Abstract
Background
Women have an increased prevalence of severe asthma compared to men. IL-17A is associated with severe asthma and requires IL-23 receptor (IL-23R) signaling, which is negatively regulated by Let-7f miRNA.
Objective
Determine the mechanism by which 17β-estradiol (E2) and progesterone (P4) increase IL-17A production.
Methods
IL-17A production was determined by flow cytometry in Th17 cells from women (n=14) and men (n=15) with severe asthma. Cytokine levels were measured by ELISA and IL-23R and Let-7f expression by qPCR in Th17 differentiated cells from healthy women (n=13) and men (n=14). In sham-operated or ovariectomized female mice, 17β-E2, P4, 17β-E2+P4, or vehicle pellets were administered for 3 weeks prior to ex vivo Th17 cell differentiation. Airway neutrophil infiltration and KC expression was also determined in OVA-challenged WT female recipient mice with an adoptive transfer of OVA-specific Th17 cells from female and male mice.
Results
In severe asthma patients and healthy controls, IL-17A production was increased in Th17 cells from women compared to men. IL-23R expression was increased and Let-7f expression was decreased in Th17 differentiated cells from women compared to men. In ovariectomized mice, IL-17A and IL-23R expression was increased and Let-7f expression was decreased in the Th17 cells from mice administered 17β-E2+P4 compared to vehicle. Further, transfer of female OVA-specific Th17 cells increased acute neutrophil infiltration in the lungs of OVA-challenged recipient mice compared to transfer of male OVA-specific Th17 cells.
Conclusions
17β-E2+P4 increased IL-17A production from Th17 cells, providing a potential mechanism for the increased prevalence of severe asthma in women compared to men.
Keywords: estrogen, IL-17A, IL-23 signaling, Let-7f, progesterone, severe asthma
INTRODUCTION
A sexual dimorphism exists in asthma, including severe asthma.(1) In children, severe asthma is more prevalent in boys than in girls, but after puberty there is a change in prevalence with women being two times more likely than men to have severe asthma.(1, 2) This change in asthma prevalence suggests a role for sex hormones in asthma pathogenesis, and it is highly likely that gender differences in airway inflammation influence the gender predilection in asthma. However, the mechanisms that regulate age-related gender differences in asthma prevalence and severity remain unclear.
Asthma has long been associated with CD4+ Th2-induced inflammation with increased secretion of IL-4, IL-5, and IL-13, as well as increased airway eosinophils.(3–6) Patients with severe asthma may have Th2 cytokine-mediated inflammation, but may also have IL-17-mediated inflammation with increased neutrophil infiltration.(3, 7, 8) IL-17A and Th17 cells are increased in the bronchoalveolar lavage (BAL) fluid of patients with severe asthma compared to healthy controls or patients with mild asthma.(9, 10) In the lungs, IL-17A is secreted by CD4+ Th17 cells, as well as by γδ T cells and group 3 innate lymphoid cells (ILC3).(11–13) Since gender has a role in the regulation of severe asthma prevalence and IL-17A is associated with severe asthma, the focus of our study was to determine the role of sex hormones in CD4+ Th17 cell differentiation and IL-17A protein expression from CD4+ Th17 cells using peripheral blood mononuclear cells (PBMCs) in humans and splenocytes in mice. We also wanted to determine the role of gender in IL-17A-mediated airway inflammation and neutrophil recruitment.
Th17 cells, a distinct subset of CD4+ T cells, secrete IL-17A and IL-17F as well as other cytokines. IL-23 signaling through the IL-23 receptor (IL-23R) is important in maintaining and stabilizing the Th17 cell phenotype and increasing IL-17A protein expression.(14, 15) Recently, Let-7f, a member of the Let-7 microRNA (miRNA) family, negatively regulated IL-17A protein expression by decreasing IL-23R surface expression on Th17 cells.(16) CD4+ T cells, including Th17 cells, express the estrogen nuclear receptors, ER-α and ER-β, progesterone receptors 1–4, and androgen receptors through which 17β-E2, P4, and testosterone signal, respectively.(17, 18) Estrogen signaling in the MCF-7 breast cancer cell line decreased Let-7f expression, suggesting a role for estrogen in regulating Let-7f expression.(19) However, the role of sex hormones in regulating Let-7f expression in CD4+ T cells has not been determined. Based on the increased prevalence of severe asthma in women compared to men and the increased airway neutrophil infiltration that is associated with IL-17A in severe asthma, we hypothesized that Th17 cells from women with severe asthma have increased IL-17A production compared to those from men with severe asthma.
RESULTS
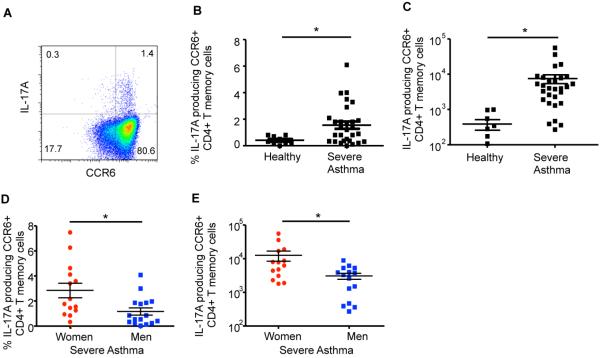
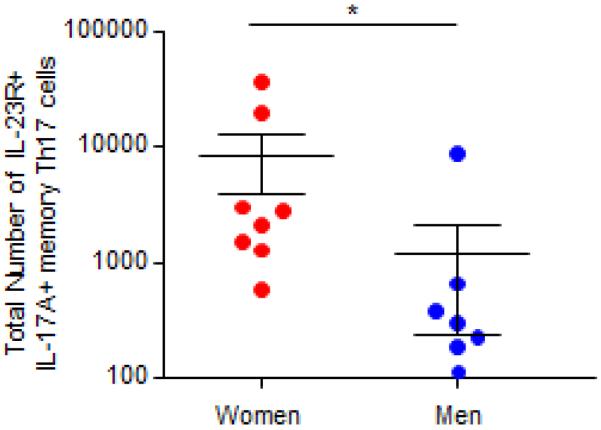
Patients with severe asthma have an increased number of Th17 cells in the peripheral blood compared to healthy controls.(9, 10, 23) Therefore, we hypothesized that IL-17A production is increased by Th17 memory cells from women with severe asthma compared to Th17 memory cells from men with severe asthma. To test our hypothesis, we recruited healthy individuals and patients with severe asthma that were clinically stable. All participants were 18–45 years old, did not have a viral or bacterial infection, or a Th17-associated disease (e.g. multiple sclerosis, psoriasis, SLE, etc.). Women were excluded if they were pregnant, breastfeeding, or taking hormonal birth control medications. Patients with severe asthma were defined based on the guidelines used by the Severe Asthma Research Program.(20) There was no significant difference in age, race, BMI, lung function, total IgE plasma levels, or severe asthma qualifying criteria between women and men with severe asthma (Tables EI and EII). CD4+ CD45RO+ memory T cells were isolated by negative selection from PBMCs, and we first determined that IL-17A+ CD4+ memory Th17 cells, identified by CCR6 surface expression,(24, 25) were increased in patients with severe asthma (n=29, both women and men) compared to healthy adult controls (n=9) (Fig. 1A–C). Next, we stratified patients with severe asthma based on sex, and women with severe asthma had increased percentages and total numbers of IL-17A+ memory Th17 cells compared to men with severe asthma (Fig. 1D–E).
Table EI.
