To the Editor
Early life respiratory syncytial virus (RSV) bronchiolitis is a major risk factor for subsequent recurrent wheezing and asthma1, 2. The highest asthma risk occurs in infants with severe bronchiolitis requiring hospitalization3: up to 75% of hospitalized infants experience at least 3 additional wheezing episodes and almost 50% are diagnosed with asthma by the age of 7 yrs1.
We recently reported the results of a proof-of-concept trial, “Azithromycin for the Prevention of Recurrent Wheezing following RSV Bronchiolitis (APW-RSV)”, in which azithromycin (AZM) treatment of infants hospitalized with RSV bronchiolitis resulted in a lower likelihood of subsequent recurrent wheezing4. Anti-inflammatory effects of AZM were detected in that study: relative to placebo, AZM treatment resulted in a greater decline in nasal lavage IL-8 levels by day 15 (p=0.03)4. However, other mechanisms through which AZM may exert this protective effect remain uncertain. Previous laboratory investigations have suggested that macrolides have in-vitro anti-viral activity, as pre-treatment of ex vivo human respiratory epithelial cells with AZM5 or clarithromycin6 reduced replication and release of RSV6 and rhinovirus5. In addition, AZM pre-treatment reduces rhinovirus replication in cystic fibrosis bronchial epithelial cells7. However, potential in vivo anti-viral effects of macrolides have not been investigated in humans infected with respiratory viruses. Therefore, we hypothesized that our finding of AZM's reduction in post-RSV recurrent wheeze4 was mediated, at least in part, by anti-viral activity.
In this current study, we aimed to address two research questions. Does AZM have antiviral activity as demonstrated by lower RSV loads in serial nasal lavage samples obtained during RSV bronchiolitis? Does this potential anti-viral activity mediate the reduction in recurrent wheeze detected in our proof-of-concept trial?
A detailed description of the APW-RSV study has been reported elsewhere4. Briefly, this was a randomized, double-masked, placebo-controlled, proof-of-concept trial involving 40 infants with RSV bronchiolitis. Eligible infants were 1-18 months of age, otherwise healthy, and hospitalized for the first episode of bronchiolitis with confirmed RSV infection. Infants with history of prematurity were excluded. Study treatments were either AZM oral suspension 10 mg/kg once daily for 7 days, followed by 5 mg/kg once daily for 7 additional days, or an oral placebo suspension. AZM therapy was well tolerated, as mild gastrointestinal adverse events (diarrhea, vomiting, or abdominal pain) during the active treatment phase were recorded in 15 children (7 azithromycin, 8 placebo); none warranted discontinuation of study medication4. The study protocol was approved by the Washington University Institutional Review Board. Participants' parents provided written informed consent, and a data and safety monitoring board monitored the study.
RSV load was measured in nasal lavage samples obtained on randomization, day 8, and day 15, using the RealStar RSV A/B RT-PCR Kit, Altona Diagnostics (Hamburg, Germany). The occurrence of wheezing episodes was assessed as we have previously done1. The log-transformed RSV loads at three time points were compared between the two treatment groups using mixed-model repeated-measures analysis of covariance with the baseline viral load and duration of respiratory symptoms prior to randomization as covariates.
