the connections they make. The firing properties and electrophysiological characteristics of a neuron are, in turn, determined by the combination, spatial distribution, and density of ion channels and receptors expressed across the cell surface (31). To maintain homeostasis in a constantly changing environment, neurons can employ a variety of mechanisms to regulate these individual features. Examples include activity-induced compensatory changes in the ratios of voltage-dependent ion channels and receptors (32-39), as well as activity-independent mechanisms (40). Theoretical models predict that neurons with a common and well-defined electrophysiological phenotype can achieve this with very different contributions of channel conductances, having only weak correlations among the conductances (41). Each measured electrophysiological property of the neuron with a given well-defined behavior can be achieved by a different subset of several maximal conductances, showing that there are many ways to arrive at the same overall outcome.
Another dimension of homeostasis is reflected in the balance of excitatory versus inhibitory input a neuron receives. An instructive example is provided by pyramidal cells of the cortex that display a fixed ratio of excitatory and inhibitory input, despite large variations in amplitude of the synaptic response. As a result, the two kinds of input remain proportional, thereby equalizing E/I ratios (22). Interestingly, parvalbumin-positive interneurons play an important role in this, participating in a bidirectional modulation of their synaptic strength onto cortical neurons to accommodate altered excitatory input and achieve the proper E/I ratio. In contrast, somatostatin-positive neurons innervating the cortical neurons do not contribute to the equalization. These observations focus attention on interneuronal subpopulations as key for determining E/I ratio and sustaining appropriate activity levels of networks.
The nicotinic cholinergic signaling system is strategically positioned to exert neuromodulatory effects on the coupling of excitatory and inhibitory balance. In the visual cortex, endogenous cholinergic signaling helps regulate the E/I balance through both nicotinic and muscarinic mechanisms (42). Nicotinic cholinergic signaling relies on a variety of nAChR subtypes, each with its own pharmacological and expression-pattern profiles. The receptors can be found both pre-and post-synaptically, as well as extra-synaptically, on excitatory and inhibitory neurons (and also on glia), where they produce a variety of actions. In the mammalian brain, the most abundantly expressed nicotinic receptors are the heteropentameric α4- and β2-containing nAChR (α4β2-nAChR) and the homopentameric α7-containing nAChR (α7-nAChR). The latter has a high relative calcium permeability (43, 44), equipping it to have many downstream effects (45, 46). Both receptor types occur at relatively high densities in the cortex and hippocampus. Notably, nicotinic signaling is well placed to provide modulation that achieves fine-tuning of circuits to select parameters within the dynamic range of acceptable values. It will be important for future research to identify the “tipping point” within a circuit that takes neuronal activity out of its acceptable range (41, 47-49).
Interneuron subtypes and nicotinic signaling
Inhibitory input is critical not only for sculpting specific firing patterns within a neural network but also for preventing network activity from escalating to dysfunctional levels. GABAergic interneurons are responsible for the vast majority of inhibitory signaling in the nervous system and are extremely diverse. They can be classified by a variety of criteria including location, anatomy, electrophysiological properties, and expression of distinct neurochemical markers.
One classification separates GABAergic cortical interneurons into three largely non-overlapping groups based on expression of parvalbumin, somatostatin, or serotonin receptor 3a (5-HT3AR). These categories include nearly all cortical interneurons (50), which account for about 20% of all neurons in the cortex (2). Further subdivisions have combined markers such as parvalbumin, calretinin, calbindin, somatostatin, vasoactive intestinal peptide (VIP), and neuropeptide Y (51, 52). With the possible exception of CHRNA2, the gene for the α2 nAChR subunit, on oriens-lacunosum moleculare (O-LM) interneurons in the hippocampal CA1 region in rodent brains (53), no single molecular marker has been found to be unique for a given interneuron subtype.