Percentage of women and men meeting each of the severe asthma criteria as defined by the Severe Asthma Research Program(1)†
| Women (%) | Men (%) | p value†† | |
|---|---|---|---|
|
Major Criteria (Must have 1)
| |||
| Oral corticosteroids <50% of year | 35.7 | 20 | 0.362 |
| High-dose inhaled corticosteroids | 78.6 | 82.4 | 0.928 |
|
Minor Criteria (Must have 2)
| |||
| Daily treatment with controller medication | 100 | 86.7 | 0.082 |
| Daily or near-daily use of short-acting β2 | 35.7 | 46.7 | 0.566 |
| FEV1 <80% predicted | 42.9 | 73.3 | 0.103 |
| 1+ urgent care visits for asthma per year | 28.6 | 6.7 | 0.127 |
| 3+ oral corticosteroid “bursts” per year | 7.1 | 6.7 | 0.961 |
| Prompt deterioration in asthma control when corticosteroids were reduced < 25% | 0 | 0 | -- |
| Near-fatal asthma event in the past | 0 | 0 | -- |
Some patients may have met more than the minimum requirements
p value was determined by Mann-Whitney U test, * p<0.05
Table EII.
Characteristics of patients with severe asthma
| Women | Men | p value† | |
|---|---|---|---|
| Number | 14 | 15 | |
| Age | 35.3 (1.8) | 35.7 (1.8) | 0.983 |
| Race (%) | |||
| African American | 14.3 | 5.9 | |
| Asian | 14.3 | 0 | |
| Caucasian | 64.3 | 88.2 | |
| Hispanic | 0 | 0 | |
| Other | 7.1 | 5.9 | |
| BMI | 34.8 (9.7) | 31.1 (4.8) | 0.418 |
| Baseline lung function | |||
| FEV1 (% predicted) | 78.0 (6.4) | 72.6 (4.5) | 0.458 |
| FVC (%) | 93.2 (5.0) | 85.2 (3.6) | 0.199 |
| FEV1/FVC | 0.86 (0.04) | 0.85 (0.03) | 0.927 |
| Total IgE levels | 465.2 (131.2) | 592.2 (162.0) | 0.76 |
data is listed as mean (SD)
p value was determined by Mann-Whitney U test, * p<0.05
Figure 1.
IL-17A+ memory Th17 cells are increased in women compared to men with severe asthma. (A–C) Percent and total number of IL-17A producing CCR6+ memory Th17 cells in healthy controls and severe asthma patients. * p<0.05, Mann-Whitney U test, n=9 healthy individuals and n=29 severe asthma patients (combined women and men). (D–E) Percent and total number of IL-17A+ or IL-4/IL-17A+ memory Th17 cells in women and men with severe asthma. * p<0.05, Mann-Whitney U test, n=14 (women) and 15 (men).
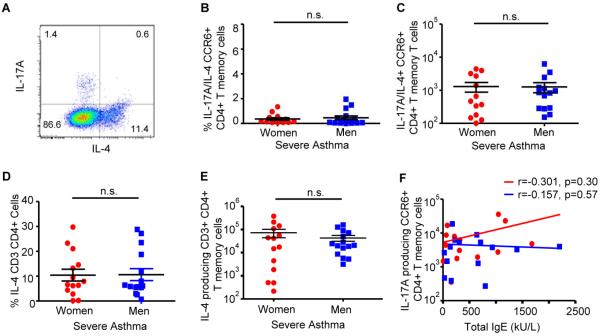
Patients with severe asthma can also have increased Th2 cytokines and increased IL-17A/IL-4 producing CD4+ T cells.(8, 26) Therefore, the total numbers of IL-17A/IL-4 dual producing, CCR6+ memory Th17 cells were also determined and no difference was found (Fig. E1A–C). There was also no difference in IL-4 production in CD3+ CD4+ memory T cells or total IgE plasma levels from women and men with severe asthma (Fig. E1D–E and Table EII). We further found no association between total IgE plasma levels and IL-17A+ memory Th17 cells (Fig. E1F). Collectively, these data showed women with severe asthma had increased IL-17A producing Th17 cells compared to men with severe asthma, but the mechanism(s) remained unclear.
Fig. E1.
IL-4+ memory T cells are similar in women and men with severe asthma. (A–C). Percent and total number of IL-17A/IL-4+ memory Th17 cells in women and men with severe asthma. (D–E). Percent and totals for IL-4+ CD3+ CD4+ memory T cells in women and men with severe asthma. * p<0.05, Mann-Whitney U test, n=14 (women) and 15 (men). (F). Spearman correlation of total IgE plasma levels and IL-17A producing CCR6+ memory Th17 cells.
IL-17A protein expression is increased in Th17 differentiated cells from women compared to men
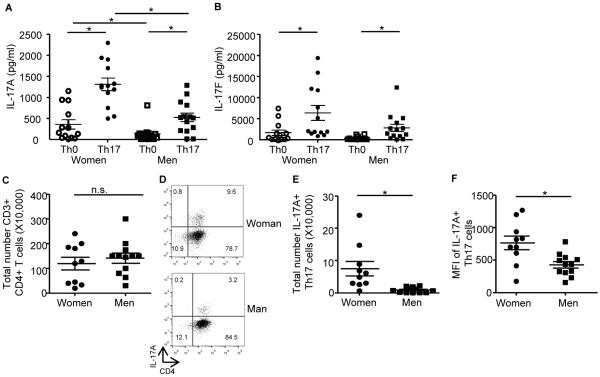
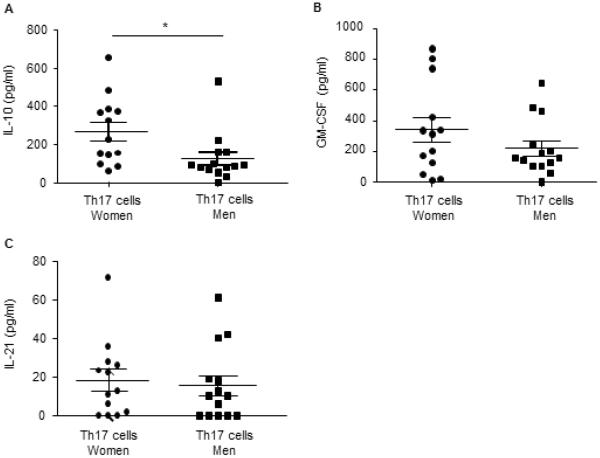
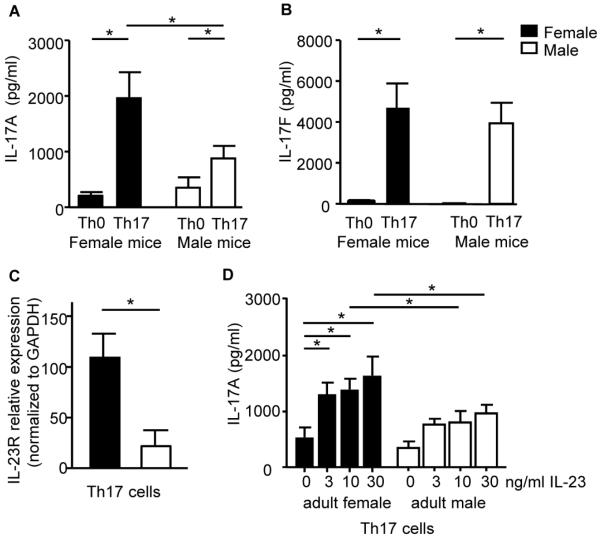
To determine the mechanisms by which gender and sex hormones regulate IL-17A protein expression and Th17 cell differentiation, we next isolated naïve T cells from healthy men and women, and differentiated these naïve T cells to Th17 cells. We differentiated naive T cells into Th17 cells from healthy patients for the following reasons: 1) to determine expression levels of transcription factors, cytokines, and cell surface receptors required for Th17 cell differentiation, and 2) to generalize the effect of sex hormones on Th17 development, independent of disease state. Th0 and Th17 culture supernatants were analyzed for IL-17A and IL-17F protein expression and as expected, IL-17A and IL-17F were significantly increased in Th17 cells compared to Th0 cells from both women and men (Fig. 2A–B). IL-17A, and not IL-17F, was significantly increased in Th17 cells from women compared to Th17 cells from men. Protein expression of other cytokines produced by Th17 cells, IL-10, GM-CSF, and IL-21, was also determined. Th17 cells from women had a significant increase in IL-10 protein expression compared to Th17 cells from men; GM-CSF and IL-21 protein expression were similar in Th17 cells from women and men (Fig. E2A–C).
