Abstract
Nicotinic acetylcholine receptors (nAChRs) are expressed widely in the CNS, and mediate both synaptic and perisynaptic activities of endogenous cholinergic inputs and pharmacological actions of exogenous compounds (e.g. nicotine and choline). Behavioral studies indicate that nicotine improves such cognitive functions as learning and memory, however the cellular mechanism of these actions remains elusive. With help from newly developed biosensors and optogenetic tools, recent studies provide new insights on signaling mechanisms involved in the activation of nAChRs. Here we will review α7 nAChR’s action in the tri-synaptic pathway in the hippocampus. The effects of α7 nAChR activation via either exogenous compounds or endogenous cholinergic innervation are detailed for spontaneous and evoked glutamatergic synaptic transmission and synaptic plasticity, as well as the underlying signaling mechanisms. In summary, α7 nAChRs trigger intracellular calcium rise and calcium-dependent signaling pathways to enhance glutamate release and induce glutamatergic synaptic plasticity.
Graphical abstract

1. Introduction
Nicotinic acetylcholine receptors (nAChRs) belong to the superfamily of cys-loop cationic ligand-gated channels (Jones et al., 1999; Dajas-Bailador & Wonnacott, 2004; Dani & Bertrand, 2007; Albuquerque et al., 2009a). They are activated by the endogenous ligand acetylcholine (ACh) and various exogenous ligands (e.g. nicotine and choline) to exert their modulatory function on synaptic excitability and plasticity (Dani & Bertrand, 2007; Yakel, 2012; 2013). The α7 nAChR subtype is prominent in the hippocampus, and has been linked with cognitive deficits and a variety of neurological disorders and diseases, such as Alzheimer's diseases and schizophrenia (Freedman & Goldowitz, 2010; Yakel, 2013; Dineley et al., 2015; Sadigh-Eteghad et al., 2015; Sinkus et al., 2015a). Schizophrenic patients are often heavy smokers (de Leon & Diaz, 2005), which has been suggested to be a form of self-medication to mitigate their cognitive dysfunctions (Levin et al., 1996). The β-amyloid peptide (Aβ1–42 or Aβ peptide), a pathological hallmark of Alzheimer's disease, has been shown to affect α7 nAChR’s function (Wang et al., 2000; Liu et al., 2001; Pettit et al., 2001; Lamb et al., 2005). Moreover, significant loss of α7 nAChR protein density was observed in the hippocampus of both traumatic brain injury and prenatal restraint stress animal models (Hoffmeister et al., 2011; Baier et al., 2015). Either activation of the α7 nAChR with agonists, or potentiation with positive allosteric modulators (PAMs) (e.g. NS-1738), has been shown to improve hippocampal-dependent learning and memory (Bitner et al., 2007; Timmermann et al., 2007; Yaguchi et al., 2009; Blake et al., 2014). Conversely, genetic deletion and pharmacological inhibition of the α7 nAChR in the hippocampus results in significant learning and memory impairments (Levin et al., 2002; Fernandes et al., 2006; Nott & Levin, 2006; Young et al., 2007; Levin et al., 2009), and worsens the learning and memory deficits in a mouse model of Alzheimer’s disease (Hernandez et al., 2010). Therefore, it is critical to understand the functional properties of nAChRs in regulating hippocampal circuitry and synaptic plasticity. Below, we will briefly review recent advances, with a particular focus on α7 nAChRs (although α4β2 nAChRs also play an important role), in the regulation of glutamatergic synaptic transmission in the hippocampal formation.
2. α7 nAChR properties and expression in the hippocampus
The nAChRs are widely expressed throughout the brain in both neurons and non-neuronal cells, and participate in a variety of physiological functions (McQuiston & Madison, 1999; Nashmi & Lester, 2006; Shen & Yakel, 2012). In the mammalian brain, there are six α (2 – 10) and three β (2 – 4) nAChR subunits, which have been shown to form either hetero- or homopentameric nAChRs (Wada et al., 1989; Sargent, 1993; Jones & Yakel, 1999; Alkondon & Albuquerque, 2004; Albuquerque et al., 2009b). The most prevalent nAChRs in the hippocampus are comprised of α7 and α4β2 subtypes (Jones et al., 1999; Alkondon & Albuquerque, 2004; Albuquerque et al., 2009a). While α7 subunits were initially thought to form mainly homopentameric α7 receptors, they have been shown to co-assemble with other subunits to form functionally distinct native α7-containing receptors (Anand et al., 1993; Yu & Role, 1998; Palma et al., 1999; Shao & Yakel, 2000; Liu et al., 2009; Murray et al., 2012; Mowrey et al., 2013). Initially, we found that α7 and β2 subunits co-assembled in vitro (Khiroug et al., 2002); subsequently it was found that basal forebrain cholinergic neurons express functional native α7β2 receptors with an enhanced sensitivity to the amyloid-β (Aβ) peptide associated with AD (Liu et al., 2009). Physiological studies have shown that α7 nAChRs have lower affinity for acetylcholine, faster desensitization kinetics, and higher calcium permeability than α4β2 receptors (Bertrand et al., 1993; Fayuk & Yakel, 2005; Albuquerque et al., 2009a). Because of its high calcium permeability, α7 nAChRs can function similar to NMDA receptors in the modulation of synaptic plasticity.
