Abstract
The precise mechanisms by which cocaine and amphetamine-like psychostimulants exert their reinforcing effects are not yet fully defined. It is widely believed, however, that these drugs produce their effects by enhancing dopamine neurotransmission in the brain, especially in limbic areas such as the nucleus accumbens, by inducing dopamine transporter-mediated reverse transport and/or blocking dopamine reuptake though the dopamine transporter. Here, we present the evidence that aside from dopamine transporter, non-dopamine transporter-mediated mechanisms also participate in psychostimulant-induced dopamine release and contribute to the behavioral effects of these drugs, such as locomotor activation and reward. Accordingly, psychostimulants could increase norepinephrine release in the prefrontal cortex, the latter then alters the firing pattern of dopamine neurons resulting in changes in action potential-dependent dopamine release. These alterations would further affect the temporal pattern of dopamine release in the nucleus accumbens, thereby modifying information processing in that area. Hence, a synaptic input to a nucleus accumbens neuron may be enhanced or inhibited by dopamine depending on its temporal relationship to dopamine release. Specific temporal patterns of dopamine release may also be required for certain forms of synaptic plasticity in the nucleus accumbens. Together, these effects induced by psychostimulants, mediated through a non-dopamine transporter-mediated mechanism involving norepinephrine and the prefrontal cortex, may also contribute importantly to the reinforcing properties of these drugs.
Keywords: psychostimulants, dopamine transporter, prefrontal cortex, dopamine neuron, firing pattern, oscillations
1. Introduction
Cocaine, and amphetamine-like psychostimulants, including methamphetamine and methylphenidate, modulate arousal and produce behavioral activation and reinforcing actions that are associated with significant abuse potential (dela Peña et al., 2010; 2011; dela Peña et al., 2013; dela Peña et al., 2014; Heal et al., 2013; Kalivas 2007; Wood et al., 2013). After the intake of a stimulant drug, temporally limited functional changes in the brain occur, which are believed to endure beyond the presence in the brain of the actual drug or metabolites in the brain (Ungless et al., 2001). Identifying the initial functional changes wrought by psychostimulants is critical in understanding further the corresponding homeostatic responses that are responsible for behavioral and subjective effects of the drug intake that outlast the presence of the drug in the brain (Koob and Le Moal, 1997; Muller et al., 2007). While previous studies have provided strong evidence that dopamine plays a key role in the reinforcing effects of psychostimulants, the precise mechanisms by which psychostimulants alter dopamine-mediated transmission remain to be fully defined. Understanding these processes will not only help explain the complex mechanism of psychostimulant addiction but also aid in the discovery of effective therapies to counteract addiction to these drugs.
2. Role of dopamine in the effects of psychostimulants
Many lines of evidence suggest that dopamine plays a central role in the above-mentioned effects of psychostimulants (for reviews see Nutt et al., 2015; Wise, 2004, 2008). In humans, for example, blockade of dopamine receptors decreased the euphoria produced by intravenous amphetamine injection (Gunne et al., 1972; Jonsson et al., 1971). In animals, dopamine receptor blockade also attenuated the reinforcing properties of amphetamine and cocaine (Davis and Smith, 1975; Pierce and Kumaresan 2006; Yokel and Wise, 1975). Through microdialysis studies in animals, it has been observed that psychostimulants increased dopamine release in the nucleus accumbens (Di Chiara and Imperato, 1988), a critical site for the reinforcing effects of addictive drugs (Di Chiara et al., 2004; Koob 1992). Further support on the importance of dopamine in psychostimulant reinforcement has been provided by findings from imaging studies which revealed that administration of psychostimulants such as methylphenidate, cocaine and amphetamine increased brain dopamine levels (Volkow et al., 1999; Volkow et al., 2007). Together, these studies implicate the role of dopamine in the behavioral and reinforcing effects of psychostimulants and that psychostimulants produce their effects by enhancing dopamine transmission in the brain, especially in limbic areas such as the nucleus accumbens (Carboni et al., 1989; Cass et al., 1992; Ikemoto 2002; 2007; Kuczenski and Segal 1992; Wu et al., 2001).
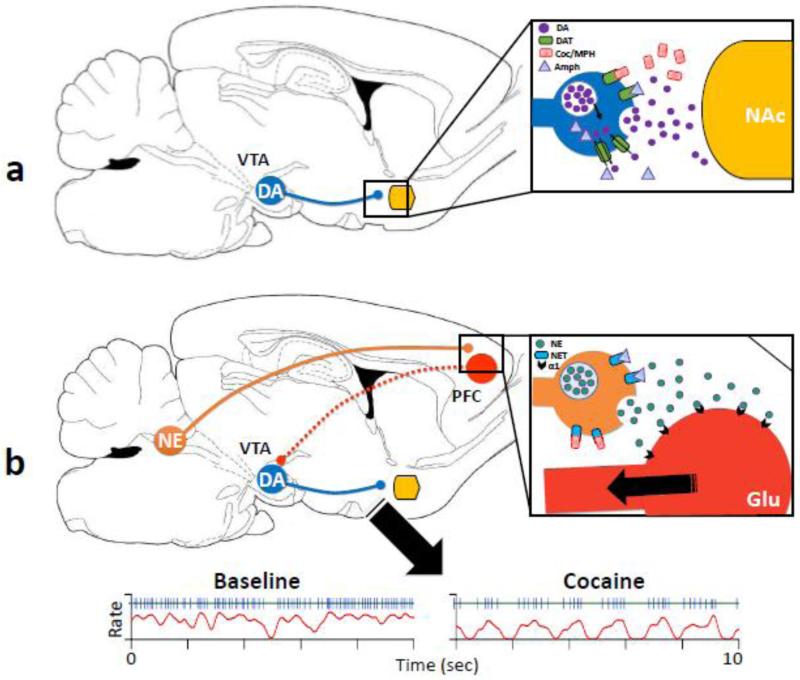
How do psychostimulants increase extracellular dopamine levels? According to the current hypothesis, psychostimulants produce this effect by binding to the dopamine transporter, a membrane protein responsible for the reuptake of synaptic dopamine and thus, the termination of dopamine signaling (Howell et al., 2008; Mortensen and Aamra, 2003; Zhu et al., 2008) (Fig. 1a). By binding to the dopamine transporter, cocaine and methylphenidate have been shown to inhibit dopamine reuptake. Amphetamine, on the other hand, causes dopamine release through dopamine transporter-mediated reverse transport, in addition to its interference with dopamine reuptake (Kahlig and Galli, 2003; Sulzer et al., 2005) (Fig. 1a). In this review, we discuss the evidence suggesting that psychostimulants also act through a dopamine transporter-independent mechanism to increase dopamine release, and propose that this non-dopamine transporter-mediated dopamine release is also critical to some of the behavioral effects of psychostimulants such as locomotor activation and reward.
