Abstract
A non-functional Cystic Fibrosis Transmembrane conductance Regulator (CFTR) leads to the disease cystic fibrosis (CF). Although the CFTR is expressed in multiple organs, pulmonary disease is the major cause of illness and death in patients with CF. Stagnant mucus, causing airway obstruction, bacterial overgrowth, persistent inflammation and tissue destruction characterizes the disease, but how the defect in CFTR function is coupled to the mucus phenotype is still controversial. We have recently shown that bicarbonate ions passing through CFTR are necessary for proper unfolding of the MUC2 mucin, thus highlighting the importance of bicarbonate ion transport via the CFTR and the ability of these ions to raise the pH and chelate calcium bound to the mucin as the important steps in forming normal mucus. In order to find potential CF treatments and expand our knowledge about the usefulness of bicarbonate as an active ingredient in formulations to alleviate mucus plugging, we used an Ussing-type chamber and explants from the F508del-CFTR mutant mouse ileum to test the effect of calcium chelators on mucus attachment, either in isolation or in combination with osmolytes such as mannitol or hypertonic saline. We found that increasing the concentration of bicarbonate, both alone or in combination with increased osmolarity of the solution, detached the otherwise attached CF mucus.
Keywords: Calcium chelator, Hyperosmolarity, Mouse ileum, Mucus attachment, Osmolyte
1. Introduction
Cystic fibrosis (CF) is caused by mutations in the ion channel Cystic Fibrosis Transmembrane conductance Regulator (CFTR) (Riordan et al., 1989), progressive bronchiectasis and lung damage being the main causes of morbidity and mortality (Chmiel and Davis, 2003). The disease causing mutations affect permeability to chloride and bicarbonate (Quinton, 2001) and lack of bicarbonate causes formation of attached mucus (Garcia et al., 2009; Gustafsson et al., 2012a). In CF the attached, stagnant mucus leads to bacterial overgrowth, infection and inflammation. The inflammatory response can increase the number of mucus secreting cells, stimulate mucus secretion and further influence mucus properties (Rogers, 1994). Many CF patients also suffer from distal intestinal obstruction syndrome (DIOS), where attached mucus obstructs the distal ileum and leads to bacterial overgrowth. Consequently, effective treatments should be aimed at preventing or reversing the attachment and stagnation of mucus.
We have shown that a MUC2 N-terminal recombinant protein consisting of the VWD1-D2-D′D3 domains formed large aggregates at pH 6.2 in the presence of Ca2+. The aggregates were dissolved upon Ca2+ chelation and pH increase. Chelation of Ca2+ was achieved by 2,2′,2″,2‴-(Ethane-1,2-diyldinitrilo)tetraacetic acid (EDTA) or bicarbonate (Ambort et al., 2012), which lead us to test Ca2+ chelation in the F508del-CFTR mutant mouse ileum (van Doorninck et al., 1995). We could show that bicarbonate ions passed through CFTR are necessary to establish proper unfolding of the Muc2 mucin, as newly formed mucus became attached in the WT mouse ileum when the basolateral buffer was depleted of bicarbonate ions. When mucus was secreted into apical isotonic 115 mM bicarbonate buffer the CF ileal mucus became easily aspirated and penetrable to beads the size of bacteria (Gustafsson et al., 2012a). Furthermore, forming mucus in an apical buffer with 20 mM EDTA normalized the attached CF mucus into a more easily aspirated phenotype (Gustafsson et al., 2012a). To find potential CF treatments, we tested calcium chelators, either in isolation or in combination with mannitol, since clinical trials on CF patients have shown that inhaled dry powder mannitol improves mucociliary clearance (Aitken et al., 2012; Robinson et al., 1999).
N-acetyl-L-cysteine (NAC) is used as a mucolytic drug in respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD) and CF (Rogers, 2007) and erdosteine is an expectorant available for treatment of chronic bronchitis and COPD (Cazzola et al., 2010). Since they are defined as mucolytics, we tested the ability of these substances to detach F508del-CFTR mutant mouse ileal mucus.
The treatments deemed promising are the ones which 1) make the F508del-CFTR mutant mouse ileal mucus as easily aspirated as in the WT mouse ileum, 2) are also effective on preformed mucus and 3) do not cause expansion of the mucus, since expansion may cause airway plugging. Our results indicate that calcium chelation by bicarbonate or EDTA in combination with hypertonic buffer, either achieved by NaCl or mannitol causes effective detachment while mucus expansion is kept at a minimum. NAC and erdosteine had no effect on mucus attachment.
2. Materials and Methods
2.1. Animals
Male and female (age 8–16 weeks) homozygous F508del-CFTR mutant mice on C57BL/6 background were bred as heterozygotes at the University of Gothenburg. Mice were housed in individually ventilated cages under controlled temperature (21–22°C), humidity and 12-h light/dark cycle under specific pathogen-free conditions, maintained as described (van Doorninck et al., 1995) and given regular water 2–3 days before the experiments. All mice were killed by cervical dislocation under isoflurane anesthesia. Ethical approval was granted by the Laboratory Animal Ethics Committee, University of Gothenburg, and experimental animal care was in accordance with their guidelines.
2.2. Explants
Intestinal explants were prepared and mounted as described previously (Gustafsson et al., 2012b). Briefly, explants were mounted between two chambers, Krebs-glucose buffer was perfused basolaterally and Krebs-mannitol was added apically. Activated charcoal particles in Krebs-mannitol buffer were added and allowed to sediment on top of the mucus to visualize the mucus surface through a stereomicroscope at ×40 magnification (Leica MZ125, Wetzlar, Germany). For full removal, mucus was aspirated with a Pipetman P200 (Gilson, Middleton, WI, USA) set to 150 μl and using a 20–200 μl tip. Tissue viability was monitored by measuring PD using electrodes (Ref201; Radiometer, Copenhagen, Denmark) connected to the chamber by agar bridges (4% agar, 0.9% NaCl).