Nicotinic input represents a prominent source of modulation for cortical interneurons, with major cholinergic projections coming from the basal forebrain (54-56). Hippocampal interneurons, which range from 4-10% of the total neurons along the ventral-dorsal axis (57), also receive extensive cholinergic innervation, largely from the medial septum (58, 59). A variety of nAChR subtypes mediate the nicotinic input both in the cortex and hippocampus (60, 61). Prominent are α7-nAChRs which are expressed on a variety of interneuronal subtypes thought to include parvalbumin-positive, somatostatin-positive, and VIP-positive interneurons, all of which have their own unique subset of connectivity patterns in cortical circuitry (62). The α7-nAChR is also expressed at high levels on interneurons in the hippocampus (63, 64). Anatomical reconstruction of interneurons in this region reveals heterogeneous populations of multiple subtypes, including those that inhibit pyramidal cells at somatic or dendritic synapses (65, 66). As a result, nicotinic activation of interneurons via α7-nAChRs could either inhibit or dis-inhibit hippocampal pyramidal neurons (the primary hippocampal output) depending on whether the activated interneuron synapses directly onto pyramidal cells or onto other interneurons that normally inhibit the pyramidal cells (67-69).
In addition to α7-nAChRs, many interneurons also express the other major nicotinic receptor subtype, namely the α4β2-nAChR. The relative abundance of receptor varies dramatically with neuron type (70), indicating a potential for lamina-specific neuromodulatory control of hippocampal function by nicotinic input. It remains unclear, however, how the number and distribution of α7-nAChRs and α4β2-nAChRs vary with interneuron subtype.
The diversity of GABAergic interneurons positions them to influence network function in complex ways (71-73). The interneuron population of the hippocampus is incredibly diverse with the precise roles played by distinct inhibitory cell types currently being unclear. Each subtype of hippocampal interneuron has distinct postsynaptic domains and is therefore positioned to differentially control input and output activity (71). Hippocampal basket and axo-axonic interneurons, both of which are parvalbumin-positive, are located in the stratum pyramidale region where they suppress pyramidal neurons via perisomatically suppressing Na+ spikes and action potentials (51, 74). Most cortical somatostatin interneurons are Martinotti cells that send their axons into superficial layers and form dense axon collateral networks in layer I (75). They are morphologically and physiologically similar to O-LM neurons of the hippocampus (76), which are highly responsive to nicotinic signaling (53, 77). Little is known about the nicotinic responsiveness of cortical somatostatin neurons.
O-LM cells (somatostatin-positive) are found in the outermost layer of the hippocampus in the stratum oriens with perpendicular projections to the stratum lacunosum-moleculare (71), and they modify the excitatory drive to the distal apical dendrites of pyramidal neurons. Nicotinic activation or potentiation of O-LM neurons in CA1 facilitates long-term potentiation through increases in calcium influx (53, 78). O-LM cells have a key role in gating information flow in CA1, differentially modulating CA3 and entorhinal inputs to hippocampal CA1 neurons, are interconnected by gap junctions, receive direct cholinergic inputs from subcortical afferents, and are primarily responsible for the effect of nicotine on synaptic plasticity of the Schaffer collateral pathway (53). They are commonly identified by somatostatin, but somatostatin is also expressed by other interneuron subtypes throughout the hippocampal formation (71). Only in the hippocampal CA1 is CHRNA2 reliable as a specific marker for O-LM cells (53).
A subpopulation of interneurons highly responsive to nicotine is the 5-HT3AR+ neurons that express VIP and are enriched in layer I of the cortex. Optogenetically it has been shown that VIP-positive cells express functional α4β2-nAChRs, but they represent an anatomically and electrophysiologically heterogeneous subgroup of cells (79). Unlike various other populations of interneurons, 5-HT3AR+ neurons do not express either Lypd6 or Lynx1 at detectable levels (80). This is noteworthy because members of the Lynx family represent a unique set of nAChR modulators (54, 81). Two members – Lynx1 and Lypd6 – are expressed in discrete GABAergic interneuron subpopulations, with Lynx1 being expressed in most parvalbumin-positive neurons, while Lypd6 is only found in somatostatin-positive interneurons (80). The distribution and function of Lynx family members in modulating nicotinic input in neural networks will be an important issue for the future.