Fig. 1. Psychostimulants affect dopamine transmission through both dopamine transporter (DAT)-dependent and independent mechanisms.
The reinforcing effects of psychostimulants have long been associated with the ability of these drugs to increase dopamine (DA) levels in the brain, especially in limbic areas such as the nucleus accumbens (NAc). a) The widely-accepted theory of psychostimulant-induced dopamine increase involves binding of stimulant drugs to the dopamine transporter to inhibit dopamine reuptake (e.g. cocaine [Coc] and methylphenidate [MPH]), and/or to induce reverse transport of dopamine via the dopamine transporter (e.g. amphetamine [Amph]). b) Some evidence, however, suggests non-dopamine transporter-mediated mechanisms of dopamine release induced by psychostimulants, one of which, as proposed in this review, involves norepinephrine transporters (NET) in the prefrontal cortex (PFC). Accordingly, psychostimulants bind to norepinephrine transporters in the prefrontal cortex, causing enhancement of norepinephrine levels in the prefrontal cortex and subsequent activation of alpha-1 adrenergic receptors. This effect may lead to activation of input derived directly or indirectly from the prefrontal cortex to dopamine neurons, resulting in changes in firing pattern of dopamine neurons in the ventral tegmental area (VTA), which then alters action potential-dependent dopamine release. Shown below b is an example of recordings from a dopamine neuron before and after cocaine injection. The blue and red traces are spike trains and smoothed rate histograms, respectively. As illustrated, cocaine significantly increased the slow oscillatory firing resembling repetitive bursting (for details see Zhou et al., 2006). As described in the text, this change in firing pattern induced psychostimulants would lead to alterations in not only the amount, but also the temporal pattern of dopamine release. Specific temporal pattern of dopamine release may be required for certain forms of synaptic plasticity in the nucleus accumbens and may facilitate or inhibit a synaptic input to nucleus accumbens neurons depending on the timing of the input relative to dopamine release. Furthermore, evidence discussed in this paper suggests that the non- dopamine transporter-mediated mechanism involving norepinephrine and the prefrontal cortex is critical in mediating some of the behavioral effects of psychostimulants such as locomotor activation and reward.
3. Evidence for non-dopamine transporter mediated dopamine release
Aside from dopamine transporters, psychostimulants also bind to the norepinephrine and serotonin transporters. Studies with the human dopamine, serotonin, and norepinephrine transporters suggest that amphetamine has very high affinity for the norepinephrine transporter, whereas methylphenidate inhibits norepinephrine and dopamine transporters equally well (Han and Gu, 2006). Moreover, both amphetamine and methylphenidate display significantly weaker binding to the serotonin transporter, while cocaine binds to all three transporters with an overall equal affinity (Han and Gu, 2006). To determine whether the dopamine transporter is the only site of action of psychostimulants, studies were conducted on dopamine transporter-knockout mice. Surprisingly, dopamine transporter-knockout mice still showed extracellular dopamine elevation in the nucleus accumbens, and cocaine and amphetamine still produced rewarding effects in these animals (Budygin et al., 2004; Carboni et al., 2001; Rocha et al., 1998; Sora et al., 1998). In the same animals, the specific dopamine transporter blocker GBR12909 produced no effect, confirming that both the increase in dopamine release and reinforcing effects induced by cocaine and amphetamine in dopamine transporter-knockout animals are mediated through a dopamine transporter-independent mechanism (Carboni et al., 2001). Because psychostimulants also have affinity for serotonin and norepinephrine transporters, it was proposed that in dopamine transporter-knockout mice, serotonin and/or norepinephrine transporters assume the responsibility to remove dopamine from the extracellular space, and psychostimulants increase extracellular dopamine by inhibiting dopamine reuptake through serotonin or norepinephrine transporters (Carboni et al., 1990; Yamamoto and Novotney, 1998). Consistent with this possibility, serotonin transporter blockers also caused dopamine elevation in the nucleus accumbens and were reinforcing in dopamine transporter-knockout mice (Hall et al., 2002; Mateo et al., 2004). A similar elevation in extracellular dopamine in dopamine transporter-knockout mice was also observed with the norepinephrine transporter inhibitor reboxetine (Carboni et al., 2001). Against this hypothesis, however, neither serotonin transporter nor norepinephrine transporter inhibition affected dopamine clearance in dopamine transporter-knockout mice (Budygin et al., 2002; Budygin et al., 2004; Mateo et al., 2004). More importantly, direct infusions of amphetamine and cocaine into the nucleus accumbens increased dopamine release in wild-type, but not dopamine transporter-knockout mice (Budygin et al., 2004; Mateo et al., 2004). Taken together, these results suggest that serotonin and norepinephrine transporters are involved in the dopamine release induced by psychostimulants. However, those serotonin and norepinephrine transporters that are responsible for the psychostimulant-induced dopamine release are located elsewhere in the brain and not within the nucleus accumbens.