2.3. Mucus thickness measurements
Mucus thickness was measured as previously described, from the mucus surface to the villi tips every 20 min for 60 min (Ermund et al., 2015; Ermund et al., 2013; Gustafsson et al., 2012a; Gustafsson et al., 2012b). All apical incubations were done for 60 min. The mucus already formed when tissue was mounted is denoted preformed mucus. In the figures, the mucus thickness at 60 min is illustrated by white bars and denoted “Pre”. To evaluate mucus properties, the whole apical volume was aspirated using a plastic Pasteur pipette (PP-101, outer tip diameter 0.9 mm, inner tip diameter 0.7 mm, maximum volume 800 μl; Cellprojects, Sutton Valence, UK). The pipette tip was placed at the edge of the circular opening without touching the mounted tissue and kept in place while the bulb was released for approximately 3 s, thus extracting all removable liquid. The remaining mucus thickness, presented by black bars and denoted “Post” in the figures, was measured after refilling the apical chamber with 150 μl Krebs-mannitol to ensure that any remaining mucus material did not collapse and adding new charcoal particles. Finally, all mucus was removed and the villus height was measured from the epithelium between the villi to the villi tips. Total mucus thickness is presented as the sum of villus height and mucus on top of the villi.
In experiments where effects on mucus secreted into buffer containing treatments were evaluated, mucus was measured at 0 min, preformed mucus was removed and villus height measured before mucus secretion was induced by basolateral perfusion with 10 μM carbachol and 10 μM PGE2 in Krebs-glucose at 20 min (blue arrow in figures) for 40 min. Mucus properties were evaluated as for preformed mucus. All apical incubations were done for 60 min.
2.4. Preparation of apical buffers
Calcium was omitted from buffers containing EDTA and osmolarity was adjusted by exchanging the corresponding molar concentration of sodium chloride for bicarbonate in the buffers with increased bicarbonate concentration. In the hyper-osmolar bicarbonate buffers, bicarbonate was added to the standard Krebs-mannitol buffer. Buffers with increased concentration of bicarbonate were first bubbled with carbogen gas (5% CO2, 95% O2) and then the pH was adjusted to 7.4. Some of the bicarbonate will evaporate as CO2 gas, but traditionally, concentrations are given as original bicarbonate concentration. For further information about bicarbonate buffers, see Gustafsson J.K. et al. 2012 (Gustafsson et al., 2012a).
2.5. Histological analysis
Tissue histology and mucus depletion from crypt goblet cells were evaluated in explants fixed in methanol-Carnoy after completed experiments. Paraffin-embedded tissue was cut in 4 μm sections and Alcian blue-Periodic Acid Schiff (Ab-PAS) stained. Images were acquired using an Eclipse E1000 microscope with a Plan-Fluor 40x/0.75 DIC objective (Nikon, Amstelveen, The Netherlands).
2.6. Reagents
Erdosteine (2-[2-Oxo-2-[(2-oxothiolan-3-yl)amino]ethyl]sulfanylacetic acid) was acquired from Abcam (Cambridge, UK). All other chemicals were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO).
2.7. Statistical analysis
Data are presented as mean ± standard error of the mean (S.E.M.) for n animals. The Mann-Whitney test was used to test differences between two groups. When the number of animals in the compared groups was too small (n = 3) for a non-parametric test, the student’s t-test was used. Statistical significance was accepted when P < 0.05.
3. Results
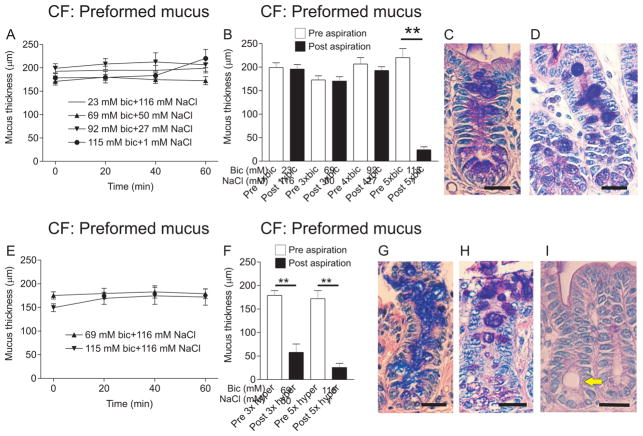
Mucus thickness was unaffected after apical incubation with an isotonic Krebs buffer containing 23, 69, 92 or 115 mM bicarbonate (“bic” in figures) for 60 min (Fig. 1A). Because the bicarbonate concentration varies with pH and pCO2 we measured the actual bicarbonate concentration and 69 mM bicarbonate was found to be 64 mM, 92 mM bicarbonate 84 mM and 115 mM bicarbonate was found to be 104 mM after 60 min (Gustafsson et al., 2012a). Mucus already formed at mounting (preformed mucus) could only be aspirated with the highest concentration of bicarbonate and the lower concentrations had no effect (Fig. 1B). After 60 min incubation with 115 mM bicarbonate, the mucus stored in the crypt goblet cells appeared porous and expanded (white arrow, Fig. 1C) but the goblet cells were still filled with mucus. When an explant was incubated in the chamber with standard apical Krebs buffer for 60 min, goblet cell content did not appear porous and expanded (white arrow, Fig. 1D). Based on PD-measurements, histological evaluation and the fact that the villi were detached by gentle aspiration, we conclude that the tissue integrity was affected when increasing the bicarbonate concentration higher than 115 mM (data not shown).
Figure 1. Effects of bicarbonate on expansion and attachment of preformed mucus in the CF mouse ileum.