Synaptic mechanisms for maintenance of excitatory-to-inhibitory balance
If the first level of homeostatic control of network activity involves neuronal thresholds for firing action potentials, the second level would appear to be the ratio of excitatory and inhibitory input (E/I ratio) neurons receive. And, of course, critical here is whether the neuron being excited is itself excitatory or inhibitory and, if the latter, whether it inhibits excitatory neurons or other inhibitory neurons. Various methods have been used to assess the E/I input a neuron receives. One is to measure the frequency of excitatory versus inhibitory synaptic events in single cells. Electrophysiologically this is usually assessed by comparing the relative contribution of glutamatergic and GABAergic synaptic events (82). Anatomical methods for identifying synapses utilize either immunostaining for glutamatergic or GABAergic synapses or ultrastructural analysis to quantify symmetrical (inhibitory) versus asymmetrical (excitatory) synapses on a neuron (82, 83).
Serial electron microscope reconstructions have shown that rat hippocampal CA1 pyramidal cell dendrites receive approximately 30,000 excitatory inputs and 1,700 inhibitory inputs, yielding an E/I ratio of about 18:1 (82). Examination of local inhibitory interneurons in the hippocampus reveal notable interneuron-subtype differences: parvalbumin-positive neurons contain about 16,000 inputs of which 6% are inhibitory (E/I = ~14:1); calbindin-positive neurons receive 4,000 inputs of which 30% are inhibitory (E/I = ~2:1); and calretinin-positive neurons maintain 2,000 inputs of which 20% are inhibitory (E/I = ~4:1; 84). Considerable differences in E/I ratios are discernable between, and even amongst, neurons of particular classes, and ratios may be highly dynamic over the entire course of development.
Absence of the α7-nAChR subtype, as found in the α7-nAChR constitutive knockout mouse (α7KO), results in decreased numbers of excitatory glutamatergic synapses forming with no change in the numbers of GABAergic synapses, assessed both electrophysiologically and by immunostaining. This suggests a decreased E/I ratio compared to wild-type control neurons, which was confirmed by a comparison of maximally evoked glutamatergic versus GABAergic synaptic responses in pyramidal cells of the hippocampal CA1 region (83). Unknown is whether this altered E/I is also found in interneurons and, if so, how it affects overall output of the CA1.
An interesting related question is whether various compensatory effects, e.g. alterations in the threshold for firing action potentials, compensate for the altered synaptic E/I ratio.
Development is a particularly challenging time for maintaining E/I balance as circuits are forming and becoming stabilized. Vast changes occur in anatomy, synaptic connections, and network dynamics. GABAergic signaling is initially excitatory/depolarizing in the immature brain, but as the brain develops it transitions to become inhibitory/hyperpolarizing as found in the adult (85, 86). An indication of the changing consequences for network function comes from the observation that during the early phase when GABA is excitatory/depolarizing, repetitive stimulation can attenuate long-term depression (LTD) in GABAergic inputs (87). In contrast, subsequently when GABA becomes inhibitory/hyperpolarizing, similar stimulation yields GABAergic long-term potentiation (LTP, 87).
Because the α7KO is constitutive, it would be expected to have effects throughout development, and this appears to be the case. Not only do the mice display fewer excitatory synapses at all times examined, but they also show a delay in the critical developmental transition when GABA converts from being excitatory/depolarizing to inhibitory/hyperpolarizing (88). A delay is also seen in the GABA transition for adult-born neurons in the α7KO dentate gyrus, and this is associated with reduced survival, maturation, and integration of the neurons (89, 90). Notably, immature granule cells in the postnatal dentate gyrus have α7-nAChRs, raising the possibility that the nicotinic effect is a direct one (91). How nicotinic signaling promotes glutamatergic synapse formation and whether the system can compensate for synaptic deficits will be important questions for the future.
Nicotinic perturbation and pathological E/I imbalance
An imbalance in the E/I ratio, occurring early in development, is increasingly being associated with neurological disorders such as epilepsy, schizophrenia, autism, and Rett syndrome (1-5). Aberrations in nicotinic signaling, particularly via α7-nAChRs, are also increasingly being linked with neurological disorders, principal among them being schizophrenia (92), Alzheimer’s disease (93, 94), and juvenile myoclonic epilepsy (95).
Deletion of human 15q13.3, including the α7-nAChR gene, is associated with autism, mental retardation, epilepsy, bipolar disorder, and intellectual disability (96-108). Additional evidence suggests that deletion of the α7-nAChR gene alone may be sufficient to cause the majority of the associated clinical features in patients with these disorders (100, 101, 104, 107).