It is important, however, to consider some of the features of the dopamine transporter-knockout mice that could have influenced data outcomes in the above-mentioned studies. For example, constitutive knockout of the dopamine transporter has been shown to produce compensatory changes in monoamine systems, especially dopamine, which led to reductions in the expression of tyrosine hydroxylase, dopamine D1 and D2 receptors, a homeostatic compensation to elevated dopamine levels, and other dopaminergic markers (Giros et al., 1996; Gainetdinov, 2008; Jaber et al., 1999; Jones et al., 1999; Fauchey et al., 2000). Neuroadaptations in dopamine system due to dopamine transporter deletion may have indirectly altered neural network activity-regulating behavior thereby complicating data interpretation (Gainetdinov, 2008). Furthermore, contrary to the above findings in dopamine transporter-knockout animals, a different line of dopamine transporter knockout mice showed attenuated cocaine self-administration (Thomsen et al., 2009) indicating complex outcomes in studies with dopamine transporter-knockout mice. Despite these limitations, the findings from dopamine transporter-knockout mice studies surely provided important hints on the existence of other potential mechanisms of psychostimulants’ actions (Gainetdinov, 2008), including those that are not mediated by the dopamine transporter (Carboni et al., 1990; Yamamoto and Novotney, 1998). In the following sections, we present one of these probable mechanisms, which involves norepinephrine transporters in the prefrontal cortex, in view of findings from studies involving mainly wild-type animals. We hypothesize that norepinephrine transporters in the prefrontal cortex, are at least, partially responsible for the increase in dopamine release in the nucleus accumbens induced by psychostimulants.
4. Involvement of norepinephrine and the prefrontal cortex in non-dopamine transporter- mediated dopamine release
In a landmark study, Darracq et al. (1998) showed that intra-nucleus accumbens dextroamphetamine (or d-amphetamine) injection did not produce behavioral activation in rats although it caused a dramatic increase in extracellular dopamine. Meanwhile, systemic administration of d-amphetamine induced an increase in locomotor activity despite producing a relatively small elevation in extracellular dopamine. Furthermore, Darracq et al. also showed that both dopamine release and locomotor activation induced by systemic injection of d-amphetamine were largely blocked by the alpha-1 adrenergic receptor antagonist prazosin administered either systemically or locally into the prefrontal cortex. Based on these findings, they proposed that d-amphetamine induces two forms of dopamine release in the nucleus accumbens: functional, which is caused by an effect of amphetamine distal from the nucleus accumbens and correlated with the development of locomotor activity, and non-functional, which is caused by a local effect of d-amphetamine in the nucleus accumbens but does not produce locomotor activation. The former, observed after systemic d-amphetamine and blocked by prazosin applied locally in the prefrontal cortex, is likely to be dopamine transporter-independent and contains synaptic information from the prefrontal cortex. On other hand, the so-called nonfunctional dopamine release, observed after intra-nucleus accumbens d-amphetamine injection, is likely to result from a direct interaction of d-amphetamine with dopamine transporters within the nucleus accumbens and lacks the information needed for locomotor activation. Hence, amphetamine acts through both dopamine transporter-dependent and independent mechanisms to increase dopamine release. The latter involves activation of alpha-1 adrenergic receptors in the prefrontal cortex and appears to be more important than the dopamine transporter-mediated dopamine release in mediating the locomotor activating effects of amphetamine.
Using the conditioned place preference protocol, a widely used experimental paradigm to measure rewarding effects drugs, Ventura et al. subsequently showed that depletion of norepinephrine in the prefrontal cortex abolished the effects of cocaine or amphetamine to induce a conditioned place preference response in animals, and to increase dopamine release in the nucleus accumbens (Ventura et al., 2003; 2007). Although results from locomotor activity studies cannot be directly compared with those obtained in conditioned place preference studies (Bardo et al.,1999; dela Peña et al., 2011), it is important to note that drugs of abuse may increase locomotor activity and produce drug reward by activating a similar dopamine substrate in the brain (Bardo et al.,1999; Di Chiara, 1995; Wise and Bozarth 1987). Hence, it appears that results of the conditioned place preference studies of Ventura et al. corroborate the findings of Darracq et al. on the role of norepinephrine in the prefrontal cortex in the behavioral effects of psychostimulants. That altering norepinephrine transmission either via administration of norepinephrine receptor antagonist or norepinephrine depletion in the prefrontal cortex (Darracq et al., 1998; Ventura et al., 2003; 2007) resulted in reduced dopamine release in the nucleus accumbens further indicates that norepinephrine in the prefrontal cortex is crucial for both locomotor activating and rewarding effects of psychostimulants.
Repeated administration of psychostimulants has been shown to progressively increase locomotor stimulating effect of a subsequent dose of the drug, a phenomenon called behavioral sensitization, which is used to detect neuroadaptations contributing to drug dependence and relapse (Robinson and Berridge, 2008). Interestingly, while repetitive (systemic) treatment of amphetamine produced behavioral sensitization (for review see Robinson and Berridge, 2008), repeated intra-nucleus accumbens administration of amphetamine did not produce an enhanced locomotor response to subsequent administration of psychostimulant drugs (e.g. cocaine) and other addictive substances (Dougherty and Ellinwood 1981, Kalivas and Weber 1988; Vezina and Stewart 1990). These results, again point to the possibility that blockade of dopamine transporter alone in the nucleus accumbens induced by local administration of psychostimulants cannot fully mimic the effects of systemic injection of these drugs.
A number of studies, however, reported contradicting findings in that intra-nucleus accumbens infusion of amphetamine, and other dopaminergic agonists induced profound locomotor and investigative behaviors (Carr and White, 1987; Kelley et al., 1989; Wise and Hoffman 1992; Ikemoto, 2002; Ikemoto et al. 2007). Nevertheless, considering that high doses of psychostimulants were used in the above-mentioned studies, local (i.e. intra-nucleus accumbens) administration of these drugs could have resulted in high concentrations in the nucleus accumbens which may be far beyond those achieved with systemic administration of psychostimulant drugs, and sufficient to produce behavioral activation. For instance, in the studies by Ikemoto (2002), the threshold dose for intra-nucleus accumbens d-amphetamine to induce locomotor activation was 30 nmol in 0.5 μl (i.e., 60 mM of d-amphetamine in concentration). Systemic administration of d-amphetamine is unlikely to reach this concentration level in the nucleus accumbens. In the studies of Darracq et al., intra-nucleus accumbens infusion of d-amphetamine at 0.5 mM or below failed to induce locomotor activation, but increased extracellular dopamine levels in the nucleus accumbens to 12,500% of baseline. Moreover, only when the concentration has been increased to 1 mM, which produced extracellular dopamine levels 25,000% above baseline, did d-amphetamine increase locomotor activity in subjects. In contrast, systemic injection of d-amphetamine (0.5 mg/kg) induced only a relatively small increase in dopamine release (350%), but significantly increased locomotor activity in subjects. Not only these findings reiterate the notion that blocking dopamine transporter alone in the nucleus accumbens induced by intra-nucleus accumbens administration of psychostimulants cannot fully simulate effects of systemic injection of these drugs, they also indicate that the behavioral effects of psychostimulant could not just be explained by the magnitude of drug-induced dopamine release in the nucleus accumbens. As mentioned above, other targets such as the norepinephrine transporters in the prefrontal cortex also appear to be very important in mediating behavioral effects of psychostimulants. Furthermore, we discuss below that the temporal pattern of dopamine release in the nucleus accumbens is also a crucial element for some of the behavioral effects induced by psychostimulant drugs (see Section 6).