A. On preformed mucus, apical incubation with 69 mM (50 mM NaCl, triangles), 92 mM (27 mM NaCl, inverted triangles) and 115 mM (1 mM NaCl, filled circles) bicarbonate for 60 min did not cause expansion of the mucus. CF ileal mucus with standard buffer (23 mM, open circles) is shown for comparison. Osmolarity was adjusted in the apical buffer by replacing NaCl for bicarbonate. B. Mucus measurement before (Pre, white bars) and after gentle aspiration (Post, black bars) of CF mouse ileal mucus was used to validate mucus attachment. On preformed mucus, 60 min apical incubation of 23 mM, 69 mM and 92 mM bicarbonate had no effect in the sense that the mucus was still attached, whereas 115 mM bicarbonate caused detachment of the CF mucus (P = 0.008, n = 5). C. As observed in Ab-PAS stained sections, crypt goblet cell mucus was expanded but the cells were not emptied of mucus after incubation with 115 mM bicarbonate for 60 min. White arrow points to a goblet cell with expanded mucus. D. Ab-PAS stained CF ileal section after mounting the explant in the chamber. Note that the goblet cell content is not expanded (white arrow). E. Even if the apical solution was substituted with 69 mM or 115 mM bicarbonate in the standard buffer (116 mM NaCl), there was no change in mucus thickness over 60 min. F. After incubation for 60 min with 69 mM or 115 mM bicarbonate (116 mM NaCl) also the lower concentration of bicarbonate was effective in making the mucus easily aspirated (P = 0.002, n = 6). The higher concentration (115 mM) of bicarbonate made the mucus easily aspirated (P = 0.008, n = 5) but tissue integrity was somewhat affected. G, H. Mucus stored in crypt goblet cells acquired a blistery, expanded appearance (white arrows) in Ab-PAS stained sections after experiments with 69 mM (G) or 115 mM (H) bicarbonate, respectively, but the goblet cells still contained mucus. I. Ab-PAS stained section of a WT mouse ileal explant stimulated with 10 μM carbachol and 10 μM PGE2 for 40 min shows an intact epithelium and emptied goblet cells. Yellow arrow points to distended crypt. Bar in C, D, G, H and I: 20 μm.
The literature suggests that increased osmolarity may help normalize mucus by increasing the hydration of the mucus gel (Tildy and Rogers, 2015). Therefore we added 69 or 115 mM bicarbonate to the apical Krebs buffer without removing NaCl, giving a hypertonic solution. When incubated for 60 min, the mucus thickness was not altered compared to timepoint 0 min (Fig. 1E) but preformed mucus became easily aspirated from the explant epithelium at a bicarbonate concentration as low as 69 mM. As expected, 115 mM was also effective (Fig. 1F), indicating that bicarbonate and increased osmolarity are more effective at normalizing CF mucus attachment than bicarbonate alone. Also after these treatments, mucus stored in crypt goblet cells became porous and expanded (white arrows in Fig. 1G and H). For comparison, an Ab-PAS stained section of a carbachol and PGE2 stimulated WT mouse ileal explant had no filled goblet cells and the crypt was distended (yellow arrow) while the epithelium was intact (Fig. 1I).
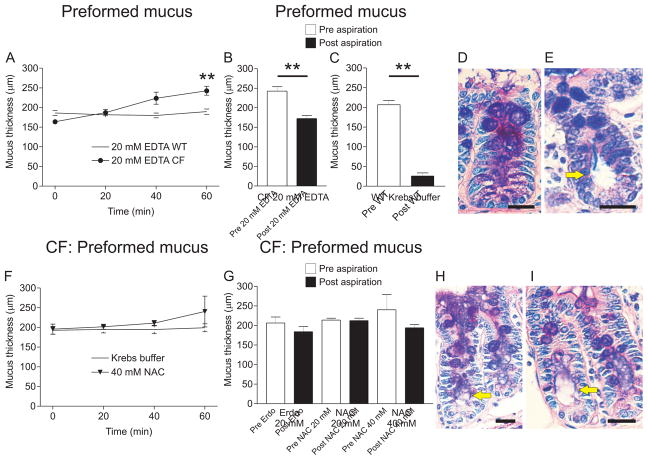
Incubation of 20 mM EDTA with preformed mucus in the CF mouse ileum for 60 min caused an increase in mucus thickness from 163±6 μm to 243±11 μm (P = 0.008, n = 5) compared to WT mouse ileum incubated with 20 mM EDTA for 60 min, where the mucus thickness did not change (Fig. 2A). Incubation of CF mouse ileum with 20 mM EDTA caused some detachment of the mucus without normalization to a fully detached mucus phenotype (Fig. 2B). After incubation with 20 mM EDTA the average mucus thickness remaining after aspiration was 172±8 μm (72±4%). This number is higher than in the normal WT ileum (Fig. 2C) where the mean mucus thickness remaining after aspiration was 26±8 μm (14±3%, P = 0.008, n = 5). It was not possible to use a higher concentration of EDTA since the tissue integrity was already affected at 20 mM as judged from tissue histology. The mucus stored in crypt goblet cells became porous and expanded upon treatment with EDTA (white arrow, Fig. 2D). A WT explant with filled goblet cells without porous mucus content (white arrow) but with distended crypt (yellow arrow, Fig. 2E) is shown for comparison.
Figure 2. Effects of EDTA and mucolytics on mucus expansion and attachment of preformed mucus in the CF mouse ileum.
A. Apical EDTA at 20 mM for 60 min on preformed CF mouse ileal mucus (filled circles) caused expansion of the mucus from 164±6 μm at time 0 to 243±11 μm at time 60 min (**, P = 0.008, n = 5). Apical EDTA at 20 mM for 60 min on preformed mucus in WT mouse ileum explants did not cause mucus expansion (open circles). B. Mucus thickness measured before (Pre, white bar) and after (Post, black bar) aspiration in CF mouse ileal explants incubated apically with EDTA at 20 mM for 60 min indicated that the treatment caused some detachment of the CF mouse ileal mucus (P = 0.008, n = 5). C. Mucus thickness measurement before (Pre, white bar) and after (Post, black bar) aspiration in WT mouse ileum showed that mucus was not attached in the WT mouse ileum. Mucus thickness after aspiration was 26±8 μm in WT ileum and 172±8 μm after aspiration in CF mouse ileum after 60 min apical incubation with 20 mM EDTA indicating that 20 mM EDTA did not detach the mucus to the level of WT mucus. D. Ab-PAS stained sections revealed expanded and blistery mucus in the crypt goblet cells after 60 min incubation with 20 mM EDTA (white arrow), but the goblet cells were not completely emptied. E. For comparison, an Ab-PAS stained section from a stimulated WT mouse ileal explant is shown. White arrow point to goblet cell with normal mucus and yellow arrow points to a distended crypt, indicating mucus release. F. The mucolytic substance NAC at 40 mM (inverted triangles) had no effect on thickness of preformed mucus when incubated apically for 60 min. Incubating CF mouse ileal explants for 60 min in the explant chamber also did not change the mucus thickness (open circles). G. Mucus attachment was not affected by apical incubation of 20 mM erdosteine (Erdo), 20 mM or 40 mM NAC. Mucus thickness before aspiration (Pre, white bars) and after aspiration (Post, black bars) was not different. H, I. Crypt goblet cell mucus was expanded and blistery after incubation with 20 mM Erdo (H) or 40 mM NAC (I) but the goblet cells were not emptied. White arrows point to expanded mucus within goblet cells and yellow arrows point to distended crypts. Bar in D, E H and I: 20 μm.