Figure 2.
IL-17A protein expression is increased in Th0 and Th17 cells from women compared to men. (A–B) Naive CD4+ T cells were differentiated into Th0 and Th17 cells. IL-17A and IL-17F protein expression was determined in Th0 and Th17 from healthy women (n=13) or men (n=14). * p<0.05, Kruskal-Wallis test with Dunn's post-test analysis. (C) Total number of CD3+ CD4+ Th17 cells. (D–F) Percent, total number, and/or gMFI for CD3 gated, CD4+, IL-17A+ Th17 cells. * p<0.05, Kruskal-Wallis test, n=10 (women) n=12 (men).
Fig. E2.
Cytokine expression in human Th17 differentiated cells from women and men. (A–C) Naïve T cells isolated from healthy women (circles) and men (squares) were differentiated into Th17 cells. Cell culture supernatants were harvested and analyzed for protein expression. * p<0.05, Mann Whitney U test, n=13 Th17 cells from women and n=14 Th17 cells from men.
We then determined whether increased IL-17A in Th17 cells from women compared to Th17 cells from men was due to increased number of Th17 differentiated cells or the amount of IL-17A produced in each Th17 cell. Four days after Th17 cell differentiation, cells were collected and restimulated with PMA and ionomycin in the presence of golgi-stop, and IL-17A producing Th17 cells were determined. While the number of CD3+ CD4+ cells was similar between women and men (Figure 2C), the percentage, as shown in the representative dot plot, and the total number of IL-17A producing CD3+ CD4+ T cells was significantly greater in the cells from women compared to the cells from men (Figure 2D–E). Further, the MFI for IL-17A+ CD3+ CD4+ cells was also increased in Th17 cells from women compared to Th17 cells from men (Figure 2F). Combined these data suggested that ovarian sex hormones increased Th17 cell differentiation and IL-17A production from Th17 cells.
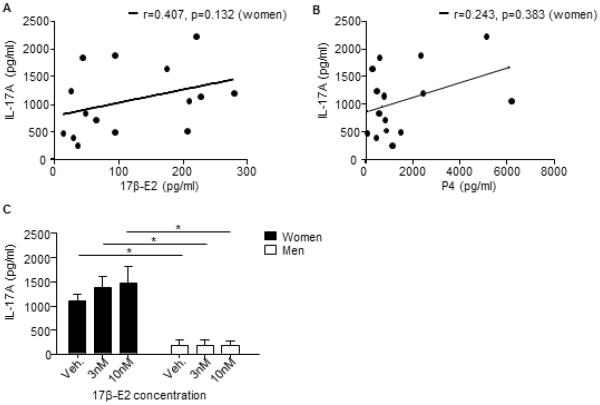
We next determined if 17β-E2 and P4 plasma levels at the time of the blood draw correlated with IL-17A protein expression in Th17 cells from women. Neither 17β-E2 nor P4 plasma levels were significantly associated with IL-17A protein expression in Th17 cells from women (Fig. E3A–B), suggesting circulating levels of 17β-E2 and P4 at the time of the blood draw were not responsible for the observed increased in IL-17A production from Th17 cells. We next determined if 17β-E2 (3–10nM) increased IL-17A protein expression from Th17 differentiated cells by adding 17β-E2 to the Th17 cell differentiation media at the time of T cell activation. Addition of 17β-E2 at the time of in vitro differentiation had no effect on IL-17A protein expression when compared to vehicle (0nM) in Th17 cells from women or men (Fig. E3C). These data suggest the increase in IL-17A production by Th17 cells from women compared to men was not an acute effect of ovarian hormones, but rather the exposure of T cells to ovarian hormones during development in vivo, prior to ex vivo Th17 cell differentiation.
Fig. E3.
IL-17A protein expression in Th17 cells from women does not correlate with 17β-E2 and P4 plasma levels. (A–B) Spearman nonparametric correlations of 17β-E2 or P4 plasma concentration and IL-17A protein expression from Th17 cell culture supernatants, r and p values are listed on the graphs. (C) Naïve T cells isolated from healthy women and men were differentiated into Th17 cells in the presence of 17β-E2 or vehicle (ethanol). Four days after differentiation, IL-17A protein expression was determined from cell culture supernatants. Data is representative from n=8 Th17 cells from women and n=5 Th17 cells from men. * p<0.05, ANOVA with Tukey post-test analysis.
IL-23R surface expression is increased and Let-7f expression is decreased in Th17 cells from women compared to Th17 cells from men
To delineate the mechanism by which ovarian hormones were increasing IL-17A protein expression from Th17 cells, we next determined phosphorylation or expression levels of transcription factors involved with Th17 cell differentiation, including STAT3, RORC2, IRF-4, and AhR. No differences in STAT3 phosphorylation or RORC2, IRF-4, and AhR mRNA expression were detected in Th17 cells from women compared to Th17 cells from men (data not shown). IL-23/IL-23R signaling is important for sustainability of the Th17 cell lineage and increased IL-17A protein expression.(14, 15) Therefore, we also determined IL-23R surface expression in Th0 and Th17 cells from women and men by flow cytometry. Th17 cells from women had a significant increase in cell surface expression of IL-23R compared to Th17 cells from men (Fig. 3A–B), and the total number of Th17 cells did not change between women and men (data not shown). Further the geometric MFI for IL-23R surface expression on Th17 cells was also significantly increased in Th17 cells from women (1537.6±297.3) compared to Th17 cells from men (602.7±181.5, n=Th17 cells from 8 women and 7 men, p<0.05, Mann-Whitney U test).
Figure 3.
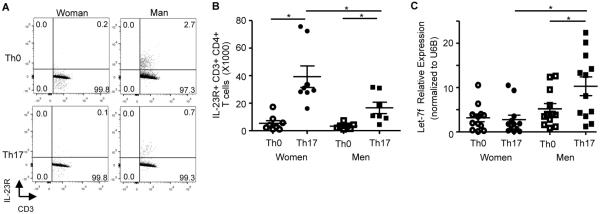
IL-23R surface expression is increased and Let-7f is decreased in Th17 cells from women compared to men. (A–B) Dot plot and total IL-23R+ CD3+ CD4+ T cells from women and men. * p<0.05, Kruskal-Wallis test with Dunn's post-test analysis, n=8 (women) n=7 (men). (C) Let-7f miRNA expression normalized to U6B expression in Th0 and Th17 cells from women and men. * p<0.05, Kruskal-Wallis test with Dunn's post-test analysis, n=12 (women and men).
Based on these data, we determined IL-23R surface expression on a subset of memory Th17 cells from women and men with severe asthma. IL-23R surface expression was also increased on CD3+ CD4+ CCR6+ memory Th17 cells from women with severe asthma compared to men with severe asthma (Figure E4). Combined these data suggest that IL-23R surface expression is increased on Th17 cells from healthy and severe asthmatic women compared to Th17 cells from healthy and severe asthmatic men.
Fig. E4.