Nicotinic signaling has long been implicated in schizophrenia. Some propose that schizophrenics self-medicate by smoking, with smoking prevalence reaching 90% (109). Post-mortem brain analysis of schizophrenic patients reveals loss of α7-nAChRs in both the prefrontal cortex and hippocampus (96, 110-114) and also deficits in both parvalbumin and somatostatin neurons (115-117), known to normally express these receptors. Alterations in α7-nAChR levels contribute importantly to the prominent changes in E/I ratio seen in schizophrenia. Rodent studies have shown that deletion of the α7-nAChR impairs cortical parvalbumin GABAergic interneuron development, and this mirrors many of the neurochemical characteristics found in the schizophrenic brain (118). The chromosome containing the α7-nAChR subunit gene is a site of heritability for schizophrenia, and also for a deficit in inhibitory neuronal function associated with this disorder. Taken together, current findings suggest that loss of interneurons expressing α7-nAChRs contributes to the pathogenesis of schizophrenia.
Neurological diseases involving aberrant nicotinic signaling and E/I imbalances are not restricted to developmental disorders. For example, the loss of excitatory cholinergic neurons in Alzheimer’s disease, a neurodegenerative disorder typically with a late onset in life, results in an unbalanced E/I ratio (119). The loss of cholinergic neurons has a profound effect on nicotinic signaling, particularly on signaling through α7-nAChRs (120).
How nicotinic signaling in general, and α7-nAChRs in particular, affect E/I and related neurological disorders is not known. In α7KO mice, the numbers of parvalbumin-positive interneurons in the cortex are decreased compared to wild-type littermates (118). The decrease in α7-nAChR-expressing parvalbumin-positive interneurons could alter the E/I ratio if not compensated. Both parvalbumin and somatostatin neurons have been implicated in governing network oscillations in the brain (121, 122). Parvalbumin interneurons are thought to play a role in the synchronization of gamma network oscillations in the hippocampus (123). Optogenetic disruption of parvalbumin cell activity can abolish gamma rhythm activity in cortical circuits (124). It is known that α7-nAChRs are required for control of gamma oscillations (125).
Nicotinic signaling is fundamental to an array of cognitive processes including attention, and learning and memory, whilst also contributing to synchronous oscillatory activity, as noted above (126-129). Network gamma oscillations are strongly correlated with cognitive activity such as working memory (130, 131) and sensorimotor gating, both of which are commonly affected in schizophrenia (81, 125, 131). Both dysfunction of parvalbumin-positive neurons in schizophrenia (5) as well as microdeletion of 15q13.3, which includes the loss of the α7-nAChR gene and is itself linked to schizophrenia (108), have been associated with perturbed gamma oscillations. Consistent with the above, α7KO mice have deficits in cortical parvalbumin interneurons (118) and show dysfunctional gamma oscillations (132).
What role might homeostatic compensation play in systems with aberrant nicotinic signaling? Deficits in behavior may not represent failure of homeostatic compensation. In fact, homeostatic compensation can result in a number of different outcomes, depending on the challenge. The homeostatic process may be working correctly but still produce pathology in response to particular perturbations or deletions (47). Using computer modeling, it has been shown that very similar network activity can be achieved with disparate circuit parameters (133), and it has been experimentally confirmed that alterations in the expression of one channel can be compensated by changes in the density of one or more other channels (40). Compensation, following genetic manipulation, can keep the network functioning within an optimal operating range (39) and prevent runaway changes that could be deleterious. As a result, compensation could produce considerable animal-to-animal variability in network parameters whilst still allowing for appropriate overall network performance (48, 49). In the case of α7KO mice, this could help explain why significant deficits in E/I ratios inferred from synaptic connections (83) produces a relatively mild behavioral phenotype (134, 135).
Conclusions
Nicotinic cholinergic signaling clearly plays important roles both during development in shaping the neural networks that form and in the adult where it modulates network function in numerous ongoing ways. Extended aberrations in nicotinic signaling have been implicated in a number of neurological disorders, and, of course, direct exposure to nicotine can produce addiction. How the nervous system responds to these challenges is only beginning to be understood. It is clear, however, that compensatory mechanisms are likely to be activated and that these can take multiple forms, at least theoretically, to ameliorate consequences, such as those disturbing the E/I balance in the network. As noted above, some of the compensatory changes in pursuit of homeostasis may unavoidably produce deleterious consequences, while others may at least partially return the system to a normal operating range. Important challenges for the future will be elucidation of network consequences when nicotinic signaling is disrupted in a sustained way and, perhaps even more interesting, will be the discovery of compensatory mechanisms the nervous system employs in an attempt to recover homeostasis in the absence of nicotinic signaling.