N-acetyl-L-cysteine (NAC) and erdosteine (Erdo) are two agents available for treatment of patients suffering from airway mucus hypersecretion, a feature typical for asthma and COPD as well as CF (Rogers, 2007). Erdosteine at 20 mM and NAC at 20 and 40 mM had no effect on expansion of preformed mucus, even though there was a tendency for NAC to cause an increase in mucus thickness at 40 mM (Fig. 2F). The mucus thickness at 60 min varied between 200 and 318 μm. Erdosteine (erdo) and NAC had no effect on the attachment of preformed mucus when incubated for 60 min (Fig. 2G). Mucus stored in crypt goblet cells became porous and expanded also after erdosteine at 20 mM (white arrow, Fig. 2H) and after NAC at 40 mM (white arrow, Fig. 2H).
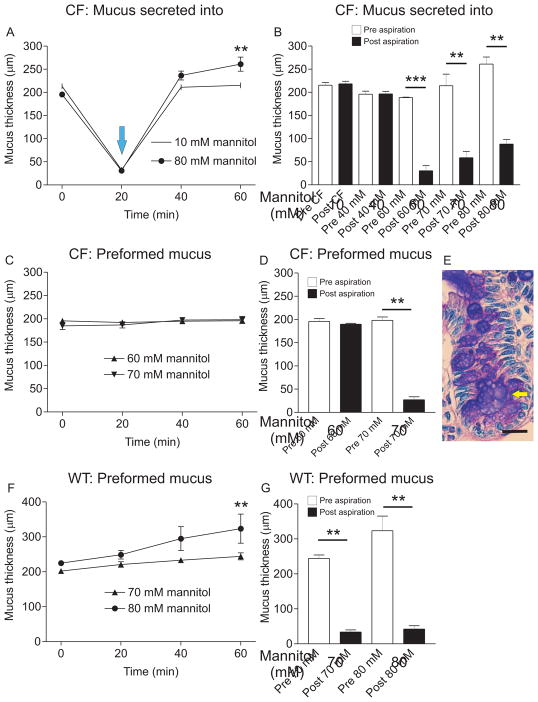
Another way of increasing buffer osmolarity is to add mannitol, a metabolically inert sugar already in use for treating CF (Aitken et al., 2012). The preformed mucus was measured, removed and then new apical buffer containing 40, 60, 70 or 80 mM mannitol was added. At 20 min, the explants were stimulated with carbachol and PGE2 (blue arrow in Fig. 3A) causing new mucus to be secreted into the hyper-osmolar buffer. With 80 mM mannitol, mucus thickness increased from 196±3 μm at 0 min to 261±15 μm at 60 min (P = 0.002, n = 6). This is in contrast to CF explants in regular Krebs buffer (10 mM mannitol) where mucus thickness was the same at 0 min and 60 min (Fig. 3A). In CF mouse ileum, the mucus thickness before (Pre, white bar) and after (Post, black bar) aspiration was the same, indicating that mucus was attached (Fig. 3B). At 40 mM mannitol there was no effect on mucus attachment but at 60 mM mannitol, the CF mucus became easily aspirated. When increasing the mannitol concentration to 70 mM the values at 60 min varied between 138 and 293 μm (white bar, Fig. 3B) and mucus was easily aspirated (Fig. 3B). At 80 mM mannitol mucus was also easily aspirated (Fig. 3B), indicating that elevated concentrations of mannitol are effective at normalizing mucus attachment in the CF mouse ileum.
Figure 3. CF mouse ileal mucus secreted into buffer with mannitol above 60 mM becomes easily aspirated.
A. Mucus was measured in CF mouse ileal explants after mounting and every 20 min for 60 min. At the blue arrow, mucus was removed and new mucus was stimulated by perfusion with 10 μM carbachol and 10 μM PGE2 for 40 min, causing the mucus to form into buffer with increased mannitol concentration. In untreated CF mouse ileum, the mucus thickness at 0 min and 60 min did not differ (10 mM mannitol, open circles). In CF mouse ileum treated with 80 mM mannitol apically for 60 min, the mucus thickness increased from 196±3 μm (0 min) to 261±15 μm (60 min), **, P = 0.008, n = 6, indicating that incubation with hyperosmolar solution causes mucus expansion (80 mM mannitol, filled circles). B. Ileal mucus in the CF mouse was attached to the epithelium (n = 6). An apical mannitol concentration of 40 mM (n = 3) did not cause any detectable change in mucus attachment. However, mucus secreted into 60, 70 and 80 mM mannitol became easily aspirated (P = 0.0001, n = 3, 0.008, n = 5 and 0.002, n = 6, respectively). C. With preformed mucus, 60 mM (triangles) and 70 mM (inverted triangles) mannitol did not affect mucus thickness over 60 min. D. There was no effect of 60 mM mannitol on attachment of preformed mucus, but 70 mM mannitol made the mucus easily aspirated (P = 0.008, n = 5). E. Ab-PAS stained sections treated with 70 mM mannitol revealed blistery and expanded mucus in the crypt goblet cells (white arrow), but the goblet cells were not emptied even if the crypt was somewhat distended (yellow arrow). F. In WT mouse ileal explants, 70 mM mannitol (triangles) did not affect preformed mucus thickness when incubated apically for 60 min. However, 80 mM mannitol caused expansion of WT mouse ileal mucus after 60 min incubation with preformed mucus (**, P = 0.008, n = 5, filled circles). G. With preformed WT mouse ileal mucus, 70 and 80 mM mannitol did not cause any change in the mucus attachment; mucus was easily aspirated in both cases (P = 0.008, n = 5 for both conditions). Bar in E: 20 μm.
When incubating 60 or 70 mM mannitol apically on preformed mucus, there was no effect on mucus thickness over 60 min (Fig. 3C). With 60 mM mannitol the mucus was still attached after 60 min incubation (Fig. 3D). To affect preformed mucus and make it easily aspirated, the mannitol concentration had to be increased to 70 mM (Fig. 3D). The mucus stored in crypt goblet cells was expanded and porous after incubation with 70 mM mannitol, but the goblet cells were still filled with mucus (white arrow, Fig. 3E). The crypt was distended, as indicated by the yellow arrow in Fig. 3E.