IL-23R surface expression is increased in memory Th17 cells from women with severe asthma compared to men with severe asthma. IL-23R surface expression was determined on CD3+ CD4+ CCR6+ memory Th17 cells from women and men with severe asthma. * p<0.05, Mann-Whitney U test, n=8 (women) and 7 (men).
IL-23R expression was previously shown to be negatively regulated by the miRNA Let-7f in CD4+ lymphocytes,(16) and Let-7f was negatively regulated by estrogen receptor signaling in the MCF-7 breast cancer cell line.(19) Therefore, we determined Let-7f miRNA expression in Th17 differentiated cells from healthy women and men. Th17 cells from women had a significant decrease in Let-7f expression compared to Th17 cells from men, but Let-7f expression was similar in Th0 cells from women and men (Fig. 3C). These data suggest that decreased Let-7f expression may be associated with increased IL-23R expression and IL-17A production in Th17 cells from women compared to Th17 cells from men.
Inhibition of Let-7f miRNA increases IL-17A production in Th17 cells from both women and men
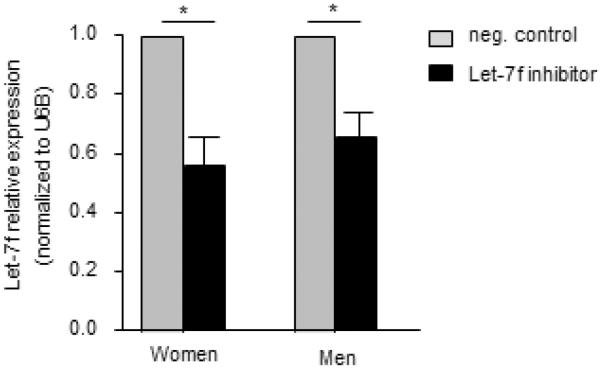
To determine if Let-7f miRNA regulated IL-17A protein expression differentially in women and men, we transfected a Let-7f inhibitor (10nM) or negative control (10nM) into naïve CD4+ T cells from healthy women and men and then differentiated the cells to Th17 cells. The Let-7f inhibitor decreased Let-7f expression by approximately 50% in Th17 cells from women and men (Fig. E5). Inhibition of Let-7f significantly increased IL-17A production in Th17 cells from both women and men compared to Th17 cells transfected with the negative control in women and men, respectively (Fig. 4A–B). We also determined IL-23R mRNA expression, and Th17 cells from women or men transfected with the Let-7f inhibitor had increased IL-23R mRNA expression compared to Th17 cells from women or men transfected with the negative control (Fig. 4C). These data show that Let-7f negatively regulates IL-23R expression and IL-17A production in Th17 cells, and that the decreased Let-7f expression in Th17 cells from women results in increased IL-17A production when compared to Th17 cells from men.
Fig. E5.
Let-7f miRNA expression is decreased in Th17 cells transfected with Let-7f inhibitor. Let-7f inhibitor (10nM) or negative control (10nM) were transfected into naïve T cells isolated from healthy women and men. Cells were differentiated to Th17 cells and Let-7f miRNA expression was determined by qPCR and normalized to U6B expression. * p<0.05, Wilcoxon matched paired test, n=9 Th17 cells from women and n=9 Th17 cells from men.
Figure 4.
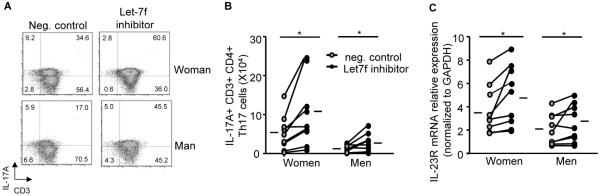
Let-7f decreased IL-23R surface expression and IL-17A levels from Th17 cells. A 10nM Let-7f inhibitor or negative (neg.) control was transfected into naïve T cells that were then differentiated to Th17 cells. (A–B) Dot plots and the total number of IL-17A+ Th17 cells. (C) IL-23R mRNA expression normalized to GAPDH. * p<0.05, Wilcoxon matched pairs test, n=9 (women) and n=9 (men), hashes are means.
IL-17A protein expression is increased in Th17 cells from female mice compared to Th17 cells from male mice
Our data from human Th17 cells strongly suggest in vivo sex hormones regulated naïve T cell development prior to isolation and affected in vitro Th17 cell differentiation. Therefore, we moved to a mouse model to test the in vivo effects of 17β-E2 and P4 on Th17 cell differentiation and IL-17A protein expression. Th17 cells from female mice had increased IL-17A, but not IL-17F, production and increased IL-23R mRNA expression compared to Th17 cells from male mice (Fig 5A–C). We also determined the requirement IL-23R signaling on increased IL-17A protein expression in Th17 cells from female and male mice by adding 0–30ng/ml of recombinant mouse (rm) IL-23 to naïve T cells during Th17 cell differentiation, keeping other Th17 differentiation conditions the same. Th17 cells from female and male mice differentiated without rmIL-23 (0ng/ml) had similar IL-17A protein expression (Fig 5D). However, IL-17A protein expression was increased in Th17 cells from female mice with the addition of 3–30ng/ml rmIL-23 compared to Th17 cells from female mice with no rmIL-23 (0ng/ml). Further, the addition of rmIL-23 (10–30ng/ml) increased IL-17A protein expression in Th17 cells from female mice to a significantly greater degree compared to Th17 cells from male mice.
Figure 5.
IL-17A protein expression is increased in Th0 and Th17 cells from female mice compared to male mice. (A–C) IL-17A and IL-17F protein expression or IL-23R mRNA expression normalized to GAPDH in Th0 and Th17 cells from female and male adult or prepubescent mice. (D). IL-17A protein expression from adult Th17 cells differentiated with rmIL-23 (0-30ng/ml). * p<0.05, ANOVA with Tukey post-test analysis, n=6, 2 experiments.
In vivo exposure to 17β-E2 and P4 increased IL-17A production from in vitro Th17 differentiated cells
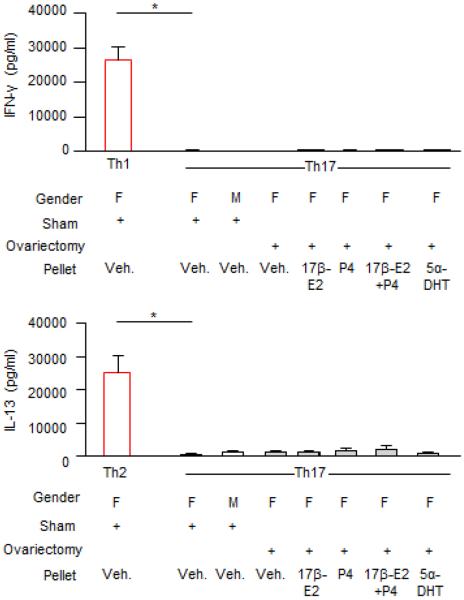
To determine the mechanism(s) by which ovarian hormones regulate IL-17A production in Th17 cells, we used female and male mice that were ovariectomized or sham-operated prior to puberty. Slow-release pellets containing 17β-E2 (0.1mg), P4 (25mg), 5α-DHT (15mg), the combination of 17β-E2 (0.1mg) and P4 (25mg), or vehicle were subcutaneously implanted into adult ovariectomized female BALB/c mice. After 21 days, splenic naïve T cells were differentiated into Th17 cells. IL-17A protein expression was increased in Th17 cells from sham-operated female mice administered vehicle pellets compared to Th17 cells from both sham-operated male mice administered vehicle pellets and ovariectomized female mice administered vehicle pellets (Fig. 6A). Further, IL-17A protein expression was similar in Th17 cells from sham-operated male mice administered vehicle pellets and Th17 cells from ovariectomized female mice administered vehicle pellets. A significant increase in IL-17A protein expression was found in Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4 compared to Th17 cells from ovariectomized female mice administered vehicle pellets (Fig. 6A). This significant increase in IL-17A protein expression was not detected in Th17 cells from ovariectomized female mice administered 17β-E2, P4, or 5α-DHT pellets. Further, IL-17A protein expression was similar in Th17 cells from sham-operated female mice administered vehicle pellets compared to Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4. IFN-γ and IL-13 production was also determined in Th17 cells from all groups, and no differences were detected (Fig E6).