ACKNOWLEDGMENTS
We thank Giordano Lippi and Seth Taylor for critical comments on the manuscript, and Taylor Berg-Kirkpatrick for help with the graphical abstract. This work was supported by grants from the National Institutes of Health (NS012601-40, NS087342-01, and DA038296-01).
Abbreviations
- ACh
Acetylcholine
- E/I
excitatory-to-inhibitory
- LTD
long-term depression
- LTP
long-term potentiation
- nAChR
nicotinic acetylcholine receptor
- (α7KO)
α7-nAChR constitutive knockout mouse
- nAChR
nicotinic acetylcholine receptor
- O-LM
oriens-lacunosum moleculare
- PV
parvalbumin
- 5-HT3AR
serotonin receptor 3a
- SST
somatostatin
- VIP
vasoactive intestinal peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Roberts E. GABA-related phenomena, models of nervous system function, and seizures. Ann Neurol. 1984;16(suppl):S77–S89. doi: 10.1002/ana.410160713. [DOI] [PubMed] [Google Scholar]
- [2].Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- [4].Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-Inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- [7].Levin ED, Wilkerson A, Jones JP, Christopher NC, Briggs SJ. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Brain Res Dev Brain Res. 1996;97:207–215. doi: 10.1016/s0165-3806(96)00144-7. [DOI] [PubMed] [Google Scholar]
- [8].Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- [9].Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatr. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- [10].Brennan P, Grekin E, Mortensen E, Mednick S. Relationship of maternal smoking during pregnancy and criminal arrest and hospitalization for substance abuse in male and female adult offspring. Am J Psychiatr. 2002;159:48–54. doi: 10.1176/appi.ajp.159.1.48. [DOI] [PubMed] [Google Scholar]
- [11].Linnet K, Dalsgaard S, Obel C, Wisborg K, Henriksen T, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Pyschiatr. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- [12].Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuro-Pyschopharm Biol Psychiat. 2003;27:501–518. doi: 10.1016/S0278-5846(03)00042-3. [DOI] [PubMed] [Google Scholar]
- [13].Button T, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Hum Dev. 2007;83:727–732. doi: 10.1016/j.earlhumdev.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hayase T. Chronologically overlapping occurrences of nicotine-induced anxiety-and depression-related behavioral symptoms: effects of anxiolytic and cannabinoid drugs. BMC Neurosci. 2007;8:76–85. doi: 10.1186/1471-2202-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90:527–536. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- [16].Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacol. 2009;56:254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eppolito AK, Bachus SE, McDonald CG, Meador-Woodruff JH, Smith RF. Late emerging effects of prenatal and early postnatal nicotine exposure on the cholinergic system and anxiety-like behavior. Neurotox Teratol. 2010;32:336–345. doi: 10.1016/j.ntt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- [18].Parameshwaran K, Buabeid MA, Karuppagounder SS, Uthayathas S, Thiruchelvam K, Shonesy B, et al. Developmental nicotine exposure induced alterations in behavior and glutamate receptor function in hippocampus. Cell Mol Life Sci. 2012;69:829–841. doi: 10.1007/s00018-011-0805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schneider T, Bizarro L, Asherson PJE, Stolerman IP. Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharm. 2012;223:401–415. doi: 10.1007/s00213-012-2728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- [21].Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- [24].Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosi. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- [25].Maffei A, Fontanini A. Network homeostasis: a matter of coordination. Curr Opin Neurobiol. 2009;2:168–173. doi: 10.1016/j.conb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;47:437–445. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- [27].Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci. 2004;24:3985–3998. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marino J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, et al. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat Neurosci. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- [29].Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- [30].Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marder E. From biophysics to models of network function. Annu Rev Neurosci. 1998;21:25–45. doi: 10.1146/annurev.neuro.21.1.25. [DOI] [PubMed] [Google Scholar]