In explants from WT mouse ileum incubation of 70 mM mannitol with preformed mucus caused expansion from 202±6 μm to 244±10 μm (P = 0.02, n = 5) and 80 mM mannitol caused mucus expansion from 225±7 μm at 0 min to 324±42 μm at 60 min (P = 0.008, n = 5, Fig. 3F). Treatment with 70 and 80 mM mannitol did not affect attachment of preformed mucus; WT mucus was still easily aspirated after incubation for 60 min (Fig. 3G).
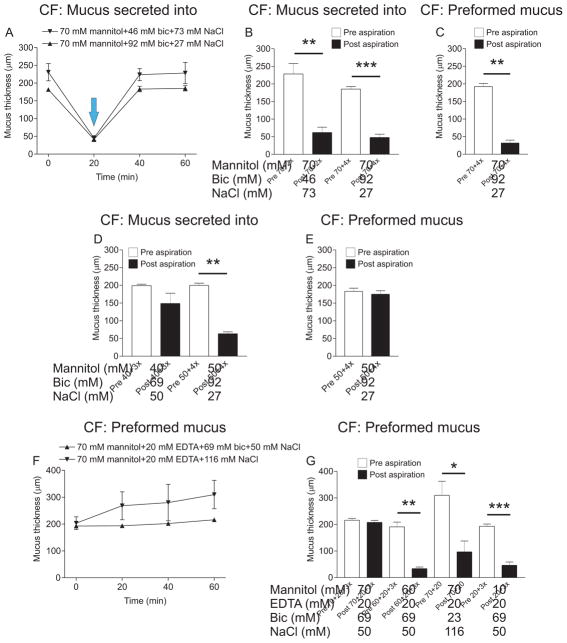
Since high osmolarity and high concentrations of calcium chelators disrupt tissue integrity, we hypothesized that combinations of EDTA, bicarbonate and mannitol may be more effective at detaching CF mucus without destroying the tissue, than each treatment alone. Therefore, we combined mannitol with bicarbonate. With mucus secreted into buffer containing combinations (stimulation indicated by the blue arrow in Fig. 4A), 70 mM mannitol and 46 mM bicarbonate did not cause expansion (Fig. 4A) but made it more easily aspirated (Fig. 4B). Increasing the concentration of bicarbonate to 92 mM maintained the detaching effect (Fig. 4B). The combination of 70 mM mannitol and 92 mM bicarbonate was also effective on preformed mucus, without causing an increase in thickness (Fig. 4C). Decreasing the mannitol concentration to 40 or 50 mM and combining it with 69 or 92 mM bicarbonate respectively caused no change in thickness when mucus was secreted into the buffer containing the combinations, but 40 mM mannitol in combination with 69 mM bicarbonate had only a minor effect on mucus attachment to the epithelium (Fig 4D). Increasing the mannitol concentration to 50 mM and the bicarbonate concentration to 92 mM made the mucus easily aspirated (Fig. 4D). However, this combination had no effect on preformed mucus (Fig. 4E). Combining 70 mM mannitol with 20 mM EDTA and 69 mM bicarbonate and incubating the solution apically for 60 min on preformed mucus did not cause any change in thickness (Fig. 4F) but completely disrupted the tissue (clearly visible through the stereo microscope) and no mucus could be aspirated (Fig. 4G). Decreasing the concentration of mannitol to 60 mM improved tissue viability, did not affect mucus thickness (Fig. 4F) and made it easily aspirated (Fig. 4G). The combination of 70 mM mannitol and 20 mM EDTA made the preformed mucus easily aspirated, but the thickness also increased in some samples. The mucus thickness varied between 189 and 508 μm after 60 min (Fig 4F). However, combining 20 mM EDTA with 69 mM bicarbonate caused no thickness increase and made the mucus easily aspirated, features desirable for potential CF treatments.
Figure 4. Some combinations of calcium chelators and osmotic agents are effective at detaching CF mucus.
A. When mucus was secreted into a buffer containing a combination of 70 mM mannitol and 46 mM bicarbonate (time point for stimulation indicated by blue arrow), mucus thickness at 60 min varied between 185 and 286 μm (inverted triangles) but there was no difference between mucus thickness at 0 min and 60 min. The combination of 70 mM mannitol and 92 mM bicarbonate also did not cause any difference in mucus thickness between 0 min and 60 min (triangles). B. Mucus secreted into 70 mM mannitol and 46 mM bicarbonate became easier to aspirate after incubation for 60 min (P = 0.008, n = 3). The bicarbonate concentration in the combination was increased to 92 mM and the variation in mucus thickness decreased (white bar and triangles, 60 min time point in A) compared to 70 mM mannitol and 46 mM bicarbonate while still easy to aspirate (P = 0.0003, n = 3). C. Mannitol at 70 mM in combination with 92 mM (4x) bicarbonate was tested on CF mouse preformed mucus and the combination made the mucus easily aspirated (P = 0.008, n = 5). D. When combining 40 mM mannitol with 69 mM bicarbonate, mucus secreted into the treatment did not expand and was not as easily aspirated as WT mucus (n = 3). Therefore, mannitol was increased to 50 mM and bicarbonate to 92 mM. Mucus formed in a buffer containing this combination did not expand and was easily aspirated (P = 0.008, n = 5). E. The combination of 50 mM mannitol and 92 mM bicarbonate had no effect on preformed mucus (n = 6). F. Mucus thickness was measured for 60 min in explants incubated with 70 mM mannitol, 20 mM EDTA and 69 mM bicarbonate (triangles) or the combination of 70 mM mannitol and 20 mM EDTA (inverted triangles) with preformed mucus. There was no difference in mucus thickness between 0 min and 60 min, but the thickness varied greatly in the explants incubated with 70 mM mannitol and 20 mM EDTA. G. When combining 70 mM mannitol with 20 mM EDTA and 69 mM bicarbonate and incubating it on preformed mucus for 60 min, the tissue started disintegrating and no mucus could be aspirated (n = 2). However, when the mannitol concentration was decreased to 60 mM, mucus was easily aspirated (P = 0.008, n = 5) without mucus expansion. The combination of 70 mM mannitol and 20 mM EDTA was incubated on the explant and mucus became easier to aspirate (P = 0.03, n = 5). The calcium chelators EDTA at 20 mM in combination with 69 mM mannitol made the preformed mucus easily aspirated (P = 0.0006, n = 7) without causing mucus expansion. Pre aspiration: white bars, post aspiration: black bars.