Figure 6.
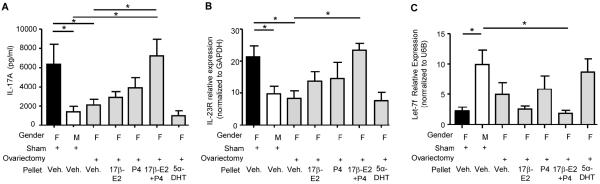
In vivo administration 17β-E2 and P4 increased IL-17A protein expression and IL-23R mRNA expression from in vitro Th17 cells. Hormones or vehicle pellets (veh.) were implanted into mice for 21 days followed by naïve T cell isolation and Th17 cell differentiation. (A) IL-17A protein expression. (B) IL-23R mRNA expression normalized to GAPDH. (C) Let-7f miRNA expression normalized to U6B expression. * p<0.05, Kruskal-Wallis test with Dunn's post-test analysis; n=Th17 cells from 5–10 mice for each group, 2 experiments.
Fig. E6.
IL-13 and IFN-γ protein expression in Th17 cells. Hormones or vehicle pellets (veh.) were implanted into mice for 21 days. Naïve T cells were isolated from the spleens of mice and Th17 cells were differentiated. As positive controls, Th1 and Th2 cells were differentiated from sham-operated female mice administered vehicle pellets. IFN-γ and IL-13 protein expression was determined by ELISA. * p<0.05 compared to Th17 cells from sham-operated female mice administered vehicle pellets, Kruskal-Wallis test with Dunn's post-test analysis; n=5–10 mice per group combined from 2 experiments.
We also determined there were no statistical differences in ROR-γT, AhR, or IRF-4 mRNA expression in Th17 cells from ovariectomized female mice administered hormone pellets compared to Th17 cells from ovariectomized female mice administered vehicle pellets (data not shown). However, Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4 had a significant increase in IL-23R mRNA expression compared to Th17 cells from sham-operated male mice administered vehicle pellets and ovariectomized female mice administered vehicle pellets (Fig. 6B). Further, IL-23R mRNA expression was similar in Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4 and Th17 cells from sham-operated female mice administered vehicle pellets.
Let-7f expression was significantly decreased in Th17 cells from sham-operated female mice administered vehicle pellets compared to Th17 cells from sham-operated male mice administered vehicle pellets (Fig. 6C). Let-7f expression was also decreased in Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4 compared to Th17 cells from sham-operated male mice administered vehicle pellets, but Let-7f expression was not significantly different in Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4 compared to Th17 cells from ovariectomized female administered vehicle pellets. Combined, these data suggest that the in vivo exposure to the combination of 17β-E2 and P4 decreased Let-7f expression in Th17 cells and therefore increased IL-23R and IL-17A protein expression.
Ovarian hormones increase IL-17A-mediated neutrophilic airway inflammation in mice
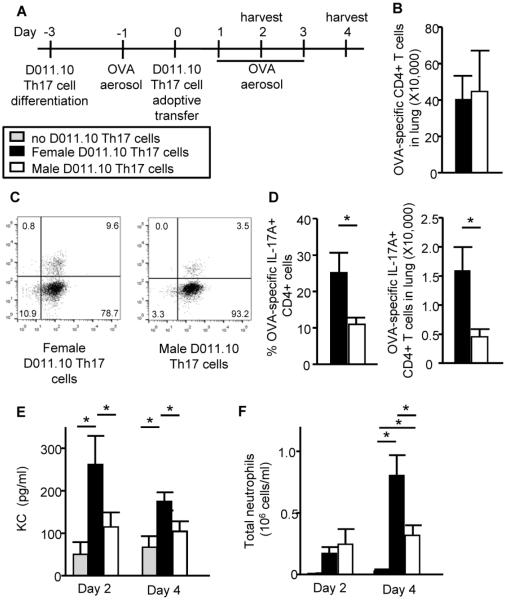
Next, we wanted to determine if females had increased IL-17A-mediated acute airway inflammation from Th17 cells compared to males. As shown in Fig. 7A, CD4+ T cells from D011.10 intact female and male donor mice, which have an OVA-specific T cell receptor, were differentiated to Th17 cells and adoptively transferred into WT female recipient mice. IL-17A protein expression was measured from pooled D011.10 Th17 culture supernatants (4484 ± 74.2 pg/ml of IL-17A from female D011.10 Th17 cells; 2377 ± 44.1 pg/ml of IL-17A from male D011.10 Th17 cells, p<0.05, t-test), similar to findings in Th17 cells from female and male WT mice. WT female recipient mice were challenged with OVA aerosol one day prior to adoptive transfer and for three days following. WT female recipient mice were euthanized on days 2 and 4 and endpoints were determined. We first determined OVA-specific (KJ126+) Th17 cell migration to the lung, and similar numbers of female or male OVA-specific CD3+ CD4+ T cells migrated to the lung at 4 days post-adoptive transfer (Figure 7B). Next IL-17A production from the OVA-specific Th17 cells was determined, and the adoptive transfer of OVA-specific Th17 cells from female D011.10 mice increased the percentages and total number of IL-17-producing CD3+ CD4+ T cells in WT female recipient mice compared to OVA-specific CD3+ CD4+ T cells from male D011.10 mice (Figure 7C–D). These data support our earlier in vitro findings in Th17 differentiated cells from women and men (Figure 2A), and showed that Th17 cell differentiation and IL-17A production was increased in CD4+ T cells from females compared to males.
Figure 7.
Adoptive transfer of female D011.10 Th17 cells increases neutrophilic infiltration in the airways of WT female recipient mice. (A) Schematic of experimental protocol. (B–D) Total number of OVA-specific CD3+ CD4+ T cells (panel B) or the percent and total number of IL-17A+ OVA-specific CD3+ CD4+ Th17 cells (panels C-D) in the lungs of recipient mice on day 4. (E) KC protein expression in whole lung homogenates. (F) Total neutrophils in BAL fluid. * p<0.05, ANOVA with Tukey post-test analysis; n=10–12 recipient mice per group administered D011.10 Th17 cells from female or male mice combined from 2 experiments.
We next determined KC protein expression, a neutrophil-attracting cytokine known to be upregulated in airway epithelial cells in response to IL-17A signaling, was significantly increased on days 2 and 4 in whole lung homogenates from WT female recipient mice adoptively transferred with D011.10 Th17 cells from female mice compared to an adoptive transfer of D011.10 Th17 cells from male mice or no cells adoptively transferred (Fig. 7E). Neutrophils were also increased on day 4 in the BAL fluid from WT female recipient mice administered D011.10 Th17 cells from female mice compared to WT female recipient mice administered D011.10 Th17 cells from male mice or no cells (Fig. 7F). These data show that the adoptive transfer of OVA-specific Th17 cells from female mice increased neutrophilic airway inflammation in recipient mice compared to adoptive transfer of OVA-specific Th17 cells from male mice.