In the hippocampal formation, distribution of mRNA for α7 nAChRs, as well as α-bungarotoxin (α-BTX) binding sites (which label α7 nAChRs), are widespread throughout the dentate gyrus (DG), CA3 and CA1 regions (Sudweeks & Yakel, 2000; Fabian-Fine et al., 2001; Adams et al., 2002b). Functional α7 nAChR-mediated currents have also been reported in CA3 and CA1 pyramidal neurons (Ji et al., 2001; Grybko et al., 2011; Gu et al., 2012), mouse dentate granule cells (John et al., 2015) and GABAergic interneurons (Jones & Yakel, 1999). Besides neuronal expression, α7 nAChRs are expressed on non-neuronal cells such as astrocytes in the hippocampal CA1 region (Shen & Yakel, 2012), which could play a role in neuroprotection and inflammation (Shen & Yakel, 2009). Ultrastructural studies in the CA1 region revealed immuno-gold particle labeling of α7 nAChRs at both pre- and postsynaptic compartments of both GABAergic and glutamatergic synapses (Fabian-Fine et al., 2001). α7 nAChRs located on presynaptic terminals can regulate release of other neurotransmitters (Jones et al. 1999; Dajas-Bailador & Wonnacott, 2004; Dani & Bertrand, 2007), while α7 nAChRs located on postsynaptic membranes mediate cholinergic synaptic transmission. Taking advantage of their high calcium permeability, functional α7 nAChRs can be mapped with sensitive calcium dyes and genetically-encoded calcium indicators, which provide complimentary information as to the locations of functional receptors beyond the reach of traditional patch-clamp studies. The calcium rise mediated by functional α7 nAChRs has been shown in dendrites of CA1 (Fayuk & Yakel, 2005; 2007a) and CA3 pyramidal neurons (Grybko et al., 2011), and dentate granule cells at their mossy fiber terminals (Cheng & Yakel, 2014). Optical recordings revealed that the amplitude of the α7 nAChR-mediated calcium response was significantly larger in the dendrites (than in the soma) despite smaller α7 nAChR current responses (Fayuk & Yakel, 2007a), suggesting that functional α7 nAChRs cluster differentially around neuronal compartments (Kawai et al., 2002). With the aid of newly developed α7 PAMs, we observed α7 nAChR-mediated calcium rise via GCaMP3 imaging in giant mossy fiber terminals (Cheng & Yakel, 2014), which is consistent with the study using the calcium dye Fura-2 (Gray et al., 1996). In addition, α7 nAChRs also distribute perisynaptically to mediate modulating effects of non-synaptically released ACh (Kasa et al., 1995; Dani & Bertrand, 2007; Albuquerque et al., 2009a; Shen & Yakel, 2009). The density and distribution of α7 nAChRs changes markedly during early development (Adams et al., 2002a; Adams, 2003), and differs among different mouse strains and species (Rubboli et al., 1994; Adams et al., 2006). Moreover, genetic polymorphism of the α7 nAChR gene (CHRNA7) linked to schizophrenia generated a partially duplicated α7 nAChR (CHRFAMA7A, dupα7) subunit either with or without a 2-bp deletion (CHRFAMA7A-Δ2bp, dupΔα7) (Gault et al., 2003; Sinkus et al., 2009), which could change the properties of the α7 nAChR and modify neuronal connections (Sinkus et al., 2015b). An in vitro study showed that both dupα7 and dupΔα7 co-assemble with the α7 subunit to form functional receptors with normal sensitivity to ACh and lower sensitivity to choline, though the calcium permeability of these mutated α7 receptors remains unclear (Wang et al., 2014).
3. The effect of α7 nAChR’s activation by exogenous agonists
Many groups have studied how α7 nAChR activation modulates glutamatergic synaptic transmission with nicotine or selective agonists to activate α7 nAChRs in the hippocampus. We will focus mainly on the direct α7 nAChR-mediated effect on glutamatergic synapses, and not the indirect impact derived from α7 nAChR activation on GABAergic neurons, which also can modulate synaptic plasticity in pyramidal cells.
For spontaneous glutamate release, acute nicotine application increased the frequency of spontaneous miniature EPSCs from dissociated hippocampal neurons (Gray et al., 1996; Radcliffe & Dani, 1998), and CA1 (Le Magueresse et al., 2006) and CA3 pyramidal neurons (Sharma & Vijayaraghavan, 2003); this suggests a presynaptic location of nicotine’s action. The nicotine-induced frequency increase of mEPSCs was blocked by MLA (an α7-selective antagonist) (Gray et al., 1996), and absent in α7 knockout mice (Le Magueresse et al., 2006), suggesting that α7 nAChRs mediated this enhancement. Moreover, a brief application of nicotine converted presynaptically silent synapses into conductive ones at immature Schaffer collateral-CA1 connections (Maggi et al., 2003). Besides its modulatory effect on mEPSC frequency, activation of α7 nAChRs could directly trigger glutamate release from mossy fiber terminals (Sharma et al., 2008). The direct measurement of glutamate release from hippocampal synaptosomes showed significant release upon choline application (Zappettini et al., 2010), which is consistent with electrophysiological findings. In vivo extracellular recordings from CA3 pyramidal neurons showed robust increases in firing induced by nicotine and blocked by MLA; this was found to be due to enhanced glutamate release (Huang et al., 2010). The α7 nAChR’s effect on spontaneous glutamate release occurred within seconds after agonist application and lasted for several minutes after agonist removal, suggesting that both immediate calcium rise through α7 nAChR activation and longer-term calcium-dependent signaling cascades are involved.