The importance of norepinephrine in mediating drug reward has long been recognized in many studies (Poschel and Ninteman, 1963; Stein, 1975; Wise, 1978, for reviews see Schimdt and Weinshenker, 2014; Sofuoglu and Sewell, 2009). In mice lacking norepinephrine, the rewarding effect of cocaine was significantly reduced (Jasmin et al., 2006). Psychostimulant-induced dopamine elevation and locomotor activity were also significantly suppressed in alpha-1b adrenergic receptor knockout mice (Auclair, et al., 2002; Drouin et al., 2002b; Wanchoo et al., 2009). Genetic ablation of the alpha-1b adrenergic receptor also diminished cocaine consumption in an oral self-administration setting (Drouin et al., 2002a). Moreover, treatment with prazosin or with the longer acting alpha-1 adrenergic antagonist doxazosin attenuated sensitized locomotor response to d-amphetamine (Vanderschuren et al., 2003), methamphetamine (White and Rauhut, 2014), cocaine (Haile et al., 2012; Jimenez-Rivera et al., 2006), or (+/−)-3,4-methylenedioxymethamphetamine (MDMA) (Selken and Nichols, 2007). Pretreatment with prazosin also attenuated cocaine-induced reinstatement of extinguished drug-seeking behavior in rats (Zhang and Kosten, 2005; 2007). In addition, administration of beta-adrenergic receptor antagonists reduced cocaine self-administration in rats (Harris et al., 1996) and in squirrel monkeys (Goldberg and Gonzalez, 1976). In human subjects, the subjective effects of amphetamine have been correlated with amphetamine-induced norepinephrine release (Rothman et al., 2001). Recent clinical studies have shown efficacy doxazosin in attenuating positive subjective effects of cocaine (Newton et al., 2012), suggesting therapeutic potential of the drug for the treatment of cocaine dependence (Newton et al., 2012; Shorter et al., 2013).
In summary, the results from the above studies suggest that psychostimulants can increase dopamine release in the nucleus accumbens through both dopamine transporter-dependent and independent mechanisms (Fig. 1). The latter could involve, among other mechanisms (see Conclusion), norepinephrine release in the prefrontal cortex and activation of alpha-1 adrenergic receptors in that brain region which appears to be an essential mechanism in mediating locomotor activating and rewarding effects of psychostimulants. Furthermore, the role of norepinephrine in mediating drug reward has been demonstrated through both knockout mice studies and the findings that treatment with alpha-1 adrenergic antagonists attenuated reinforcing effects of various psychostimulant drugs. In succeeding sections we will discuss how the prefrontal cortex regulates dopamine release in the nucleus accumbens and explain why dopamine release mediated through the prefrontal cortex is important for the actions of psychostimulants.
5. Prefrontal cortical regulation of dopamine neurons
The prefrontal cortex is highly developed in humans and primates and critical to the executive functions of brain, including decision making (Euston et al., 2012), learning and memory (Rugg et al., 1996; Tomita et al., 1999), social behavior (Avale et al., 2011; Forbes and Grafman, 2010) and emotional regulation (Davidson and Irwin, 1999; Kennis et al., 2013). Prefrontal cortical dysfunction has been implicated in a number of neuropsychiatric disorders, including drug addiction (Goldstein and Volkow, 2011).
Anatomical studies have shown that prefrontal cortex sends and receives projections from virtually all cortical sensory systems, motor systems, as well as many subcortical structures. In particular, projections from the prefrontal cortex to the ventral tegmental area play an important role in regulating the activity of ventral tegmental area neurons and the extracellular levels of dopamine within forebrain regions (Carr and Sesack, 2000). Dopamine neurons in the ventral tegmental area have been shown to respond to various stimuli by changing their firing rate and pattern. Two main types of firing patterns have been described in dopamine neurons recorded in vivo: irregular single-spike firing and burst firing, the latter of which has been suggested to be critically driven by afferent input (for review see Grace et al., 2007). Moreover, using spectral analysis, we have shown that burst firing in dopamine neurons can also be described as slow oscillatory firing (see below).