DISCUSSION
The increased prevalence of severe asthma in females begins around the time of puberty,(1, 2) suggesting a role for sex hormones. IL-17A is associated with severe asthma, but the role of gender and sex hormones on Th17 cell differentiation remains unclear. Determining if sex hormones regulate Th17 cell differentiation is vital for understanding the mechanisms that are responsible for severe asthma. Prior research showed estrogen signaling negatively regulated Let-7f in the MCF-7 breast cancer cell line.(19) Let-7f also decreased IL-23R surface expression and IL-17A protein expression in Th17 cells.(16) Our study is the first to show that: (1) CD4+ T cells from women have increased Th17 cell differentiation and IL-17A protein expression, and (2) Let-7f is differentially expressed in Th17 cells from women and men and alters Th17 cell expression of IL-23R and production of IL-17A. We extended our findings in human Th17 cells, and showed that Th17 cells from ovariectomized female mice exposed to the combination 17β-E2 and P4 had decreased Let-7f expression, increased IL-23R mRNA expression, and increased IL-17A protein expression compared to Th17 cells from sham-operated male mice and/or Th17 cells from female ovariectomized mice administered vehicle pellets (Fig E7). Further, we showed that adoptive transfer of OVA-specific Th17 cells from female mice into WT female recipient mice followed by OVA aerosolized challenge resulted in increased neutrophilic inflammation compared to adoptive transfer of OVA-specific Th17 cells from male mice. Combined, these data strongly suggest that in vivo exposure to both 17β-E2 and P4 may differentially regulate IL-17A protein expression in Th17 cells through a Let-7f/IL-23R pathway.
Fig. E7.
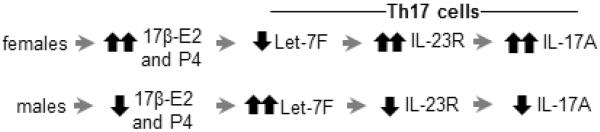
Role of 17β-E2 and P4 in IL-17A protein expression from Th17 cells. Schematic proposing how increased 17β-E2 and P4 concentrations in women impact IL-17A protein expression from Th17 cells.
Th17 cells are also associated with autoimmune conditions including multiple sclerosis, systemic lupus erythematosus (SLE) and rheumatoid arthritis. A gender disparity also exists in these autoimmune conditions, with women being two to three times more likely to have multiple sclerosis and nine times more likely to have SLE compared to men.(27) However, the role of sex hormones in Th17-associated diseases remains unclear, with variable findings in both in vitro and in vivo studies. Similar to our in vitro findings, IL-17A protein expression was increased in Th17 cells from mice administered 17β-E2 compared to Th17 cells from mice administered vehicle.(28) However, other in vitro studies reported 17β-E2 signaling decreased IL-17A production in CD4+ T cells. These variable findings may be due to different T cell activation and differentiation protocols.(28–33) In vivo mouse models of multiple sclerosis and rheumatoid arthritis showed 17β-E2 signaling decreased Th17-mediated inflammation.(28–34) CCR6+, CXCR3+ IL-17A+, IFN-γ+ cells are vital for the pathogenesis of multiple sclerosis and rheumatoid arthritis,(35) but not severe asthma. Therefore, the divergence of our in vivo data and the data from mouse models of IL-17A-mediated autoimmune conditions suggests that severe asthma and IL-17A-mediated autoimmune diseases are mediated by different subsets of IL-17A-producing Th17 cells.
The il17a and il17f genes are found on chromosome 1 in a tail-to-tail configuration.(36) IL-17A and IL-17F have approximately 50% homology, the most closely related members of the IL-17 family of proteins.(36) Many studies have shown that IL-17A and IL-17F are increased by similar mechanisms, but our findings and others show differential production of IL-17A and IL-17F. In our study, we found that IL-17A, but not IL-17F, protein expression was increased in Th17 cells from women and female mice compared to Th17 cells from men and male mice, respectively. Further, in the mouse models of multiple sclerosis and rheumatoid arthritis, il-17a−/−, but not il-17f−/−, have decreased disease severity compared to WT mice.(37, 38) These results suggest that 17β-E2 and P4 may differentially regulate IL-17A and IL-17F protein expression, potentially through an epigenetic mechanism or different enhancer/promoter states of the genes.
Let-7f was significantly decreased in Th17 cells from women and female mice compared to Th17 cells from men and male mice, respectively. In vivo administration of pellets containing the combination of 17β-E2 and P4 decreased Let-7f expression in Th17 cells from ovariectomized female mice compared to Th17 cells from sham-operated male mice administered vehicle pellets. However, there was not a significant difference in Let-7f expression between Th17 cells from ovariectomized female mice administered the vehicle pellet and Th17 cells from ovariectomized female mice administered the combination of 17β-E2 and P4. These data suggests that other factors, in conjunction with or independent of Let-7f, also regulate IL-23R expression. Sgk1 and Foxo1 were recently described to increase and decrease, respectively, IL-23R expression and IL-17A protein expression in Th17 cells.(39) We determined Sgk1 and Foxo1 mRNA expression in Th17 cells from women and men as well as Th17 cells from sham-operated and ovariectomized female mice administered hormone or vehicle pellets. No differences in Sgk1 and Foxo1 mRNA expression were detected in Th17 cells from humans or mice (data not shown). Further, we also examined expression of Let-7e, another Let-7 family member which increased allergic airway inflammation in mice,(40) and found no differences in Let-7e expression in Th17 cells from healthy women compared to men (data not shown). Combined, these results suggest that 17β-E2 and P4 regulate IL-23R expression partially through Let-7f, but that other unidentified factors are likely involved in regulating IL-17A protein expression.
IL-17A can also be produced by other cells, including γδ T cells and group 3 innate lymphoid cells.(11, 12) IL-23/IL-23R signaling is important for γδ T cell and IL-C3 production of IL-17A, (11, 12) but it is unknown if Let-7f regulates IL-23R expression in γδ T cells and ILC3. Further studies are needed elucidate theses mechanisms. Therapeutics targeting IL-17A and IL-17RA are currently undergoing clinical trials for patients with multiple sclerosis, psoriasis, or severe asthma. Brodulamab, a humanized IL-17RA monoclonal antibody, decreased disease severity in patients with moderate to severe plaque psoriasis,(41) but brodulamab had little effect in decreasing asthma symptoms in patients with moderate to severe asthma.(42) However, the brodulamab asthma clinical trial did not specifically recruit patients with high IL-17A production,(42) and brodulamab may have a more pronounced effect on asthma symptoms in patients with IL-17A-mediated airway inflammation and asthma. Our findings suggest that women with severe asthma may respond better to medications which target IL-17A and IL-17RA signaling.
ONLINE REPOSITORY METHODS
Recruitment of participants
Patients with severe asthma (ages 18–45), as defined by the Severe Asthma Research Program,(1) were recruited from the Vanderbilt University. These patients with severe asthma were clinically stable, not undergoing an exacerbation, and continued taking their asthma medications. Table EI shows the percentage of women and men in our study meeting the severe asthma criteria, including use of oral and inhaled corticosteroids, daily controller medications, and short-acting β-agonist. Participants were excluded for viral or bacterial symptoms in the previous week or any Th17-associated disease, including multiple sclerosis, psoriasis, SLE, or rheumatoid arthritis. Women were excluded if they were pregnant, breastfeeding, taking hormonal birth control medications, on estrogen replacement therapy, menopausal, or had undergone an oophorectomy or hysterectomy. At the time of the blood draw, the first day of the last menstrual period was recorded for each woman. Healthy participants (ages 18–45) with no diagnosis of asthma were also recruited with the same exclusion criteria described for patients with severe asthma. All participants were consented in accordance with Vanderbilt University's Institutional Review Board policies.