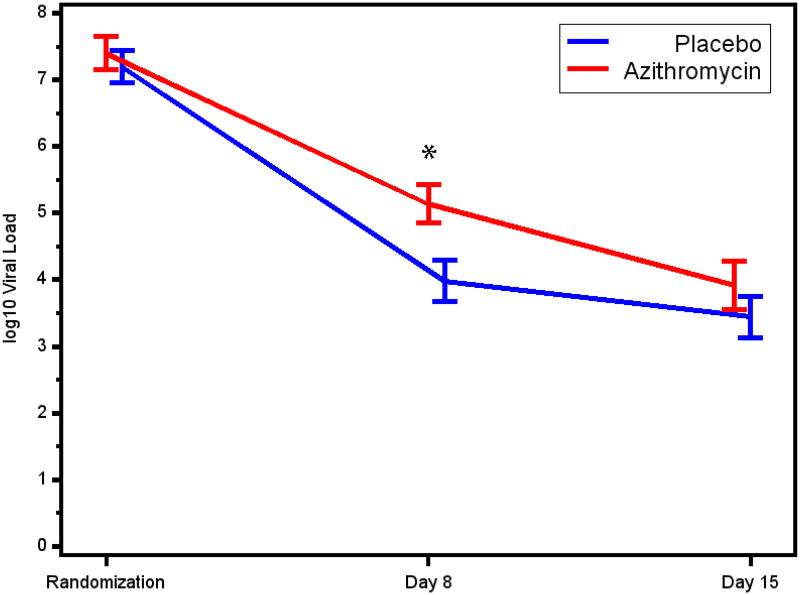
Complete sets of nasal wash samples obtained before (at randomization), during (day 8), and after (day 15) the study treatments were available for 39 participants (19 AZM, 20 placebo). Study participants were 3.8±2.9 months of age, and 59% were males. Other characteristics of study participants are described in Table 1. No significant differences were noted in baseline characteristics between the treatment groups. Nasal RSV loads during the study period are presented in Figure 1. Mean RSV loads at randomization did not differ between the treatment groups (p=0.80). Mean viral loads declined in both groups from randomization to day 8, and further to day 15. Compared to placebo, the AZM group had a higher RSV load on day 8 (p<0.01), whereas RSV loads were comparable between groups on day 15 (p=0.33).
Table 1. Baseline characteristicsa of the APW-RSV study participants.
| All participants | Azithromycin (n=19) | Placebo (n=20) | |
|---|---|---|---|
| Age at enrollment (months)b | 3.8 (2.9) | 3.7 (3.7) | 3.9 (2.0) |
|
| |||
| Gender (male) | 59.0% | 47.4% | 70.0% |
|
| |||
| Race (Caucasian) | 64.1% | 63.2% | 65.0% |
|
| |||
| Birth weight (kg) b | 3.4 (0.5) | 3.3 (0.6) | 3.4 (0.4) |
|
| |||
| Length of pregnancy (wks) a | 38.8 (1.4) | 38.9 (1.3) | 38.7 (1.4) |
|
| |||
| Maternal smoking during pregnancy | 7.7% | 10.5% | 5.0% |
|
| |||
| History of breast feeding | 28.2% | 31.6% | 25.0% |
|
| |||
| Tobacco smoke exposure | 35.9% | 42.1% | 30% |
|
| |||
| Pet exposure | 61.5% | 63.1% | 60.0% |
|
| |||
| History of eczema | 17.9% | 21.1% | 15.0% |
|
| |||
| Parental history of asthma | 41.0% | 42.1% | 40.0% |
|
| |||
| Duration of respiratory symptoms prior to randomization (days) b | 5.4 (1.4) | 5.2 (1.3) | 5.5 (1.4) |
|
| |||
| Duration of hospitalization (hours) b | 63.4 (53.2) | 58.0 (41.4) | 68.5 (63.2) |
|
| |||
| Lowest O2 saturation on room airb | 90.8 (4.3) | 91.4 (4.9) | 90.3 (3.6) |
|
| |||
| RSV type: | |||
| RSV A | 77% | 70% | 84% |
| RSV B | 23% | 30% | 16% |
Data are expressed as proportion of children in each group except as noted.
p>0.05 for the baseline characteristics comparisons between the treatment groups.
Data represents the mean (SD).
Figure 1.
Mean log10 RSV nasal lavage loads on randomization, day 8, and day 15 among the APW-RSV trial participants. * p<0.01.
In this clinical scenario, AZM therapy did not exert anti-viral effects in terms of accelerating the reduction in RSV load among infants with severe bronchiolitis. AZM therapy was associated with less RSV clearance from the airway, albeit in a transient and time-dependent manner, as higher RSV loads were measured in the AZM group on day 8, while comparable RSV loads were measured in the AZM and placebo groups on day 15. The reason for this unexpected finding is unknown, but we speculate that it might be related to the presumptive anti-inflammatory effects of AZM, which could result in reduced levels of anti-viral mediators. Nevertheless, the effect of the intervention was very temporary, as comparable RSV loads were measured in the two groups on day 15. To our knowledge, this is the first study investigating the potential in-vivo anti-viral activity of macrolide in humans infected with respiratory viruses. The differences between our findings and previous reports describing ex-vivo anti-viral activity of macrolides5-7 could be related to difference in timing of interventions, as the ex- vivo studies used pre-treatment of the epithelial cell before the infection, while our clinical trial involved treatment during ongoing RSV infection4. Therefore, a follow up study may incorporate an earlier initiation of macrolide therapy.