4. Discussion
In healthy airways, mucus secretion and removal have a protective role (Munkholm and Mortensen, 2014). This is true also in the small intestine (Jacobs et al., 2013). However, when mucus secretion becomes excessive and adhesive, mucus stagnates in the airways and bowel, bacterial overgrowth can occur and inflammatory processes eventually lead to tissue damage (Cohen-Cymberknoh et al., 2013). This is a major problem in the lungs of CF patients and often the cause of mortality in this group (Rowe et al., 2005). In conditions when the mucus adheres to the epithelium, increased ciliary beat frequency may be insufficient to clear the airways and additional treatment becomes necessary.
Gel-forming mucins are packed in goblet cell granules with calcium and hydrogen ions. This is a common feature of MUC2 in the intestine, MUC5AC and MUC5B in the airways. We have previously shown that the purified MUC2 N-terminus forms aggregates at high calcium and low pH, aggregates which can be dissolved by calcium chelation and raised pH (Ambort et al., 2012). In a physiological setting, this is achieved by bicarbonate. Impaired bicarbonate transport in CF, due to malfunctions in the CFTR ion channel, causes improper mucus unfolding, resulting in attached and impenetrable mucus in the CF mouse ileum (Gustafsson et al., 2012a). In normal WT mouse ileum, mucus is easily aspirated and penetrable to beads the size of bacteria (Ermund et al., 2015; Gustafsson et al., 2012a). We have also shown that the enzyme meprin beta, expressed by the ileal epithelium is responsible for detachment of mucus in WT mouse ileum by cleaving in Muc2. The cleavage site must be exposed by sufficient mucus unfolding. Since the mucus is not properly unfolded in CF, meprin beta cannot cleave Muc2 and hence the mucus is attached (Schütte et al., 2014). In CF mouse ileum, we could normalize both attachment and penetrability by apical incubation with isotonic 115 mM bicarbonate (Gustafsson et al., 2012a). Furthermore, when mucus was secreted into 92 mM isotonic apical bicarbonate, the mucus was only partly detached (Gustafsson et al., 2012a). In contrast, we observed no effect of isotonic 92 mM bicarbonate on preformed mucus. Thus we conclude that it is more difficult to affect preformed mucus than mucus secreted into an isotonic buffer with elevated bicarbonate concentration. Increasing also the osmolarity together with the bicarbonate concentration detached the mucus from the epithelium, indicating normalization in terms of attachment.
The stronger calcium chelator EDTA dissolved the aggregates formed by purified MUC2N (Ambort et al., 2012) and EDTA detached mucus secreted into EDTA-containing buffer (Gustafsson et al., 2012a). Hence, we wanted to test EDTA on preformed mucus. Unfortunately, concentrations of EDTA necessary to detach preformed mucus caused PD deterioration and inhibited stimulated mucus release. Furthermore, the villi had a tendency to detach at gentle aspiration. We could use up to 20 mM EDTA, which caused an increase in mucus thickness over 60 min, due to mucus expansion. At 20 mM, EDTA did not normalize mucus attachment.
Mucolytics are commonly used to alleviate bronchiectasis in CF as well as other obstructive pulmonary diseases. These compounds are believed to reduce disulfide bonds, thereby decreasing mucus viscosity (Seagrave et al., 2012). In a clinical setting inhalation of nebulized 20% NAC solution has been studied (Tam et al., 2013). If assuming an inhaled volume of 4 ml, a 15% distribution and 2 ml airway liquid volume (Ermund et al., 2015), the concentration of NAC achieved by this treatment would be 370 mM in proximal airways. This is much higher than the concentrations used here (20 and 40 mM). Presuming that erdosteine can be inhaled at the same concentration as NAC and assuming the same distribution, the concentration in proximal airways would be 240 mM, also much higher than used here (20 mM). Incubation of primary human epithelial cells with 10–300 μM NAC for three h caused a decrease in the amount of MUC5AC released and 10–100 μM NAC also decreased the amount of intracellular mucin (Seagrave et al., 2012). In addition, 30 and 100 μM NAC decreased both elasticity and viscosity of the secretions from these cells at both 8 and 24 h of incubation (Seagrave et al., 2012). Erdosteine increased mucociliary clearance at doses above 600 mg/kg in normal rats and at 300 mg/kg in LPS-treated rats (Hosoe et al., 1998). Thus, the concentrations used here are intermediate. In our system, we observe no effects of the mucolytics after 60 min incubation. Reported effects of NAC and erdosteine were observed at three (Seagrave et al., 2012) and two h (Hosoe et al., 1998), respectively. Our incubation time may be too short but we cannot increase incubation times beyond 60 min due to loss of tissue viability. Clinical trials have pointed to a beneficial effect of both these drugs in decreasing exacerbations and fewer days spent in the hospital, but no effects on lung function or mucociliary clearance were found (Rahman and MacNee, 2012). Thus, even though NAC and erdosteine did not have a direct effect on CF mucus attachment, they could still be beneficial. Several of the treatments resulted in expanded and blistery mucus in the crypt goblet cells. This observation does not appear to be correlated with detachment of the mucus from the epithelium, since both effective treatments, for example 115 mM bicarbonate and 70 mM mannitol as well as ineffective treatments such as 20 mM EDTA, 20 mM Erdosteine and 40 mM NAC resulted in this type of expansion. In addition, there was no correlation between expansion of the mucus in the crypt goblet cells and expansion of the extracellular mucus layer as 115 mM bicarbonate did not cause mucus expansion whereas 20 mM EDTA did cause an increase in mucus thickness over 60 min. We conclude that mucus expansion in crypt goblet cells can occur without concomitant expansion of already released mucus and also without detachment of attached CF mucus.