For the evoked release of glutamate, α7 nAChR activation can increase the amplitude of evoked EPSCs in cultured hippocampal neurons (Radcliffe & Dani, 1998), for CA3 to CA1 pyramidal neuron synapses, DG to CA3 synapses, and perforant path to DG glutamate synapses (Sola et al., 2006; Ondrejcak et al., 2012; Cheng & Yakel, 2014). The increase in amplitude was associated with a significant increase in the probability of release along with a significant decrease of the PPR (paired-pulse ratio) (Sola et al., 2006). Our study (Cheng & Yakel, 2014) of CA3 pyramidal neurons showed that α7 nAChR activation enhanced eEPSC amplitude, which persisted for 5-10 minutes after the removal of agonist. Similar long-lasting effects of nAChR agonists on neurotransmitter release were observed by others as well (Lena & Changeux, 1997; Zhong et al., 2008). Studies from both spontaneous and evoked glutamatergic synaptic transmission showed that presynaptic α7 nAChRs mediated enhancement of glutamate release, suggesting that α7 nAChRs could be activating the same calcium-dependent signaling cascades to sustain the nicotinic enhancement of glutamate release. In the prefrontal cortex, nicotine induced an increase in frequency and amplitude of spontaneous EPSCs, but did not affect the amplitude of evoked glutamatergic transmission from layer II/III to layer V pyramidal neurons (Couey et al., 2007), suggesting that multiple calcium-dependent pathways could be involved in its action.
Hippocampal LTP is a well-accepted cellular model for learning and memory. The effect of α7 nAChR’s activation on synaptic plasticity is dictated by the timing of its activation and electrical stimulation on glutamatergic synaptic currents. When α7 nAChR agonists were bath perfused, the activation of α7 nAChRs facilitated the induction of LTP (Hunter et al., 1994; Fujii et al., 1999), and enhanced both early and late LTP (Kroker et al., 2011) in the CA1 hippocampal region. When α7 nAChRs were activated via brief application of ACh, which coincided or preceded (by 1-5 s) high frequency stimulation (HFS), this produced LTP under mild presynaptic stimulation conditions at CA1 pyramidal neurons (Ji, 2001). Outside of these time frames, the mismatch of nAChR activity and stimulation led to short-term potentiation (STP) (Ge & Dani, 2005). The α7 nAChR-mediated enhancement of LTP was due to its ability to increase presynaptic release of glutamate and postsynaptic depolarization. Activation of α7 nAChRs has also been shown to suppress LTD induction at CA3-CA1 synapses (Fujii & Sumikawa, 2001; Nakauchi & Sumikawa, 2014). In the DG, acute α7 nAChR activation enhanced HFS-induced LTP, and α7 nAChR PAMs reduced the threshold for LTP induction, an effect which required ERK and PKA activity (Welsby et al., 2009). For mossy fiber LTP, α7 nAChR agonists rescued LTP that was deficient in BACE1 knock-out mice, but had very little effect on LTP in wildtype mice (Wang et al., 2010). The rescue by α7 nAChR agonists was abolished by blockers of ryanodine-sensitive calcium stores, suggesting that calcium-induced calcium release played a key role in this regulation. However, the effect of α7 nAChR activation on STP needs to be tested on mossy fiber transmission. These studies also pointed to a calcium rise induced by α7 nAChR activation as the central mechanism for these actions, and α7 nAChR activation also triggers differential signaling molecules to produce various influences in the hippocampus.
4. α7 activation via cholinergic synapses in the hippocampus
Under physiological conditions, α7 nAChRs are activated by endogenous ACh released from cholinergic neurons. The primary cholinergic input to the hippocampus arises from the medial septum and diagonal band of Broca (Amaral & Kurz, 1985; Dutar et al., 1995), although a small population of cholinergic interneurons do exist in the hippocampus as recently reconfirmed from ChAT-tauGFP and ChAT-CRE/Rosa-YFP transgenic mice (Yi et al., 2015). Initial studies using electrical cholinergic stimulation to dissect the function of endogenous ACh showed prominent muscarinic receptor’s effect of Schaffer collateral input to CA1 pyramidal neurons (Fernandez de Sevilla & Buno, 2003). The α7 nAChR-dependent modulation of synaptic plasticity of CA3-CA1 glutamatergic synapses was uncovered with localized application of ACh, which is sensitive to the location and timing of α7 nAChR activation (Ji et al., 2001; Ge & Dani, 2005). The development of optogenetic techniques allows both cell-type specific and temporally precise stimulation of cholinergic inputs to the hippocampus. Taking advantage of this approach, our lab investigated how activation of endogenous cholinergic inputs may regulate hippocampal synaptic plasticity (Gu & Yakel, 2011). We found that α7 nAChRs mediated two forms of hippocampal synaptic plasticity with a timing precision in the millisecond range. When the cholinergic input to the CA1 hippocampal region was activated 100 ms prior to activation of the Schaffer collateral (SC) pathway, this induced an α7 nAChR-dependent form of LTP. When the cholinergic input was activated only 10 ms prior to the SC pathway, this induced an α7 nAChR-dependent short-term depression (STD) that was mediated primarily through the presynaptic inhibition of glutamate release. Furthermore, we observed α7 nAChR-dependent enhancement of calcium responses during this LTP both pre- and post synaptically, and short-term depression of calcium responses during STD (Gu et al., 2012). In aged α7-nAChR KO mice, both evoked field synaptic potentials and LTP were significantly reduced in hippocampal CA3-CA1 synapses (Ma et al., 2014), suggesting that cholinergic modulation of LTP required the activation of α7 nAChRs.