Because the prefrontal cortex projects to both the ventral tegmental area and nucleus accumbens (Carr and Sesack, 2000), there are at least two possible mechanisms by which the prefrontal cortex influences dopamine release. First, via its projection to the ventral tegmental area, the prefrontal cortex may regulate the firing rate or pattern of dopamine neurons and thus the action potential-dependent dopamine release in the nucleus accumbens. Second, in the nucleus accumbens, glutamate released from prefrontal cortex terminals may directly depolarize dopamine terminals to induce dopamine release without affecting the firing of dopamine cells in the ventral tegmental area. Several lines of evidence, however, suggest that prefrontal cortex regulates dopamine release through the first mechanism. Thus, electrical or chemical stimulation of the prefrontal cortex has been shown to increase not only dopamine release in the nucleus accumbens, but also burst firing of dopamine neurons (Karreman and Moghaddam, 1996; Murase et al., 1993; Taber and Fibiger, 1995; Tong et al., 1996; You et al., 1998, but see Jackson et al., 2001). Studies using microdialysis further showed that dopamine release in the nucleus accumbens induced by prefrontal cortex activation is blocked by glutamate antagonists locally applied to the ventral tegmental area, but not to the nucleus accumbens (Karreman and Moghaddam, 1996; Taber and Fibiger, 1995; Taber et al., 1995), indicating that the effect is mediated by ventral tegmental area glutamate release and subsequent activation of dopamine neurons. However, since prefrontal cortex terminals in the ventral tegmental area preferentially synapse with dopamine neurons projecting back the prefrontal cortex and not those projecting to the nucleus accumbens (Carr and Sesack, 2000; Sesack et al., 2003), the excitatory influence of the prefrontal cortex on nucleus accumbens-projecting dopamine neurons is likely to be indirectly mediated via other brain areas that are innervated by the prefrontal cortex and provide glutamate input to dopamine neurons (Geisler, 2007; Omelchenko and Sesack, 2007). Nevertheless, it is also possible that prefrontal cortex terminals in the ventral tegmental area regulate dopamine neurons through extrasynaptic volume transmission (for review see Taber and Hurley, 2014), instead of synaptic transmission. In other words, glutamate released from prefrontal cortex terminals induced by e.g., prefrontal cortex alpha-1 adrenergic receptor stimulation, may diffuse out from the synaptic cleft to the extrasynaptic space and activate extrasynaptic glutamate receptors located on dopamine neurons.
In addition to an excitatory influence, the prefrontal cortex appears to also have an inhibitory control over dopamine neurons (Gao et al., 2007). Prefrontal cortex projections have been shown to form direct synaptic contacts onto γ-aminobutyric acid (GABA)ergic cells in the ventral tegmental area (Carr and Sesack, 2000; Sesack et al., 2003). Some of the GABA neurons may form synapses with dopamine neurons. The prefrontal cortex may also inhibit dopamine cells through GABA neurons in other brain areas, such as the nucleus accumbens, that are innervated by the prefrontal cortex and provide GABA projections to the ventral tegmental area. Consistent with a bidirectional regulation of dopamine neurons by the prefrontal cortex, prefrontal cortex stimulation-induced bursts in dopamine neurons were often preceded by a period of inhibition lasting for more than 150 ms (Gariano and Groves, 1988; Overton, and Clark, 1997).
Under non-stimulated baseline conditions, the activity of dopamine neurons in the ventral tegmental area also appears to be regulated by the prefrontal cortex (Gao et al., 2007). Spectral analysis suggests that most dopamine neurons display repetitive bursting or slow oscillatory firing (Shi, 2005; 2009; Zhang, et al., 2008). Coherence analysis shows that the slow oscillation (0.5-1.5 Hz) in dopamine neurons is highly correlated with the activity of prefrontal cortex cells (Gao et al., 2007). Furthermore, inactivation of the prefrontal cortex not only inhibits bursting, but also the slow oscillation in dopamine neurons, suggesting that the slow oscillation in firing in dopamine neurons is driven by inputs derived either directly or indirectly from the prefrontal cortex and, therefore, contains synaptic information from the prefrontal cortex (Gao et al., 2007).
6. Importance of prefrontal cortex regulation of dopamine neurons in the effects of psychostimulants
Dopamine neurons in the ventral tegmental area receive synaptic information from many brain areas including inputs directly from the prefrontal cortex and from other areas that are under the control of the prefrontal cortex (e.g. hippocampus, amygdala, and ventral pallidum). Information from different inputs is processed and integrated in the dendrites and soma of dopamine neurons and then transmitted in the form of spike trains to axon terminals where action potentials would trigger Ca2+-dependent exocytosis of dopamine. In contrast with single-spike firing, rhythmic bursting or slow oscillation in firing has been reported to induce more dopamine release (Gonon, 1988; for review see Heien and Wightman, 2006). Oscillatory firing may also lead to an oscillatory dopamine release, thus, variations in firing pattern of dopamine neurons may lead to not only different amounts, but also different temporal patterns of dopamine release. We have previously shown that psychostimulants including d-amphetamine and cocaine increased the slow oscillation in dopamine neurons (Fig.1b) (Shi et al., 2000; Shi et al., 2004, Zhou 2006). Considering that the slow oscillation in dopamine neurons is regulated by the prefrontal cortex, psychostimulants, by acting through the prefrontal cortex, may change the firing pattern of dopamine neurons, and induce a specific temporal pattern of dopamine release. In dopamine terminal areas such as the nucleus accumbens, psychostimulant-induced oscillatory dopamine release may produce effects which are very different from those produced by a tonic dopamine release. If, for example, dopamine inhibits the response of a nucleus accumbens neuron to glutamate inputs, an oscillatory dopamine release would selectively inhibit inputs to that cell that coincide with dopamine release. Inputs that are not synchronized with dopamine release would be less affected by dopamine. Therefore, information carried by those inputs is selectively transmitted to downstream nuclei. A tonic dopamine release, on the other hand, would equally inhibit all glutamate inputs to the nucleus accumbens neuron. In support of these statements, spine enlargement of nucleus accumbens neurons was only observed during specific time points at which dopamine was released in accordance with glutamate. Accordingly, physiologically relevant phasic release of dopamine induced by optogenetic stimulation of dopamine fibers caused maximal spine enlargement only when introduced at about 600 milliseconds after glutamatergic input. Stimulation of dopamine fibers prior to and after the critical time window (0.3 to 2 seconds) produced much smaller or no dendritic spine enlargement (Yagishita et al., 2014).