In order to facilitate clearance, osmotic agents are commonly inhaled. Hypertonic saline is used to clear mucus from the lungs of CF patients and clinical trials have established that the treatment is effective in increasing mucociliary clearance (Elkins et al., 2006). We have tested up to 5% NaCl and seen detachment of CF mucus at 1.75% NaCl, verifying that this treatment works also in our model (Ermund et al., 2015). Sodium can probably replace the Muc2 N-terminally bound calcium ion and the increased hydration may expand the mucin to expose the meprin beta cleavage sites (Schütte et al., 2014). Dry powder mannitol is another osmotic agent with beneficial effects in the airways. It was first used to diagnose bronchial hyperresponsiveness (Anderson et al., 1997), but seems to be well tolerated in patients with CF and induces only mild coughing (Daviskas et al., 1997; Robinson et al., 1999). Inhaled dry powder mannitol at amounts between 300 and 420 mg twice daily has been tested in clinical trials in CF patients, where mucus clearance was measured for 60 min post intervention by analyzing residual radioaerosol with a gamma camera (Daviskas et al., 2002; Robinson et al., 1999). This treatment gave a mean improvement in forced expiratory volume in one second (Aitken et al., 2012; Jaques et al., 2008) and increased forced vital capacity (Teper et al., 2011). Here we show a direct effect of mannitol on mucus attachment in the CF mouse model but also increased mucus thickness, which may cause mucus plugging and decreased gas exchange. Hyperosmolar solutions increase mucus hydration and pull the N-terminal von Willebrand D-domains apart, thus exposing meprin beta cleavage sites to detach the mucus. The difficulty in affecting preformed mucus attachment was also evident in the fact that mucus secreted into 60 mM mannitol was detached, whereas the same concentration of mannitol was ineffective on preformed mucus.
In patients, 400 mg mannitol is a common dose (Burness and Keating, 2012) and using the same model for dilution and distribution of inhaled drug as for NAC and erdosteine, inhalation of 400 mg dry powder mannitol results in a concentration of 1 M in proximal airways. The highest concentration mannitol tested here (80 mM) corresponds to 1.5%. This can be compared to 7% NaCl, inhaled by many CF patients on a regular basis (Elkins et al., 2006). Thus, the concentration of mannitol used here is not particularly high. One drawback of mannitol as a drug candidate is that that hyperosmotic solutions may induce mucus secretion (Peatfield et al., 1986) but we see no obvious mucus release from crypt goblet cells in our experimental setting.
Since neither calcium chelators nor hyperosmolar solutions were ideal for detaching CF mucus on their own, combinations were tested in the explant system. Both preformed mucus and mucus secreted into 70 mM mannitol and 92 mM bicarbonate were detached. Decreasing the mannitol concentration to 50 mM, while keeping 92 mM bicarbonate, detached mucus secreted into the buffer, but had no effect on preformed mucus, again highlighting the relative difficulty in affecting the attachment of preformed mucus. Combining EDTA at 20 mM with bicarbonate at 69 mM gave a very good effect on preformed mucus. The mucus was detached after 60 min apical incubation while keeping the same thickness throughout. This again emphasizes the importance of calcium chelation in the formation of a normal, detached mucus layer.
Recently, it was demonstrated that CFTR is also less active in COPD patients, suggesting that CF and COPD may share common features (Dransfield et al., 2013). Thus the mucus phenotype in CF and COPD might be caused by similar mechanisms and treatments effective in detaching the stagnant mucus in CF might also be used as COPD treatment.
5. Conclusion
We have used the CF mouse ileum to investigate how calcium chelation, hyperosmolarity and combined treatments could be used to detach stagnant mucus. We found that it is more difficult to affect preformed mucus than mucus secreted into buffer containing treatment. The most effective combinations were hypertonic solutions with calcium chelators, for example 70 mM mannitol with 92 mM bicarbonate or 116 mM NaCl with 69 mM bicarbonate.
Acknowledgments
This work was supported by the Swedish Research Council (no. 7461 and 21027), The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, The Sahlgrenska University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), The Cystic Fibrosis Foundation, The Swedish CF Foundation, Erica Lederhausen’s Foundation, Lederhausen’s Center for CF Research at the University of Gothenburg, and The Swedish Foundation for Strategic Research – The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program.
Abbreviations
- Ab-PAS
Alcian blue-Periodic Acid Schiff
- CF
cystic fibrosis
- CFTR
Cystic Fibrosis Transmembrane conductance Regulator
- COPD
chronic obstructive pulmonary disease
- DIOS
distal intestinal obstruction syndrome
- EDTA
2,2′,2″,2‴-(Ethane-1,2-diyldinitrilo)tetraacetic acid
- F508del-CFTR
Fenylalanin 508 deletion in CFTR
- MUC2/MUC5AC
mucin 2/mucin 5AC
- NAC
N-acetyl-L-cysteine
- PGE2
prostaglandin E2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jenny K. Gustafsson, Email: Jenny.Gustafsson@medkem.gu.se.
Gunnar C. Hansson, Email: Gunnar.Hansson@medkem.gu.se.