- [32].LeMasson G, Marder E, Abott LF. Activity-dependent regulation of conductances in model neurons. Science. 1993;259:1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- [33].Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- [34].Turrigiano G, Abbott LF, Marder E. Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci. 1995;15:3640–3652. doi: 10.1523/JNEUROSCI.15-05-03640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- [36].Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- [37].Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- [38].Golowasch J, Abbott LF, Marder E. Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab Cancer borealis. J Neurosci. 1999;19:RC33. doi: 10.1523/JNEUROSCI.19-20-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spitzer NC. New dimensions of neuronal plasticity. Nat Neurosci. 1999;2:489–491. doi: 10.1038/9132. [DOI] [PubMed] [Google Scholar]
- [40].MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- [41].Taylor AL, Goaillard JM, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci. 2009;29(17):5573–5586. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lucas-Meunier E, Monier C, Amar M, Baux G, Fregnac Y, Fossier P. Involvement of nicotinic and muscarinic receptors in the endogenous cholinergic modulation of the balance between excitation and inhibition in the young rat visual cortex. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn258. doi:10.1093/cercor/bhn258. [DOI] [PubMed] [Google Scholar]
- [43].Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;1993:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rasmussen H, Barrett PQ. Calcium messenger system: an integrated view. Physiol Rev. 1984;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- [46].Kennedy MB. Regulation of neuronal function by calcium. Trends Neurosci. 1989;12(11):417–420. doi: 10.1016/0166-2236(89)90089-1. [DOI] [PubMed] [Google Scholar]
- [47].O’Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron. 2014;82:809–821. doi: 10.1016/j.neuron.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Marder E, Goeritz G, Otopalik AG. Robust circuit rhythms in small circuits arise from variable circuit components and mechanisms. Curr Opin Neurobiol. 2015;31:156–163. doi: 10.1016/j.conb.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hamood AW, Marder E. Animal-to-animal variability in neuromodulation and circuit function. Cold Spring Harb Symp Quant Biol. 2015 doi: 10.1101/sqb.2014.79.024828. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic interneurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Leão RN, Mikulovic S, Leão KE, Munguba H, Gezelius H, Enjin A, et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15:1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali R, Role LW, et al. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- [55].Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci. 2012;6:79. doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bloem B, Poorthuis RB, Mansvelder HD. Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity. Front Neural Circuits. 2014;8:17. doi: 10.3389/fncir.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jinno S, Kosaka T. Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus. 2010;20:829–840. doi: 10.1002/hipo.20685. [DOI] [PubMed] [Google Scholar]
- [58].Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- [59].Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- [60].Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-alpha7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 2012;32:3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23:148–161. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Arroyo S, Bennett C, Hestrin S. Nicotinic modulation of cortical circuits. Front Neural Circuits. 2014;8:30. doi: 10.3389/fncir.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Buhler AV, Dunwiddie TV. Regulation of the activity of hippocampal stratum oriens interneurons by alpha 7 nicotinic acetylcholine receptors. Neuroscience. 2001;106:55–67. doi: 10.1016/s0306-4522(01)00257-3. [DOI] [PubMed] [Google Scholar]
- [66].McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- [67].Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- [70].Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- [71].Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [72].Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- [73].Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- [75].McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classificiation of somatostain-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits. 2010;4:12. doi: 10.3389/fncir.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Heys JG, Schultheiss NW, Shay CF, Tsuno Y, Hasselmo ME. Effects of acetylcholine on neuronal properties in entorhinal cortex. Front Behav Neurosci. 2012;6:32. doi: 10.3389/fnbeh.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur J Neurosci. 2007;25:2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- [78].Jia Y, Yamazaki Y, Nakauchi S, Ito K, Sumikawa K. Nicotine facilitates long-term potentiation induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-alpha7 nicotinic acetylcholine receptors. Eur J Neurosci. 