Flow cytometry of memory T cells from participants with severe asthma
In Figure 1 and Figure E1, one million restimulated memory CD4+ T cells from healthy individuals or patients with severe asthma were harvested, and cells were surface stained in the dark for 45 minutes at 4°C with FITC-conjugated anti-CD4 (5 μl per 1×106 cells, BD Biosciences catalog #555346), pacific blue conjugated anti-CD3 (0.5μl per 1×106 cells, BD Biosciences catalog #558117), and biotinylated anti-CCR6 (2μl per 1×106 cells, BD Biosciences catalog #559561). For Figure E4, cells were also surface stained with PE conjugated anti-IL-23R (2μl per 1×106 cells, R&D Systems, catalog # FAB14001P). Cells were then stained with PE-Cy7 conjugated to streptavidin (1:500 diluted, BD Biosciences catalog #557598), permeabolized with cytofix/cytoperm in the dark at 4°C for 20 minutes to overnight (eBiosciences catalog #00-5523-00), washed thoroughly, and intracellularly stained in the dark with PerCp Cy5.5 anti-IL-17A (5μl per 1×106 cells, eBiosciences catalog #45-7179-41) and PE conjugated anti-IL-4 (2μl per 1×106 cells, BD Biosciences catalog #554435). In Figure 3A, Th17 differentiated cells from healthy women and men were surface stained with FITC-conjugated anti-CD4, pacific blue conjugated anti-CD3, and PE conjugated IL-23R. For experiments in Figure 4, Th17 differentiated cells from healthy women and men were surface stained with FITC-conjugated anti-CD4 and pacific blue conjugated anti-CD3. Cells were then fixed and permeabolized with cytofix/cytoperm solution and intracellular stained in the dark with PerCp Cy5.5 anti-IL-17A. For all experiments, cells were analyzed using a LSR II flow cytometer (BD Biosciences), and data were analyzed using Flow Jo 7.2 software.
Hormone analysis
17β-E2 and testosterone levels were determined by radioimmunoassay double antibody procedure at the Vanderbilt University Hormone Assay Core. Final analysis was accomplished by quantifying the bound radioactive counts with a Packard Gamma counter connected to a computerized data reduction station. Progesterone levels were determined by ELISA (Alpco, catalog #11-PROHU-E01).
Th17 cell differentiation from human CD4+ naïve T cells
Human peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats of participants by Ficoll-paque Plus (GE Healthcare catalog #17-1440-02). Naïve CD4+ T cells were isolated from the PBMCs using a naïve T cell isolation kit (Miltenyi Biotec catalog # 130-094-131) per manufacturer's instruction. CD4+ naïve T cells were activated using an activation/expansion kit (Miltenyi Biotec catalog #130-091-441) with anti-CD3 and anti-CD28 bound to bead particles at the ratio of 1 bead particle per 2 cells. CD4+ T cells were cultured differentiated to become Th17 cells with hIL-2 (10ng/ml), rhIL-1β (10ng/ml), rhTGF-β (1ng/ml), rhIL-6 (10ng/ml), rhIL-23 (10ng/ml), anti-IFN-γ (10μg/ml), and/or anti-IL-4 (10μg/ml) in T cell culture media. T cell culture media was RPMI containing 10% FBS, 1% penicillin/streptomycin, 2mM l-glutamine, 10mM HEPES, 0.1mM non-essential amino acids, and 1mM sodium pyruvate (Gibco, Carlsbad, CA). All antibodies and rhIL-1β, rhIL-6, and rhIL-23 were purchased from R&D Systems. rhIL-2 and rhTGF-β was purchased from PeproTech (Rocky Hill, NJ).
Cytokine measurements
Cytokine levels were measured from Th0 and Th17 cell culture supernatants, BAL fluid, or whole lung homogenates with available Duoset ELISA kits (R & D Systems) following the manufacturer's instructions or by cytokine bead array (BD Technologies) from the Vanderbilt Immunology Core. Cell culture supernatants were collected from 400,000–500,000 Th0 or Th17 cells, and no differences were detected in Th0 and Th17 cell numbers from men and women at the time of supernatant collection. Any value below the lower limit of detection (L.O.D.) was assigned half the value of the lowest detectable standard.
IgE measurements
Total and allergen-specific plasma IgE concentrations were determined using a commercially available Phadiatop assay (ImmunoCAP, Phadia, Kalamazoo, MI) per manufacturer's directions.
RNA/miRNA isolation and qPCR
Total RNA was isolated using a RNeasy Mini Kit (Qiagen catalog #74106). For studies on human T cells, cDNA was generated using 100–500 ng of total RNA, with the same concentration of total RNA used within each independent experiment. Taqman qPCR analysis of IL-23R and GAPDH mRNA expression was conducted using commercially available primers and FAM/MGB probes (Applied Biosystems, catalog #4331182 Hs00332759_m1 for IL-23R and catalog #4352934E for GAPDH). For studies on mouse T cells, cDNA was generated using 100–200 ng of total RNA, with the same concentration of total RNA used within each independent experiment. qPCR was conducted using SYBR green mix (BioRad, catalog #170-8882) and IL-23R and GAPDH primers from Integrated DNA Technologies with the following sequences: IL23R forward 5'-GGTCCAAGCTGTCAATTCCCTAGGC -3', IL-23R reverse 5'-AGCCCTGGAAATGATGGACGCA -3', GAPDH forward 5'-GGCCCCTCTGGAAAGCTGTGG-3', GAPDH reverse 5'-CCCGGCATCGAAGGTGGAAGA-3'. Data were reported as relative expression normalized to the housekeeping gene, GAPDH.
For the detection and quantification of miRNA, we used the NCode miRNA first-strand cDNA synthesis and qRT-PCR kit (Life Technologies, catalog #MIRQ-100). Per the manufacturer's instructions, 500 ng of total RNA underwent a polyadenylation reaction to add a poly(A) 3' tail to miRNAs. cDNA synthesis was then performed using 4 μl of polyadenylated RNA, Superscript III reverse transcriptase, and a Universal reverse transcriptase primer. Finally, qPCR was conducted using SYBR green mix and a provided universal qPCR reverse primer (200 nM) and forward primers for miRNA (200 nM). Forward primer sequences were: Let7f forward primer: TGAGGTAGTAGATTGTATAGTT and U6B forward primer: TGACACGCAAATTCGTGAAG (Integrated DNA Technologies). Data were reported as relative expression normalized to the housekeeping gene, U6B.
Transfection of Let-7f miRNA inhibitor
Two million isolated human CD4+ naïve T cells were transfected, using nucleoporation, with the commercially available mirVana Let-7f inhibitor (10 nM) (Life Technologies, catalog #4464084 hsa-let-7f-) or mirVana Negative Control #1 (10nM) (Life Technologies, catalog #4464076) according to manufacturer's protocol (Lonza) and as previously described.(2) Cells rested for 4 hours after transfection at 37°C/5% CO2, and transfected cells were activated and differentiated into Th17 cells as described above for 3 days.
CD4+ Th17 cell differentiation from mice
Naïve CD4+ T cells isolated from the spleens of WT mice were activated using anti-CD3 (5 μg/ml) (BD Biosciences, catalog #553057) and anti-CD28 (1 μg/ml) (BD Biosciences, catalog #553295) in 96-well plates. Naïve CD4+ T cells from D011.10 mice were activated using OVA peptide 323-339 (5 μg/ml; Sigma Chemical Co., St. Louis, MO, catalog #O164) and anti-CD28 (1 μg/ml). CD4+ T cells from both WT and D011.10 mice were differentiated into Th17 cells by adding rmIL-23 (10 ng/ml), rhTGF-β (1 ng/ml), rmIL-6 (20 ng/ml), anti-IFN-γ (10 g/ml), and anti-IL-4 (10 μg/ml). In select experiments, Th17 cells were differentiated with varying concentrations of rmIL-23 ranging from 0-30ng/ml. All antibodies and rmIL-23 were purchased from R&D Systems. rmIL-6 and rhTGF-β were purchased from PeproTech (Rocky Hill, NJ).