5. Second messenger signaling upon activation of α7 nAChRs-
The α7 nAChRs have a high calcium permeability, comparable to that of NMDA receptors (Seguela et al., 1993; Uteshev, 2012); this confers the ability of α7 nAChRs to produce long-lasting modulations of synaptic transmission (Fagen et al., 2003; McKay et al., 2007). The intracellular calcium rise alters the amount of neurotransmitter release directly (Neher & Sakaba, 2008), and triggers multiple signaling cascades to modify the efficacy of synaptic transmission. Indeed, we observed a prominent calcium rise upon activation of α7 nAChRs at mossy fiber terminals (using the genetically-encoded calcium sensor GCaMP3), and found that the peak of the action potential (AP)-evoked calcium transient was enhanced by the α7 nAChR agonist PNU-282987 (Cheng & Yakel, 2014; 2015). Similar actions of presynaptic α7 nAChRs on AP-evoked calcium transients were also reported in CA1 pyramidal neurons (Szabo et al., 2008). Changes in the AP-induced calcium transients would lead to changes in the amount of neurotransmitter released at presynaptic terminals (Jackson et al., 1991). The source of the calcium increase would be mainly comprised of the calcium influx directly through the α7 nAChR itself (Fayuk & Yakel, 2007a; Gilbert et al., 2009), and any calcium-induced calcium release from intracellular calcium stores (Weisskopf & Nicoll, 1995; Sharma & Vijayaraghavan, 2003; Sharma et al., 2008). Although calcium influx through voltage-gated calcium channels (VGCC) previously was shown not to contribute significantly to the α7 nAChR-mediated presynaptic effect (Dickinson et al., 2008), other studies reported that calcium influx through VGCCs could contribute to some degree (Barrantes et al., 1995; Fayuk & Yakel, 2007b; del Barrio et al., 2011). In addition, recent studies showed that α7 nAChR activation enhanced NMDAR-mediated whole-cell currents and glutamate release from hippocampal synaptosomes (Zappettini et al., 2010; Li et al., 2013). Lastly, membrane depolarization induced by α7 nAChR activation could release the magnesium blockade of NMDARs to reduce the threshold for NMDAR-dependent synaptic plasticity.
Although activation of the α7 nAChRs induces transient calcium rises, the kinetics of this do not explain the long-lasting effects caused by α7 nAChR agonists on synaptic plasticity (Lena & Changeux, 1997; Zhong et al., 2008; Zhong et al., 2013). Many calcium signaling cascades have been linked to α7 nAChRs, including the CaMKII (Sharma et al., 2008) and cAMP-PKA pathways (Dajas-Bailador et al., 2002; Welsby et al., 2009). Recently, we reported that the action of α7 nAChRs at mossy fiber terminals depended on PKA activity (Cheng & Yakel, 2014). Since it was shown that the α7 nAChR and adenylyl cyclase 1 (AC1) are associated physically and functionally in epithelium (Maouche et al., 2013), we investigated any connection between the activation of the α7 nAChR and cAMP signaling in hippocampal neurons. Using a FRET-based cAMP sensor, we found that activation of α7 nAChRs significantly increased intracellular cAMP levels. The cAMP rise was abolished by the α7 nAChR antagonist MLA, the calcium chelator BAPTA, the selective AC1 inhibitor CB-6673567, and siRNA-mediated AC1 knockdown; this suggested that the calcium-dependent AC1 is required for linking the activation of α7 nAChRs to a cAMP rise (Cheng & Yakel, 2015). We also found that the activation of α7 nAChRs leads to the widespread increase of phosphorylated synapsin I (via its PKA site), which is a prominent presynaptic PKA substrate (Huttner & Greengard, 1979) and can regulate neurotransmitter release and calcium-dependent synaptic plasticity (Hilfiker et al., 1999; Hilfiker et al., 2005; Menegon et al., 2006; Fiumara et al., 2007; Cousin & Evans, 2011). The PKA pathway was also shown to be required for the α7 nAChR-mediated enhancement of LTP in dentate granule cells (Welsby et al., 2009). Moreover, α7 nAChR activation was shown to interact with G proteins and G protein-regulated inducer of neurite outgrowth 1 to reduce axonal growth (Nordman & Kabbani, 2014; Nordman et al., 2014). With the advancement of new technologies, novel signaling pathways might be linked to α7 nAChRs to interpret its complex actions in the brain.