2010;31:463–476. doi: 10.1111/j.1460-9568.2009.07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bell LA, Bell KA, McQuiston AR. Acetylcholine release in mouse hippocampal CA1 preferentially activates inhibitory-selective interneurons via α4β2* nicotinic receptor activation. Front Cell Neurosci. 2015;9:115. doi: 10.3389/fncel.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Demars MP, Morishita H. Cortical parvalbumin and somatostatin GABA neurons express distinct endogenous modulators of nicotinic acetylcholine receptors. Molecular Brain. 2014;7:75. doi: 10.1186/s13041-014-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, et al. Novel modulation of neuronal nicotinic acetylcholine receptors in association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- [82].Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- [83].Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci. 2012;32:7651–7661. doi: 10.1523/JNEUROSCI.6246-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat rev. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- [86].Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [87].Liu Y, Zhang LI, Tao HW. Heterosynaptic scaling of developing GABAergic synapses: dependence on glutamatergic input and developmental stage. J Neurosci. 2007;27:5301–5312. doi: 10.1523/JNEUROSCI.0376-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- [89].Campbell NR, Fernandes CC, Halff A, Berg DK. Endogenous signaling through α7-containing nicotinic receptors promotes maturation and integration of adultborn neurons in the hippocampus. J Neurosci. 2010;30:8734–8744. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Campbell NR, Fernandes CC, John D, Lozada AF, Berg DK. Nicotinic control of adult-born neuron fate. Biochem Pharmacol. 2011;82:820–827. doi: 10.1016/j.bcp.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].John D, Shelukhina I, Yanagawa Y, Deuchars J, Henderson Z. Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res. 2015;1601:15–30. doi: 10.1016/j.brainres.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, et al. Schizophrenia and nicotinic receptor. Harvard Rev Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- [93].Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. Brain Res. 2000;863:259–265. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- [94].Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. β-amyloid1-42 binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- [95].Elmslie FV, Rees M, Williamson MP, Kerr M, Kjeldsen MJ, Pang KA, et al. Genetic mapping of a major susceptibility locus for juvenile myoclonic epilepsy on chromosome 15q. Hum Mol Genet. 1997;6:1329–1334. doi: 10.1093/hmg/6.8.1329. [DOI] [PubMed] [Google Scholar]
- [96].Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl. Acad. Sci. U.S.A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sharp AJ, Mefford HC, Li K, Baker C, Skinner C, Stevenson RE, et al. A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat. Genet. 2008;40:322–328. doi: 10.1038/ng.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Helbig L, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A, et al. 15q13.3 microdeletions increased risk of idiopathic generalized epilepsy. Nat. Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, et al. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J. Med. Genet. 2009;46:242–248. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shinawi M, Schaaf CP, Bhatt SS, Xia Z, Patel A, Cheung SW, et al. a small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat. Genet. 2009;41:1269–1271. doi: 10.1038/ng.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Endris V, Hackman k, Neuhann TM, Grasshoff U, Bonin M, Haug U, et al. Homozygous loss of CHRA7 on chromosome 15q13.3 causes severe and encephalopathy with seizures and hypotonia. Am. J. Med. Genet. A. 2010;152A:2908–2911. doi: 10.1002/ajmg.a.33692. [DOI] [PubMed] [Google Scholar]
- [102].Lepichon JB, Bittel DC, Graf WD, Yu S. 15q13.3 homozygous microdeletion associated with a severe neurodevelopmental disorder suggests putative functions of the TRPM1, CHRNA7, and ever homozygously deleted genes. Am. J.Med. Genet. A. 2010;152A:1300–1304. doi: 10.1002/ajmg.a.33374. [DOI] [PubMed] [Google Scholar]
- [103].Ancin L, Cabranes JA, Santos JL, Sanchez-Morla E, Vazquez-Alvarez B, Rodriguez-Moya L, et al. CHRA7 haplotypes are associated with impaired attention in euthymic bipolar disorder. J Affect Disord. 2011;133:340–345. doi: 10.1016/j.jad.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [104].Liao J, DeWard SJ, Madan-Khetarpal S, Surti U, Hu J. a small homozygous microdeletion of 15q13.3 including the CHRNA7 in a girl with a spectrum of severe neurodevelopmental features. Am. J. Med. Genet. A. 2011;155A:2795–2800. doi: 10.1002/ajmg.a.34237. [DOI] [PubMed] [Google Scholar]