Oscillatory firing of dopamine neurons may also enable dopamine neurons to select inputs and regulate synaptic plasticity within the ventral tegmental area. Two forms of synaptic plasticity have been identified in ventral tegmental area dopaminergic neurons: long-term potentiation and long-term depression (Jones and Bonci 2005). The ability of psychostimulants to induce long-term potentiation of glutamate synapses on dopamine neurons has been previously reported (for reviews see Bowers et al., 2010; Jones and Bonci 2005; Wolf et al., 2004), although the mechanism for this effect is not yet known. Modulation of synaptic transmission has been observed as a function of the precise temporal relations between input and output spikes or the so-called spike-timing dependent plasticity (for review see Feldman 2012). Thus, psychostimulants, by inducing the slow oscillation in dopamine neurons through e.g., alpha-1 adrenergic receptors in the prefrontal cortex (see below), may induce long-term potentiation only in a subset of glutamate inputs that coincide with the firing of a dopamine neuron during the slow oscillation. An important aspect of future research is to determine whether glutamate synapses formed by prefrontal cortex terminals are among those that are potentiated by psychostimulants.
Of note, psychostimulant-induced increase in slow oscillation was not blocked by dopamine antagonists, suggesting that the increase in slow oscillation is non-dopamine transporter-mediated (Shi et al., 2000, 2004). The effect, however, depends on activation of alpha-1 adrenergic receptors since it is blocked by prazosin, which has also been shown to block amphetamine-induced dopamine release and locomotor activation (Darracq et al. 1998). Importantly, the effect of d-amphetamine is mimicked by all psychostimulants tested, but not by non-psychostimulant dopamine agonists including L-3,4-dihydroxyphenylalanine (L-dopa) (Shi et al., 2004). These results suggest that the increase in the slow oscillation is an effect unique to psychostimulants and may play an important role in the behavioral effects induced by these drugs. The blockade of the effect by prazosin further suggests that enhancement of the slow oscillation may be responsible for the non-dopamine transporter-mediated dopamine release induced by amphetamine as described by Darracq et al. and caused by an increased norepinephrine release and the subsequent activation of alpha-1 adrenergic receptors in the prefrontal cortex (Darracq et al., 1998; Shi et al., 2004; Shi et al., 2007; Ventura et al., 2003; Zhou et al., 2006). This increase in slow oscillation in firing induced by d-amphetamine may provide part of the explanation as to why the non-dopamine transporter-mediated dopamine release induced by d-amphetamine is behaviorally effective and different from those mediated by a dopamine transporter-dependent mechanism induced by the same drug.
In summary, the above results, together with the fact that psychostimulants, but not dopamine agonists and L-dopa, induce the slow oscillation in dopamine neurons, suggest that the reinforcing efficacy of a drug may depend not only on its ability to enhance dopamine release, but also on its capacity to induce a specific temporal pattern of dopamine release. The importance of the prefrontal cortex in mediating the above-mentioned effects of psychostimulants cannot be underscored, by virtue of its involvement in regulating the activity of dopamine neurons.
7. Concluding remarks
It has long been believed that psychostimulants produce rewarding effects via dopamine transporter-mediated reverse transport and/or blocking dopamine reuptake though the dopamine transporter, thereby enhancing dopamine neurotransmission. The evidence presented in this paper indicates that in addition to dopamine transporter, non- dopamine transporter mediated mechanisms of dopamine release are also involved and appears to be critical for the behavioral effects of stimulant drugs such as locomotor activation and reward. Whereas dopamine transporter-mediated dopamine elevation by psychostimulants is a local effect, a non- dopamine transporter-mediated mechanism involves a complex interaction among brain areas and neurotransmitter systems that act in a coordinated manner to regulate the firing pattern of dopamine neurons, leading to changes not only in the amount, but also the temporal pattern of dopamine release (Fig. 1b). Specific temporal pattern of dopamine release may be required for certain forms of synaptic plasticity in the nucleus accumbens and may facilitate or inhibit a synaptic input to nucleus accumbens neurons depending on its temporal relationship to dopamine release. This may explain in part, why the so-called non-dopamine transporter-mediated dopamine release is required for psychostimulants to produce some of their behavioral effects.
While this review focuses primarily on non- dopamine transporter-dependent mechanism of psychostimulants’ actions, we cannot totally disregard the role of the dopamine transporter in mediating effects of psychostimulants. Notably, the studies of Thomsen et al. in dopamine transporter-knockout mice (Thomsen et al., 2009a, see Section 3) as well in mice expressing a cocaine-insensitive dopamine transporter (Thomsen et al., 2009b), revealed attenuation or elimination of cocaine self-administration, respectively, in these animals, emphasizing the indispensable participation of dopamine transporter blockade in the reinforcing effects of psychostimulants. Differing outcomes can be explained by a number of factors (see Thomsen et al., 2009a), including the different lines of dopamine transporter-knockout mice used in the above studies (Carboni et al., 2001; Thomsen et al., 2009a; 2009b). Moreover, we propose that when dopamine transporters are not blocked by cocaine in cocaine-insensitive dopamine transporter mice, the non-dopamine transporter-mediated dopamine release is rapidly removed by dopamine transporter and is too small to produce a significant effect. In normal animals, however, when dopamine transporters are blocked by a psychostimulant, the same dopamine release achieved through a non-dopamine transporter-mediated mechanism would lead to a larger increase in synaptic dopamine concentration and thus, an increased stimulation of dopamine receptors in nucleus accumbens neurons.
Although our discussion has been limited to the roles of norepinephrine and the prefrontal cortex, it is very likely that psychostimulants may also act through other brain areas and other neurotransmitter systems to regulate dopamine release in the nucleus accumbens. Indeed, many brain areas that project to the ventral tegmental area are also under the control of the prefrontal cortex (e.g. hippocampus, amygdala, and ventral pallidum), thus, psychostimulants may also alter the firing pattern of dopamine neurons through these pathways. Aside from norepinephrine, the serotonergic system has been shown to participate in psychostimulant-induced dopamine release, as evidenced by the findings that serotonin transporter blockers which enhanced dopamine levels in the nucleus accumbens of dopamine transporter-knockout mice (Hall 2002; Mateo et al., 2002), and that serotonin receptors positively modulated dopamine outflow (De Deurwaerdere et al., 1997; De Deurwaerdère and Spampinato, 1999; Dray 1981). It is possible that these various inputs and neurotransmitters act in concert with norepinephrine and the prefrontal cortex to modulate dopamine release and produce behavioral changes associated with psychostimulant reward. In addition to alpha-1 adrenergic receptor, norepinephrine also binds to alpha-2, beta 1-3 adrenergic receptors. Although our previous studies suggest that these receptors do not play a significant role in the increase in the slow oscillation of dopamine neurons induced by psychostimulants (Shi et al., 2000; Shi et al., 2004), they may contribute to the behavioral effects of these drugs through dopamine-independent mechanisms. It is also probable that norepinephrine contributes to the rewarding effect of psychostimulants beyond its effect on dopamine transmission.