Administration of hormone pellet in vivo to mice
At 7 weeks of age, 60-day slow-release pellets (Innovate Research of America) containing 17β-E2 (0.1 mg), P4 (25 mg), 5α-DHT (15 mg) or a combination of 17β-E2 (0.1 mg) and P4 (25 mg) were surgically placed subcutaneously into ovariectomized BALB/c mice as previously described.(3) As a control, vehicle pellets (Innovative Research of America) were surgically placed into intact males, intact females, or ovariectomized female mice. Three weeks (21 days) after pellets were implanted; naïve CD4+ T cells were harvested from the spleens of mice and differentiated into Th17 cells. Serum was also collected and analyzed for hormone levels using the Vanderbilt Hormone Core Facility as described in the section above. Four days after Th17 cell differentiation, cell culture supernatants and cells were collected for various endpoints.
Adoptive Transfer of D011.10 Cells into WT female recipient mice
As depicted in Figure 7A, naïve CD4+ T cells were isolated from the spleens of female and male D011.10 donor mice using a commercially available CD4 negative selection isolation kit from Miltenyi (catalog #130-093-227) per manufacturer's directions. Naïve CD4+ T cells from donor mice were stimulated with 5 μg/ml of OVA peptide 323-339 and 1 μg/ml of anti-CD28 and differentiated into Th17 cells for 4 days as described in section above. One day prior to the adoptive transfer (day -1), recipient WT female BALB/c mice were aerosol challenged with 1% OVA for 40 minutes to facilitate migration of adoptively transferred CD4+ D011.10 Th17 cells to the lung. On day 0, 2×106 Th17 differentiated cells from male or female donor D011.10 mice were adoptively transferred via tail vein injection into WT female recipient BALB/c mice. As a control, a group of recipient WT female BALB/c mice received no adoptive transfer of cells (depicted by the gray bars in Figure 7). On days 1, 2, and 3 after the adoptive transfer of D011.10 OVA-specific Th17 cells, all recipient mice were challenged with 1% OVA protein aerosol for 40 minutes each day to induce Th17-mediated airway inflammation. BAL fluid and lungs were collected on Days 2 and 4 for analysis of KJ126+, OVA-specific Th17 cells in the lung (see methods below), inflammatory cell infiltrate (see methods below), and cytokine or chemokine expression in whole lung homogenates measured by ELISA.
Analysis of inflammatory cell infiltration into the airway
BAL was performed by instilling 800 μl of saline through a tracheostomy tube and then withdrawing the fluid with gentle suction via syringe as previously described.(4) The total cell count in the BAL fluid was determined using a hemocytometer and Trypan Blue Exclusion dye (Sigma, catalog #T8154). Cells from the BAL fluid were then fixed to a slide and stained using a Three-Step Stain (Richard-Allan Scientific, catalog # 22-050-272). Two hundred leukocytes were classified as neutrophils, eosinophils, lymphocytes, or monocytes using standard morphologic criteria, and a percentage of neutrophils was determined. Total numbers of neutrophils were determined by multiplying percentage of neutrophils times the total number of viable cells in the BAL fluid.
Key Messages.
17β-E2 plus P4 increase IL-17A production in Th17 cells by decreasing Let-7f miRNA expression and increasing IL-23R expression, providing a potential mechanism for the increased prevalence of severe asthma in women compared to men.
Transfer of female OVA-specific Th17 cells increased neutrophil infiltration in the lungs of OVA-challenged recipient mice compared to transfer of male OVA-specific Th17 cells.
Capsule Summary.
Severe asthma prevalence is increased in women and associated with IL-17A. Ovarian hormones increased IL-17A production in Th17 cells through a Let-7f/IL-23R pathway, and ovarian hormones increased airway neutrophils in mice with acute IL-17A-mediated inflammation.
ACKNOWLEDGEMENTS
Declaration of all sources of funding: National Institute of Health (U19 AI 095227, R01 AI 111820, R01 HL122554, K12HD043483-08), Veteran Affairs (2I01BX000624), and Vanderbilt Institute for Clinical and Translational Research (VICTR) grant VR576. We would also like to thank Thomas Aune, M.D. and Taylor Sherrill for technical advice and use of equipment.
Abbreviations
- (BAL)
bronchoalveolar lavage
- (E2)
estradiol
- (ER)
estrogen receptors
- (ILC3)
group 3 innate lymphoid cells
- (miRNA)
microRNA
- (neg.)
negative
- (n.s.)
not significant
- (PBMCs)
peripheral blood mononuclear cells
- (P4)
progesterone
- (R)
receptor
- (rm)
recombinant mouse
- (SLE)
systemic lupus erythematosus
- (veh.)
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Lung Association . Trends in Asthma Morbidity and Mortality. 2012. [Google Scholar]
- 2.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127(2):382–389. e381–313. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 4.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 5.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111(4):677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 6.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 10.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 11.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 12.Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25(6):755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 15.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Wu F, Brant SR, Kwon JH. IL-23 receptor regulation by Let-7f in human CD4+ memory T cells. J Immunol. 2011;186(11):6182–6190. doi: 10.4049/jimmunol.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol. 2010;120(2–3):105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 19.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour EF, Liu Y, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37(14):4850–4861. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, Khurana Hershey GK, Kolls JK, Peebles RS., Jr Human T(H)17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol. 2011;127(4):1006–1013. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, Peebles RS., Jr A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182(9):5317–5321. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 25.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R. Increased frequency of dual-positive T2/T17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283(5406):1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 28.Khan D, Dai R, Karpuzoglu E, Ahmed SA. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur J Immunol. 2010;40(9):2549–2556. doi: 10.1002/eji.201040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 30.Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, Pelletier L, Engelhardt B, Guery JC. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187(5):2386–2393. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 31.Relloso M, Aragoneses-Fenoll L, Lasarte S, Bourgeois C, Romera G, Kuchler K, Corbi AL, Munoz-Fernandez MA, Nombela C, Rodriguez-Fernandez JL, et al. Estradiol impairs the Th17 immune response against Candida albicans. J Leukoc Biol. 2012;91(1):159–165. doi: 10.1189/jlb.1110645. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res. 2010;11:166. doi: 10.1186/1465-9921-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, et al. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA. 2012;109(24):9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173(3):600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, Levings MK. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J Immunol. 2011;187(11):5615–5626. doi: 10.4049/jimmunol.1101058. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Zhang Y, Yang XO, Nurieva RI, Chang SH, Ojeda SS, Kang HS, Schluns KS, Gui J, Jetten AM, et al. Transcription of Il17 and Il17f is controlled by conserved noncoding sequence 2. Immunity. 2012;36(1):23–31. doi: 10.1016/j.immuni.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008 doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C, Benham AL, Kim J, Soibam B, Harris RA, et al. Proinflammatory role for let-7 microRNAS in experimental asthma. J Biol Chem. 2010;285(39):30139–30149. doi: 10.1074/jbc.M110.145698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J RespirCrit Care Med. 2013;188(11):1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 1.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collier SP, Collins PL, Williams CL, Boothby MR, Aune TM. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J Immunol. 2012;189(5):2084–2088. doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177(1):621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, Lukacs NW, Kolls JK, Peebles RS., Jr IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol. 2012;188(3):1027–1035. doi: 10.4049/jimmunol.1102216. [DOI] [PMC free article] [PubMed] [Google Scholar]