- [105].Spielman M, Reichelt G, Hertzberg C, Trimborn M, Mundlos S, Hom D, et al. Homozygous deletion of chromosome 15q13.3 including CHRNA7 causes severe mental retardation, seizures, muscular hypotonia, and the loss of KLF13 and TRPM1 potentially cause macrocytosis and congenital retinal dysfunction in siblings. Eur. J. Med. Genet. 2011;54:e441–e445. doi: 10.1016/j.ejmg.2011.04.004. [DOI] [PubMed] [Google Scholar]
- [106].Yasui DH, Scholes HA, Horike S, Meguro-Horike M, Dunaway KW, Schroeder DL, et al. 15q11.2-13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum. Mol. Genet. 2011;20:4311–4323. doi: 10.1093/hmg/ddr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hoppman-Chaney N, Wain K, Seger P, Superneau D, Hodge J. Identification of single gene deletions at 15q13.3: further evidence that CHRA7 causes the 15q13.3 microdeletion syndrome phenotype. Clin. Genet. 2013;83:345–351. doi: 10.1111/j.1399-0004.2012.01925.x. [DOI] [PubMed] [Google Scholar]
- [108].Freedman R. Alpha7-nicotinic acetylcholine receptor agonist for cognitive enhancement in schizophrenia. Annu. Rev. Med. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- [109].Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- [110].Freedman R, Hall M, Adler LE, Leonard S. Evidence in post-mortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- [111].Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, et al. nicotinic receptor function in schizophrenia. Schizophr. Bull. 1996;22:431–445. doi: 10.1093/schbul/22.3.431. [DOI] [PubMed] [Google Scholar]
- [112].Freedman R, Adams CE, Leonard S. the alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J. Chem. Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- [113].Freedman R, Goldowitz D. Studies on the hippocampal formation: from basic development to clinical applications: Studies on schizophrenia. Prog. Neurobiol. 2010;90:263–275. doi: 10.1016/j.pneurobio.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ross RG, Stevens KE, proctor WR, Leonard S, Kisley MA, Hunter SK, et al. research review: cholinergic mechanisms, early brain development, and risks of schizophrenia. J. Child Psychol. Psychiatry. 2010;51:535–549. doi: 10.1111/j.1469-7610.2009.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Mellios N, Huang HS, Maker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- [116].Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- [117].Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lin H, Hsu FC, Baumann BH, Coulter DA, Anderson SA, Lynch DR. Cortical parvalbumin GABAergic deficits with α7 nicotinic acetylcholine receptor depletion: implications for schizophrenia. Mol Cell Neurosci. 2014;61:163–175. doi: 10.1016/j.mcn.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Small DH. Neural network dysfunction on Alzheimer’s disease: a drug development perspective. Drug News Perspect. 2007;20:55763. doi: 10.1358/dnp.2007.20.9.1162245. [DOI] [PubMed] [Google Scholar]
- [120].Hicks D, John D, Makova NZ, Henderson Z, Nalivaeva NN, Turner AJ. Membrane targeting, shedding and protein interactions of brain acetylcholinesterase. J Neurochem. 2011;116:742–746. doi: 10.1111/j.1471-4159.2010.07032.x. [DOI] [PubMed] [Google Scholar]
- [121].Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;10:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Song C, Murray TA, Kimura R, Wakui M, Ellsworth K, Javedan SP, et al. Role of alpha7-nicotinic acetylcholine receptors in tetanic stimulation-induced gamma oscillations in rat hippocampal slices. 2005;48:869–880. doi: 10.1016/j.neuropharm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [126].Guillem K, Bloem B, Poorthuis RB, Los M, Smit AB, Maskos U, et al. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- [127].Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Janiesch PC, Kruger HS, Poschel B, Hanganu-Opatz IL. Cholinergic control in developing prefrontal-hippocampal networks. J Neurosci. 2011;31:17955–17970. doi: 10.1523/JNEUROSCI.2644-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sivarao DV, Frenkel M, Chen P, Healy FL, Lodge NJ, Zaczek R. MK-801 disrupts and nicotine augments 40 Hz auditory steady state responses in the auditory cortex of the urethane-anesthetized rat. 2013;73:1–9. doi: 10.1016/j.neuropharm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- [130].Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- [131].Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, et al. Region-specific reduction in entorhinal gamma oscillations and Parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB, et al. A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry. 2014;76:128–137. doi: 10.1016/j.biopsych.2013.08.014. [DOI] [PubMed] [Google Scholar]
- [133].Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7(12):1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- [134].Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn. Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- [135].Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav. Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]