References
- Aitken ML, Bellon G, De Boeck K, Flume PA, Fox HG, Geller DE, Haarman EG, Hebestreit HU, Lapey A, Schou IM, et al. Long-Term Inhaled Dry Powder Mannitol in Cystic Fibrosis. Am J Resp Crit Care. 2012;185:645–652. doi: 10.1164/rccm.201109-1666OC. http://dx.doi.org/10.1164/rccm.201109-1666OC. [DOI] [PubMed] [Google Scholar]
- Ambort D, Johansson MEV, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJB, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. P Natl Acad Sci USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. http://dx.doi.org/10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Brannan J, Spring J, Spalding N, Rodwell L, Chan KIM, Gonda I, Walsh A, Clark A. A New Method For Bronchial-provocation Testing in Asthmatic Subjects Using a Dry Powder of Mannitol. Am J Resp Crit Care. 1997;156:758–765. doi: 10.1164/ajrccm.156.3.9701113. http://dx.doi.org/10.1164/ajrccm.156.3.9701113. [DOI] [PubMed] [Google Scholar]
- Burness C, Keating G. Mannitol Dry Powder for Inhalation. Drugs. 2012;72:1411–1421. doi: 10.2165/11208950-000000000-00000. http://dx.doi.org/10.2165/11208950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Floriani I, Page CP. The therapeutic efficacy of erdosteine in the treatment of chronic obstructive bronchitis: a meta-analysis of individual patient data. Pulm Pharmacol Ther. 2010;23:135–144. doi: 10.1016/j.pupt.2009.10.002. http://dx.doi.org/10.1016/j.pupt.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Chmiel J, Davis P. State of the Art: Why do the lungs of patients with cystic fibrosis become infected and why can’t they clear the infection? Respiratory Research. 2003;4:8. doi: 10.1186/1465-9921-4-8. http://dx.doi.org/10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68:1157–1162. doi: 10.1136/thoraxjnl-2013-203204. http://dx.doi.org/10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- Daviskas E, Anderson S, Brannan J, Chan H, Eberl S, Bautovich G. Inhalation of dry-powder mannitol increases mucociliary clearance. Eur Respir J. 1997;10:2449–2454. doi: 10.1183/09031936.97.10112449. http://dx.doi.org/10.1183/09031936.97.10112449. [DOI] [PubMed] [Google Scholar]
- Daviskas E, Robinson M, Anderson SD, Bye PTP. Osmotic Stimuli Increase Clearance of Mucus in Patients with Mucociliary Dysfunction. J Aerosol Med. 2002;15:331–341. doi: 10.1089/089426802760292681. http://dx.doi.org/10.1089/089426802760292681. [DOI] [PubMed] [Google Scholar]
- Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in copd. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. http://dx.doi.org/10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PTP. A Controlled Trial of Long-Term Inhaled Hypertonic Saline in Patients with Cystic Fibrosis. New Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. http://dx.doi.org/10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- Ermund A, Meiss LN, Scholte BJ, Hansson GC. Hypertonic saline releases the attached small intestinal cystic fibrosis mucus. Clin Exp Pharmacol P. 2015;42:69–75. doi: 10.1111/1440-1681.12322. http://dx.doi.org/10.1111/1440-1681.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermund A, Schütte A, Johansson MEV, Gustafsson JK, Hansson GC. The gastrointestinal mucus layers have different properties depending on location within the tract – 1. Studies of mucus in mouse stomach, small intestine, Peyer’s patches and colon. Am J Physiol Gastrointest Liver Physiol. 2013;305:G341–347. doi: 10.1152/ajpgi.00046.2013. http://dx.doi.org/10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MAS, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator–dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. http://dx.doi.org/10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, Hebert H, Sjövall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012a;209:1263–1272. doi: 10.1084/jem.20120562. http://dx.doi.org/10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Johansson MEV, Schütte A, Hansson GC, Sjövall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol. 2012b;302:G430–G438. doi: 10.1152/ajpgi.00405.2011. http://dx.doi.org/10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoe H, Kaise T, Ohmori K. Erdosteine enhances mucociliary clearance in rats with and without airway inflammation. J Pharmacol Toxico. 1998;40:165–171. doi: 10.1016/s1056-8719(98)00053-7. http://dx.doi.org/10.1016/S1056-8719(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Coss Adame E, Attaluri A, Valestin J, Rao SSC. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther. 2013;37:1103–1111. doi: 10.1111/apt.12304. http://dx.doi.org/10.1111/apt.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques A, Daviskas E, Turton JA, McKay, Cooper P, Stirling, Robertson CF, Bye PTP, LeSouëf PN, Shadbolt B, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest. 2008;133:1388–1396. doi: 10.1378/chest.07-2294. http://dx.doi.org/10.1378/chest.07-2294. [DOI] [PubMed] [Google Scholar]
- Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clinical Physiology and Functional Imaging. 2014;34:171–177. doi: 10.1111/cpf.12085. http://dx.doi.org/10.1111/cpf.12085. [DOI] [PubMed] [Google Scholar]
- Peatfield AC, Richardson PS, Wells UM. The effect of airflow on mucus secretion into the trachea of the cat. J Physiol. 1986;380:429–439. doi: 10.1113/jphysiol.1986.sp016295. http://dx.doi.org/10.1113/jphysiol.1986.sp016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM. The neglected ion: HCO3. Nat Med. 2001;7:292–293. doi: 10.1038/85429. http://dx.doi.org/10.1038/85429. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Antioxidant pharmacological therapies for COPD. Curr Opin Pharmacol. 2012;12:256–265. doi: 10.1016/j.coph.2012.01.015. http://dx.doi.org/10.1016/j.coph.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B-s, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the Cystic Fibrosis Gene: Cloning and Characterization of Complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. http://dx.doi.org/10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robinson M, Daviskas E, Eberl S, Baker J, Chan H, Anderson S, Bye P. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J. 1999;14:678–685. doi: 10.1034/j.1399-3003.1999.14c30.x. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Airway goblet cells: responsive and adaptable front-line defenders. Eur Respir J. 1994;7:1690–1706. http://dx.doi.org/10.1183/09031936.94.07091678. [PubMed] [Google Scholar]
- Rogers DF. Mucoactive Agents for Airway Mucus Hypersecretory Diseases. Respir Care. 2007;52:1176–1197. [PubMed] [Google Scholar]
- Rowe SM, Miller S, Sorscher EJ. Cystic Fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. http://dx.doi.org/10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- Schütte A, Ermund A, Becker-Pauly C, Johansson MEV, Rodriguez-Pineiro AM, Bäckhed F, Müller S, Lottaz D, Bond JS, Hansson GC. Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proceedings of the National Academy of Sciences. 2014;111:12396–12401. doi: 10.1073/pnas.1407597111. http://dx.doi.org/10.1073/pnas.1407597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrave J, Albrecht H, Hill D, Rogers D, Solomon G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Resp Res. 2012;13:98. doi: 10.1186/1465-9921-13-98. http://dx.doi.org/10.1186/1465-9921-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Nash Edward F, Ratjen F, Tullis E, Stephenson A. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2013. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. http://dx.doi.org/10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper A, Jaques A, Charlton B. Inhaled mannitol in patients with cystic fibrosis: A randomised open-label dose response trial. J Cyst Fibros. 2011;10:1–8. doi: 10.1016/j.jcf.2010.08.020. http://dx.doi.org/10.1016/j.jcf.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Tildy BE, Rogers DF. Therapeutic Options for Hydrating Airway Mucus in Cystic Fibrosis. Pharmacology. 2015;95:117–132. doi: 10.1159/000377638. [DOI] [PubMed] [Google Scholar]
- van Doorninck JH, French PJ, Verbeek E, Peters HHPC, Morreau H, Bijman J, Scholte B. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J. 1995;14:4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]