6.Significance and conclusion
The changes in α7 nAChR expression and density in the hippocampus have been observed in many cognitive impairment disorders, such as schizophrenia (Freedman & Goldowitz, 2010) and Alzheimer’s disease (Dani & Bertrand, 2007; Dineley et al., 2015; Sadigh-Eteghad et al., 2015). However, the physiological mechanism and consequences of these changes are still unclear, in terms of how these receptors regulate neuronal excitability, synaptic plasticity and brain circuit function. Furthermore, the outcome of α7 nAChR activation on the hippocampal circuit is complicated by the extensive distribution of α7 nAChRs on both glutamatergic and GABAergic neurons. With the newly-generated floxed α7 nAChR transgenic mouse (Hernandez et al., 2014) combining with Cre and Cre-ER dependent systems, selective deletion of α7 nAChRs from distinct cell populations at specific developmental stages will allow us to unravel systematically the impact of α7 nAChR activation on hippocampal network both in vitro and in vivo. This will also help us to understand how the alteration of α7 nAChR function will impact cognitive function.
The agonists and modulators of α7 nAChRs have been attractive candidates for developing therapeutic treatments for these neuronal disorders (Deutsch et al., 2013; Deutsch et al., 2014). Indeed, several drugs targeting α7 nAChRs demonstrated their potential in treating schizophrenic patients (Freedman et al., 2008; Preskorn et al., 2014; Keefe et al., 2015). With the development of improved biosensors and emerging tools such as optogenetics (combined with electrophysiology), we will continue to probe cellular and molecular mechanisms of α7 nAChR’s functions in neural circuits. The insights gained from these studies will lead to new treatments to mitigate cognitive impairments.
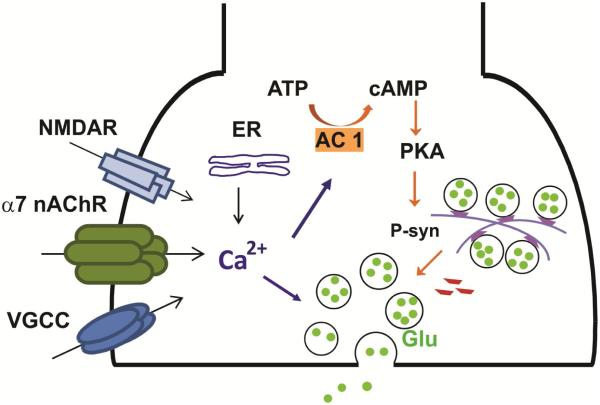
Figure 1.
Mechanisms of α7 nAChR-mediated presynaptic enhancement of glutamate release. The intracellular calcium level at presynaptic terminals is increased by calcium influx through α7 nAChRs, VGCCs, and NMDARs from the outside, and calcium release from intracellular calcium stores. The raised calcium levels could increase vesicular glutamate release directly, and trigger cAMP-PKA-dependent pathways for prolonged enhancement of glutamate release. Phosphorylation of synapsins by PKA leads to synaptic vesicle mobilization from the reserve pool to the readily releasable pool, hence increasing glutamate release. (Purple trapeziods depict unphosphorylated synapsins, while red trapezoids depict phosphorylated synapsins.)
Acknowledgements
We would like to thank Drs. Serena Dudek and Christian Erxleben for the helpful reading and suggestions on our manuscript. This research was funded by the NIEHS Intramural Research Program/NIH. The authors declare no competing financial interests.
Abbreviations
- ACh
acetylcholine
- DG
dentate gyrus
- HFS
high frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- nAChR
nicotinic ACh receptor
- STP
short-term potentiation
- PAM
positive allosteric modulator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CE. Comparison of alpha7 nicotinic acetylcholine receptor development in the hippocampal formation of C3H and DBA/2 mice. Brain Res Dev Brain Res. 2003;143:137–149. doi: 10.1016/s0165-3806(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002a;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002b;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Adams CE, Yonchek JC, Stitzel JA. Development of hippocampal alpha7 nicotinic receptors in C3H and DBA/2 congenic mice. Brain Res. 2006;1122:27–35. doi: 10.1016/j.brainres.2006.08.113. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009a;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009b;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Anand R, Peng X, Lindstrom J. Homomeric and native alpha 7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- Baier CJ, Pallares ME, Adrover E, Monteleone MC, Brocco MA, Barrantes FJ, Antonelli MC. Prenatal restraint stress decreases the expression of alpha-7 nicotinic receptor in the brain of adult rat offspring. Stress. 2015:1–11. doi: 10.3109/10253890.2015.1022148. [DOI] [PubMed] [Google Scholar]
- Barrantes GE, Murphy CT, Westwick J, Wonnacott S. Nicotine increases intracellular calcium in rat hippocampal neurons via voltage-gated calcium channels. Neurosci Lett. 1995;196:101–104. doi: 10.1016/0304-3940(95)11859-u. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MG, Krawczyk MC, Baratti CM, Boccia MM. Neuropharmacology of memory consolidation and reconsolidation: Insights on central cholinergic mechanisms. J Physiol Paris. 2014;108:286–291. doi: 10.1016/j.jphysparis.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL. Presynaptic alpha7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J Neurosci. 2014;34:124–133. doi: 10.1523/JNEUROSCI.2973-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL. Activation of alpha7 nicotinic acetylcholine receptors increases intracellular cAMP levels via activation of AC1 in hippocampal neurons. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Evans GJ. Activation of silent and weak synapses by cAMP-dependent protein kinase in cultured cerebellar granule neurons. J Physiol. 2011;589:1943–1955. doi: 10.1113/jphysiol.2010.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Soliakov L, Wonnacott S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem. 2002;80:520–530. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- del Barrio L, Egea J, Leon R, Romero A, Ruiz A, Montero M, Alvarez J, Lopez MG. Calcium signalling mediated through alpha7 and non-alpha7 nAChR stimulation is differentially regulated in bovine chromaffin cells to induce catecholamine release. Br J Pharmacol. 2011;162:94–110. doi: 10.1111/j.1476-5381.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Benson AD. Targeting the alpha nicotinic acetylcholine receptor to prevent progressive dementia and improve cognition in adults with Down's syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54C:131–139. doi: 10.1016/j.pnpbp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Schwartz BL, Schooler NR, Brown CH, Rosse RB, Rosse SM. Targeting alpha-7 nicotinic neurotransmission in schizophrenia: a novel agonist strategy. Schizophr Res. 2013;148:138–144. doi: 10.1016/j.schres.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36:96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen ZM, Mansvelder HD, Keath JR, McGehee DS. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann N Y Acad Sci. 2003;1003:185–195. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones. J Physiol. 2005;566:759–768. doi: 10.1113/jphysiol.2005.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Dendritic Ca2+ signalling due to activation of alpha 7-containing nicotinic acetylcholine receptors in rat hippocampal neurons. J Physiol. 2007a;582:597–611. doi: 10.1113/jphysiol.2007.135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Dendritic Ca2+ signalling due to activation of alpha 7-containing nicotinic acetylcholine receptors in rat hippocampal neurons. J Physiol. 2007b;582:597–611. doi: 10.1113/jphysiol.2007.135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes Brain Behav. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- Fernandez de Sevilla D, Buno W. Presynaptic inhibition of Schaffer collateral synapses by stimulation of hippocampal cholinergic afferent fibres. Eur J Neurosci. 2003;17:555–558. doi: 10.1046/j.1460-9568.2003.02490.x. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Milanese C, Corradi A, Giovedi S, Leitinger G, Menegon A, Montarolo PG, Benfenati F, Ghirardi M. Phosphorylation of synapsin domain A is required for post-tetanic potentiation. J Cell Sci. 2007;120:3228–3237. doi: 10.1242/jcs.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Goldowitz D. Studies on the hippocampal formation: From basic development to clinical applications: Studies on schizophrenia. Prog Neurobiol. 2010;90:263–275. doi: 10.1016/j.pneurobio.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. The American journal of psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Fujii S, Sumikawa K. Nicotine accelerates reversal of long-term potentiation and enhances long-term depression in the rat hippocampal CA1 region. Brain Res. 2001;894:340–346. doi: 10.1016/s0006-8993(01)02058-3. [DOI] [PubMed] [Google Scholar]
- Gault J, Hopkins J, Berger R, Drebing C, Logel J, Walton C, Short M, Vianzon R, Olincy A, Ross RG, Adler LE, Freedman R, Leonard S. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:39–49. doi: 10.1002/ajmg.b.20061. [DOI] [PubMed] [Google Scholar]
- Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Lecchi M, Arnaudeau S, Bertrand D, Demaurex N. Local and global calcium signals associated with the opening of neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium. 2009;45:198–207. doi: 10.1016/j.ceca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Grybko MJ, Hahm ET, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S. A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci. 2011;33:1786–1798. doi: 10.1111/j.1460-9568.2011.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Lamb PW, Yakel JL. Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity. J Neurosci. 2012;32:12337–12348. doi: 10.1523/JNEUROSCI.2129-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Cortez I, Gu Z, Colon-Saez JO, Lamb PW, Wakamiya M, Yakel JL, Dineley KT. Research tool: Validation of floxed alpha7 nicotinic acetylcholine receptor conditional knockout mice using in vitro and in vivo approaches. J Physiol. 2014;592:3201–3214. doi: 10.1113/jphysiol.2014.272054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Benfenati F, Doussau F, Nairn AC, Czernik AJ, Augustine GJ, Greengard P. Structural domains involved in the regulation of transmitter release by synapsins. J Neurosci. 2005;25:2658–2669. doi: 10.1523/JNEUROSCI.4278-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister PG, Donat CK, Schuhmann MU, Voigt C, Walter B, Nieber K, Meixensberger J, Bauer R, Brust P. Traumatic brain injury elicits similar alterations in alpha7 nicotinic receptor density in two different experimental models. Neuromolecular Med. 2011;13:44–53. doi: 10.1007/s12017-010-8136-4. [DOI] [PubMed] [Google Scholar]
- Huang LT, Sherwood JL, Sun YJ, Lodge D, Wang Y. Activation of presynaptic alpha7 nicotinic receptors evokes an excitatory response in hippocampal CA3 neurones in anaesthetized rats: an in vivo iontophoretic study. Br J Pharmacol. 2010;159:554–565. doi: 10.1111/j.1476-5381.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BE, de Fiebre CM, Papke RL, Kem WR, Meyer EM. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci Lett. 1994;168:130–134. doi: 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979;76:5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991;88:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- John D, Shelukhina I, Yanagawa Y, Deuchars J, Henderson Z. Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res. 2015;1601:15–30. doi: 10.1016/j.brainres.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Inhibitory interneurons in hippocampus. Cell Biochem Biophys. 1999;31:207–218. doi: 10.1007/BF02738173. [DOI] [PubMed] [Google Scholar]