Our measured endpoint for the antiviral effect was the level of RSV RNA as measured by RT-PCR. While quantitative RT-PCR has been found to correlate with the level of infectious virus8, it is possible but not likely that direct measurement of viable virus by quantitative culture might have shown an effect that was missed by the measurement of viral RNA.
Previous reports correlated higher RSV load in respiratory secretions, measured during the first 3 days of hospitalization9 or within 2-4 days from onset of symptoms10, with more severe courses of acute bronchiolitis. We found no significant difference in the duration of hospitalization between treatment groups (mean (SD) 58.0 (41.4) hrs in AZM group vs. 68.5 (63.2) hrs in placebo group, p=0.54). Our results do not contradict these previous reports since at randomization, at a mean of 5.5 days into the illness (Table 1), viral load did not differ between the treatment groups. The transient increase in RSV load in the AZM group was noted only in the recovery phase (day 8) and likely did not affect acute bronchiolitis course. However, as we did not perform serial daily measurements of RSV loads, it remains unknown whether peak RSV load among our study participants was associated with acute bronchiolitis severity.
Viral load measurements were performed on upper airway samples since technical and ethical limitations precluded obtaining lower respiratory tract samples in young infants. We recognize upper airway samples may not directly represent the viral load in the lower airway. Nevertheless, we believe that if azithromycin had an antiviral effect, it would be evident in either the upper or lower airway. In addition, previous reports9, 10 support the premise that RSV load measurements in the upper airway are informative as these were related to acute disease severity, and thus may serve as a valuable surrogate of lower airway viral load.
A major strength of our study is the use of a homogenous study population, as we enrolled only otherwise healthy full-term infants experiencing acute RSV bronchiolitis and the prospective collection of biological samples and clinical outcomes in a well-characterized cohort. The relatively small sample size is a limitation; nevertheless, the detection of significant difference in RSV load on day 8 suggests adequate power to detect differences in viral loads between the treatment groups. However, a definitive exclusion of anti-viral properties of macrolides will require confirmation in a larger cohort. In average, the AZM treatment was initiated at day 6 of illness; therefore, we cannot completely exclude that earlier initiation of therapy would demonstrate an antiviral effect. While the homogeneity of our study population is strength of this study, it is also possible that an antiviral effect might be evident in different populations, including other age or ethnic groups. In addition, it is possible that an antiviral effect might be more evident in certain RSV subtypes that might not have been represented in our study population. Finally, while AZM does not appear to exert anti-viral effects in this population, the mechanism(s) by which macrolides provide anti-inflammatory effects remains uncertain. Future studies including a non-macrolide antibiotic may be helpful in elucidating whether these effects are independent of antimicrobial actions.
In summary, AZM therapy in infants with severe bronchiolitis did not facilitate RSV clearance from the upper airway. As such, we conclude that the beneficial effect of AZM on the reduction in the occurrence of post-RSV recurrent wheeze noted in our proof-of-concept study4 was not mediated by direct anti-viral activity of AZM, and may have been mediated by other mechanisms, such as anti-inflammatory effects and airway microbiome modification.
Acknowledgments
Source of funding: Supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences- sub award KL2 TR000450, and the Children's Discovery Institute of Washington University and St. Louis Children's Hospital. Supported in part (REDCap data base) by the CTSA Grant UL1 TR000448 and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Abbreviations
- AZM
azithromycin
- RSV
Respiratory syncytial virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100 e3. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol. 2013;13:211–6. doi: 10.1097/ACI.0b013e32835eb6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–8 e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36:646–54. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 6.Asada M, Yoshida M, Suzuki T, Hatachi Y, Sasaki T, Yasuda H, et al. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antiviral Res. 2009;83:191–200. doi: 10.1016/j.antiviral.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Schogler A, Kopf BS, Edwards MR, Johnston SL, Casaulta C, Kieninger E, et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45:428–39. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 8.Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol. 2003;41:4160–5. doi: 10.1128/JCM.41.9.4160-4165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houben ML, Coenjaerts FE, Rossen JW, Belderbos ME, Hofland RW, Kimpen JL, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–71. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]