In conclusion, we described a non- dopamine transporter-mediated dopamine release mechanism of psychostimulant action, involving norepinephrine and the prefrontal cortex, which we suggest to be one of the mechanisms critically associated with the reinforcing effects of psychostimulants. From the clinical standpoint, such mechanism of action of psychostimulant drugs provides a rational basis for the use of noradrenergic medications as treatments for psychostimulant addiction (for reviews see Schimdt and Weinshenker, 2014; Sofuoglu and Sewell, 2009). Of note, recent clinical studies have shown promising results for the use of alpha-1 adrenergic antagonists (e.g. doxazosin) for the treatment of cocaine dependence (Shorter et al., 2013; Newton et al., 2012) further corroborating the involvement of norepinephrine in mediating psychostimulant reinforcement.
Acknowledgments
This research was supported, in part, by the National Institutes of Health, National Institute of Drug Abuse DA032857 and Loma Linda University School of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auclair A, Cotecchia S, Glowinski J, Tassin JP. D-amphetamine fails to increase extracellular dopamine levels in mice lacking alpha 1b-adrenergic receptors: relationship between functional and nonfunctional dopamine release. J Neurosci. 2002;22(21):9150–9154. doi: 10.1523/JNEUROSCI.22-21-09150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, Chabout J, Pons S, Serreau P, De Chaumont F, Olivo-Marin JC, Bourgeois JP, Maskos U, Changeux JP, Granon S. Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 2011. 2011;25(7):2145–2155. doi: 10.1096/fj.10-178558. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valoney JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacol. (Berl) 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67(1):11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc. Natl. Acad. Sci. U S A. 2004;101(20):7781–7786. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Jones SR. Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J. Neurosci. 2002;22(10):RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J. Neurosci. 2001;21(9):RC141, 1–4. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 1990;55(3):1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 2000;20(10):3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD, White NM. Effects of systemic and intracranial amphetamine injections on behavior in the open field: a detailed analysis. Pharmacol. Biochem. Behav. 1987;27(1):113–122. doi: 10.1016/0091-3057(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J. Neurochem. 1992;59:259–266. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J. Neurosci. 1998;18(7):2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Effect of haloperidol on (+)-amphetamine self-administration. J. Pharm. Pharmacol. 1975;27(7):540–542. doi: 10.1111/j.2042-7158.1975.tb09502.x. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, L'hirondel M, Bonhomme N, Lucas G, Cheramy A, Spampinato U. Serotonin stimulation of 5-HT4 receptors indirectly enhances in vivo dopamine release in the rat striatum. J. Neurochem. 1997;68(1):195–203. doi: 10.1046/j.1471-4159.1997.68010195.x. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Spampinato U. Role of serotonin(2A) and serotonin(2B/2C) receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J. Neurochem. 1999;73(3):1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- dela Peña I, Ahn HS, Choi JY, Shin CY, Ryu JH, Cheong JH. Reinforcing effects of methamphetamine in an animal model of attention-deficit/hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Funct. 2010;6:72. doi: 10.1186/1744-9081-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Peña I, Jeon SJ, Lee E, Ryu JH, Shin CY, Noh M, Cheong JH. Neuronal development genes are key elements mediating the reinforcing effects of methamphetamine, amphetamine, and methylphenidate. Psychopharmacology (Berl) 2013;230(3):399–413. doi: 10.1007/s00213-013-3168-8. [DOI] [PubMed] [Google Scholar]
- dela Peña I, Kim BN, Han DH, Kim Y, Cheong JH. Abuse and dependence liability analysis of methylphenidate in the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder (ADHD): what have we learned? Arch. Pharm. Res. 2013;36(4):400–410. doi: 10.1007/s12272-013-0037-2. [DOI] [PubMed] [Google Scholar]
- dela Peńa IC, Ahn HS, Choi JY, Shin CY, Ryu JH, Cheong JH. Methylphenidate self-administration and conditioned place preference in an animal model of attention-deficit hyperactivity disorder: the spontaneously hypertensive rat. Behav. Pharmacol. 2011;22(1):31–9. doi: 10.1097/FBP.0b013e328342503a. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Psychobiology of the role of dopamine in drug-abuse and addiction. Neurosci. Res. Commun. 1995;17:133–143. [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty GG, Ellinwood EH. Chronic d-amphetamine in nucleus accumbens: lack of tolerance or reverse tolerance of locomotor activity. Life Sci. 1981;28:2295–2298. doi: 10.1016/0024-3205(81)90582-8. [DOI] [PubMed] [Google Scholar]
- Dray A. Serotonin in the basal ganglia: functions and interactions with other neuronal pathways. J Physiol (Paris) 1981;77(2-3):393–403. [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villégier AS, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002a;43(1):51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J. Neurosci. 2002b;22(7):2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchey V, Jaber M, Bloch B, Le Moine C. Dopamine control of striatal gene expression during development: relevance to knockout mice for the dopamine transporter. Eur J Neurosci. 2000;12:3415–3425. doi: 10.1046/j.1460-9568.2000.00220.x. [DOI] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75(4):556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu. Rev. Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377(4-6):301–13. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Gonzalez FA. Effects of propranolol on behavior maintained under fixed-ratio schedules of cocaine injection or food presentation in squirrel monkeys. J. Pharmacol. Exp. Ther. 1976;198:626–634. [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1998;24(1):19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge D. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gunne LM, Anggård E, Jönsson LE. Clinical trials with amphetamine-blocking drugs. Eur. J. Pharmacol. 1972;19(1):52–55. [PubMed] [Google Scholar]