- Kasa P, Hlavati I, Dobo E, Wolff A, Joo F, Wolff JR. Synaptic and non-synaptic cholinergic innervation of the various types of neurons in the main olfactory bulb of adult rat: immunocytochemistry of choline acetyltransferase. Neuroscience. 1995;67:667–677. doi: 10.1016/0306-4522(95)00031-d. [DOI] [PubMed] [Google Scholar]
- Kawai H, Zago W, Berg DK. Nicotinic alpha 7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci. 2002;22:7903–7912. doi: 10.1523/JNEUROSCI.22-18-07903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Meltzer HA, Dgetluck N, Gawryl M, Koenig G, Moebius HJ, Lombardo I, Hilt DC. Randomized, Double-Blind, Placebo-Controlled Study of Encenicline, an Alpha-7 Nicotinic Acetylcholine Receptor Agonist as a Treatment for Cognitive Impairment in Schizophrenia. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroker KS, Rast G, Rosenbrock H. Differential effects of subtype-specific nicotinic acetylcholine receptor agonists on early and late hippocampal LTP. Eur J Pharmacol. 2011;671:26–32. doi: 10.1016/j.ejphar.2011.09.167. [DOI] [PubMed] [Google Scholar]
- Lamb PW, Melton MA, Yakel JL. Inhibition of neuronal nicotinic acetylcholine receptor channels expressed in Xenopus oocytes by beta-amyloid1-42 peptide. J Mol Neurosci. 2005;27:13–21. doi: 10.1385/JMN:27:1:013. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Safiulina V, Changeux JP, Cherubini E. Nicotinic modulation of network and synaptic transmission in the immature hippocampus investigated with genetically modified mice. J Physiol. 2006;576:533–546. doi: 10.1113/jphysiol.2006.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Li S, Nai Q, Lipina TV, Roder JC, Liu F. alpha7nAchR/NMDAR coupling affects NMDAR function and object recognition. Mol Brain. 2013;6:58. doi: 10.1186/1756-6606-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kawai H, Berg DK. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci U S A. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Turner D, Zhang J, Wang Q, Wang M, Shen J, Zhang S, Wu J. Deficits of synaptic functions in hippocampal slices prepared from aged mice null alpha7 nicotinic acetylcholine receptors. Neurosci Lett. 2014;570:97–101. doi: 10.1016/j.neulet.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature "silent" connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maouche K, Medjber K, Zahm JM, Delavoie F, Terryn C, Coraux C, Pons S, Cloez-Tayarani I, Maskos U, Birembaut P, Tournier JM. Contribution of alpha7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc Natl Acad Sci U S A. 2013;110:4099–4104. doi: 10.1073/pnas.1216939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon A, Bonanomi D, Albertinazzi C, Lotti F, Ferrari G, Kao HT, Benfenati F, Baldelli P, Valtorta F. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J Neurosci. 2006;26:11670–11681. doi: 10.1523/JNEUROSCI.3321-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrey DD, Liu Q, Bondarenko V, Chen Q, Seyoum E, Xu Y, Wu J, Tang P. Insights into distinct modulation of alpha7 and alpha7beta2 nicotinic acetylcholine receptors by the volatile anesthetic isoflurane. J Biol Chem. 2013;288:35793–35800. doi: 10.1074/jbc.M113.508333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TA, Bertrand D, Papke RL, George AA, Pantoja R, Srinivasan R, Liu Q, Wu J, Whiteaker P, Lester HA, Lukas RJ. alpha7beta2 nicotinic acetylcholine receptors assemble, function, and are activated primarily via their alpha7-alpha7 interfaces. Mol Pharmacol. 2012;81:175–188. doi: 10.1124/mol.111.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi S, Sumikawa K. Endogenous ACh suppresses LTD induction and nicotine relieves the suppression via different nicotinic ACh receptor subtypes in the mouse hippocampus. Life Sci. 2014;111:62–68. doi: 10.1016/j.lfs.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. J Mol Neurosci. 2006;30:181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Kabbani N. Microtubule dynamics at the growth cone are mediated by alpha7 nicotinic receptor activation of a Galphaq and IP3 receptor pathway. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:2995–3006. doi: 10.1096/fj.14-251439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman JC, Phillips WS, Kodama N, Clark SG, Del Negro CA, Kabbani N. Axon targeting of the alpha 7 nicotinic receptor in developing hippocampal neurons by Gprin1 regulates growth. J Neurochem. 2014;129:649–662. doi: 10.1111/jnc.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A, Levin ED. Dorsal hippocampal alpha7 and alpha4beta2 nicotinic receptors and memory. Brain Res. 2006;1081:72–78. doi: 10.1016/j.brainres.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Ondrejcak T, Wang Q, Kew JN, Virley DJ, Upton N, Anwyl R, Rowan MJ. Activation of alpha7 nicotinic acetylcholine receptors persistently enhances hippocampal synaptic transmission and prevents Ass-mediated inhibition of LTP in the rat hippocampus. Eur J Pharmacol. 2012;677:63–70. doi: 10.1016/j.ejphar.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the alpha7 and beta3 subunits. J Biol Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. beta-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. Journal of psychiatric practice. 2014;20:12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F. Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci. 1994;6:1596–1604. doi: 10.1111/j.1460-9568.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Majdi A, Talebi M, Mahmoudi J, Babri S. Regulation of nicotinic acetylcholine receptors in Alzheimers disease: A possible role of chaperones. Eur J Pharmacol. 2015;755:34–41. doi: 10.1016/j.ejphar.2015.02.047. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Yakel JL. Single channel properties of neuronal nicotinic ACh receptors in stratum radiatum interneurons of rat hippocampal slices. J Physiol. 2000;527:507–513. doi: 10.1111/j.1469-7793.2000.00507.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Grybko M, Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J Neurosci. 2008;28:2563–2575. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30:673–680. doi: 10.1038/aps.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. Functional alpha7 nicotinic ACh receptors on astrocytes in rat hippocampal CA1 slices. J Mol Neurosci. 2012;48:14–21. doi: 10.1007/s12031-012-9719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkus ML, Graw S, Freedman R, Ross RG, Lester HA, Leonard S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology. 2015a doi: 10.1016/j.neuropharm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkus ML, Graw S, Freedman R, Ross RG, Lester HA, Leonard S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology. 2015b;96:274–288. doi: 10.1016/j.neuropharm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkus ML, Lee MJ, Gault J, Logel J, Short M, Freedman R, Christian SL, Lyon J, Leonard S. A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;1291:1–11. doi: 10.1016/j.brainres.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola E, Capsoni S, Rosato-Siri M, Cattaneo A, Cherubini E. Failure of nicotine-dependent enhancement of synaptic efficacy at Schaffer-collateral CA1 synapses of AD11 anti-nerve growth factor transgenic mice. Eur J Neurosci. 2006;24:1252–1264. doi: 10.1111/j.1460-9568.2006.04996.x. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SI, Zelles T, Vizi ES, Lendvai B. The effect of nicotine on spiking activity and Ca2+ dynamics of dendritic spines in rat CA1 pyramidal neurons. Hippocampus. 2008;18:376–385. doi: 10.1002/hipo.20401. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Gronlien JH, Kohlhaas KL, Nielsen EO, Dam E, Jorgensen TD, Ahring PK, Peters D, Holst D, Christensen JK, Malysz J, Briggs CA, Gopalakrishnan M, Olsen GM. An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Uteshev VV. alpha7 nicotinic ACh receptors as a ligand-gated source of Ca(2+) ions: the search for a Ca(2+) optimum. Adv Exp Med Biol. 2012;740:603–638. doi: 10.1007/978-94-007-2888-2_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang H, Song L, Lee A, Laird F, Wong PC, Lee HK. Mossy fiber long-term potentiation deficits in BACE1 knock-outs can be rescued by activation of alpha7 nicotinic acetylcholine receptors. J Neurosci. 2010;30:13808–13813. doi: 10.1523/JNEUROSCI.1070-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiao C, Indersmitten T, Freedman R, Leonard S, Lester HA. The duplicated alpha7 subunits assemble and form functional nicotinic receptors with the full-length alpha7. J Biol Chem. 2014;289:26451–26463. doi: 10.1074/jbc.M114.582858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. Eur J Neurosci. 2009;29:65–75. doi: 10.1111/j.1460-9568.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- Yaguchi T, Nagata T, Nishizaki T. Dilinoleoylphosphatidylcholine ameliorates scopolamine-induced impairment of spatial learning and memory by targeting alpha7 nicotinic ACh receptors. Life Sci. 2009;84:263–266. doi: 10.1016/j.lfs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Yakel JL. Nicotinic ACh receptors in the hippocampus: role in excitability and plasticity. Nicotine Tob Res. 2012;14:1249–1257. doi: 10.1093/ntr/nts091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL. Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch. 2013;465:441–450. doi: 10.1007/s00424-012-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Catudio-Garrett E, Gabriel R, Wilhelm M, Erdelyi F, Szabo G, Deisseroth K, Lawrence J. Hippocampal "cholinergic interneurons" visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Frontiers in synaptic neuroscience. 2015;7:4. doi: 10.3389/fnsyn.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the alpha7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol. 1998;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappettini S, Grilli M, Salamone A, Fedele E, Marchi M. Pre-synaptic nicotinic receptors evoke endogenous glutamate and aspartate release from hippocampal synaptosomes by way of distinct coupling mechanisms. Br J Pharmacol. 2010;161:1161–1171. doi: 10.1111/j.1476-5381.2010.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J Neurosci. 2008;28:9111–9116. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Talmage DA, Role LW. Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PLoS One. 2013;8:e82719. doi: 10.1371/journal.pone.0082719. [DOI] [PMC free article] [PubMed] [Google Scholar]