- Haile CN, Hao Y, O'Malley PW, Newton TF, Kosten TA. The α1 antagonist doxazosin alters the behavioral effects of cocaine in rats. Brain Sci. 2012;2(4):619–633. doi: 10.3390/brainsci2040619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115(1):153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 3. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present--a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol. Disord. Drug Targets. 2006;5(1):99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem. Pharmacol. 2008;75(1):196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, d-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Dumartin B, Sagné C, Haycock JW, Roubert C, Giros B, Bloch B, Caron MG. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur. J. Neurosci. 1999;11(10):3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem. 2001;78(4):920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Narasaiah M, Tien D. Noradrenaline is necessary for the hedonic properties of addictive drugs. Vascul. Pharmacol. 2006;45(4):243–250. doi: 10.1016/j.vph.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Jiménez-Rivera CA, Feliu-Mojer M, Vázquez-Torres R. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann N Y Acad Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr. Opin. Pharmacol. 2005;5(1):20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson LE, Anggård E, Gunne LM. Blockade of intravenous amphetamine euphoria in man. Clin. Pharmacol. Ther. 1971;12(6):889–896. doi: 10.1002/cpt1971126889. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur. J. Pharmacol. 2003;479(1-3):153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin. Neurosci. 2007;9(4):389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: An effect mediated by ventral tegmental area. J. Neurosci. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav. Brain Res. 1989;35:27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, van Rooij SJ, Kahn RS, Geuze E. Altered functional connectivity in posttraumatic stress disorder with versus without comorbid major depressive disorder: a resting state fMRI study. Version 2. F1000Res. 2013;2:289. doi: 10.12688/f1000research.2-289.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;28(654):171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Differential effects of amphetamine and dopamine uptake blockers (cocaine, nomifensine) on caudate and accumbens dialysate dopamine and 3-methoxytyramine. J. Pharmacol. Exp. Ther. 1992;262:1085–1094. [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc. Natl. Acad. Sci. U S A. 2004;101(1):372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur. J. Pharmacol. 2003;479(1-3):159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog. Neurobiol. 2007;81(3):133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon F, Svensson T. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci. Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, Brown G, Kosten TR, Mahoney JJ, Haile CN. Noradrenergic α1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7(2):e30854. doi: 10.1371/journal.pone.0030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16(5):305–312. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146(3):1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res. Brain Res. Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Poschel BP, Ninteman FW. Norepinephrine: a possible excitatory neurohormone of the reward system. Life Sci. 1963;10:782–788. doi: 10.1016/0024-3205(63)90087-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1(2):132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119(Pt 6):2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Schmidt KT, Weinshenker D. Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol Pharmacol. 2014;85(4):640–650. doi: 10.1124/mol.113.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selken J, Nichols DE. Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of (+/−)-3,4-methylenedioxymethamphetamine(MDMA) in rats. Pharmacol Biochem Behav. 2007;86(4):622–630. doi: 10.1016/j.pbb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann. N.Y. Acad. Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shi WX. Slow oscillatory firing: a major firing pattern of dopamine neurons in the ventral tegmental area. J Neurophysiol. 2005;94(5):3516–22. doi: 10.1152/jn.00317.2005. [DOI] [PubMed] [Google Scholar]
- Shi WX. Electrophysiological characteristics of dopamine neurons: a 35-year update. J Neural Transm Suppl. 2009;(73):103–19. doi: 10.1007/978-3-211-92660-4_8. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J. Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 2004;29:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Shi WX, Zhang XY, Pun CL, Bunney BS. Clozapine blocks D-amphetamine-induced excitation of dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2007;32(9):1922–1928. doi: 10.1038/sj.npp.1301334. [DOI] [PubMed] [Google Scholar]
- Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: A pilot study. Drug Alcohol Depend. 2013;131(1-2):66–70. doi: 10.1016/j.drugalcdep.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14(2):119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl G,R. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. U S A. 1998;95(13):7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. Norepinephrine reward pathways: role of self-stimulation, memory consolidation, and schizophrenia. Nebr. Symp. Motiv. 1975;22:113–159. [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA. Volume transmission in the brain: beyond the synapse. J. Neuropsychiatry Clin. Neurosci. 2014;26(1):iv, 1–4. doi: 10.1176/appi.neuropsych.13110351. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J. Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J. Neurosci. 1995;(5 Pt 2):3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009a;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009b;331(1):204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401(6754):699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411(6837):583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Beemster P, Schoffelmeer AN. On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology (Berl) 2002;169(2):176–185. doi: 10.1007/s00213-003-1509-8. [DOI] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J. Neurosci. 2003;23(5):1879–1885. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. U S A. 2007;104(12):5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291(1):409–415. [PubMed] [Google Scholar]
- Wanchoo SJ, Swann AC, Dafny N. Descending glutamatergic pathways of PFC are involved in acute and chronic action of methylphenidate. Brain Res. 2009;1301:68–79. doi: 10.1016/j.brainres.2009.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AO, Rauhut AS. Time-dependent effects of prazosin on the development of methamphetamine conditioned hyperactivity and context-specific sensitization in mice. Behav Brain Res. 2014;15(263):80–89. doi: 10.1016/j.bbr.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152(2):215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2-3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. 2013;66(1):193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J. Neurosci. 2001;21(16):6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345(6204):1616–1620. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J. Neurochem. 1998;71(1):274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187(4176):547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- You Z, Tzschentke T, Brodin E, Wise R. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vitro microdialysis study in freely moving rats. J. Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an α-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang S, Jin GZ, Bunney BS, Shi WX. Oscillatory firing of dopamine neurons: differences between cells in the substantia nigra and ventral tegmental area. Synapse. 2008;62(3):169–75. doi: 10.1002/syn.20479. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology. 2007;32(3):638–645. doi: 10.1038/sj.npp.1301120. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bunney BS, Shi WX. Differential effects of cocaine on firing rate and pattern of dopamine neurons: role of alpha1 receptors and comparison with L-dopa and apomorphine. J. Pharmacol. Exp. Ther. 2006;317(1):196–201. doi: 10.1124/jpet.105.094045. [DOI] [PubMed] [Google Scholar]
- Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol. Disord. Drug Targets. 2008;7(5):393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]