Abstract
Cholinergic signaling via the nicotinic acetylcholine receptors (nAChRs) in the mesolimbic circuitry is involved in the rewarding effects of abused drugs such as cocaine and opioids. In mouse studies, nonselective nAChR antagonist mecamylamine blocks cocaine-induced conditioned place preference (CPP) and behavioral sensitization. Among subtype-selective nAChR antagonists, the β2-selective antagonist dihydrobetaerythroidine and α7 nAChR antagonist methyllycaconitine (MLA), but not MLA alone prevent behavioral sensitization to cocaine. Since the role of the α3β4 nAChR subtype in the rewarding and behavioral effects of cocaine is unknown, the present study investigated the effect of two potent and selective α3β4 nAChR ligands, AT-1001 and AT-1012, on the acquisition of cocaine-induced CPP and behavioral sensitization in mice. At 5–30 mg/kg, cocaine produced robust CPP, whereas behavioral sensitization of locomotor activity was only observed at the higher doses (20–30 mg/kg). Pretreatment with AT-1001 (1–10 mg/kg) or AT-1012 (3–10 mg/kg) blocked CPP induced by 5 mg/kg cocaine, but not by 30 mg/kg cocaine. Lower doses of AT-1001 (0.3–3 mg/kg) and AT-1012 (1–3 mg/kg) did not affect the increase in locomotor activity induced by 5 or 30 mg/kg cocaine. But AT-1001, at these doses, blocked locomotor sensitization induced by 30 mg/kg cocaine. These results indicate that the α3β4 nAChR play a role in the rewarding and behavioral effects of cocaine, and that selective α3β4 nAChR ligands can attenuate cocaine-induced behavioral phenomena. Since the selective α3β4 nAChR functional antagonist AT-1001 has also been shown to block nicotine self-administration in rats, the present results suggest that α3β4 nAChRs may be a target for the treatment of cocaine addiction as well as for cocaine-nicotine comorbid addiction.
Keywords: α3β4 nAChRs, cocaine-induced behavior, α3β4 nAChR-selective ligands, cocaine-induced conditioned place preference, cocaine addiction
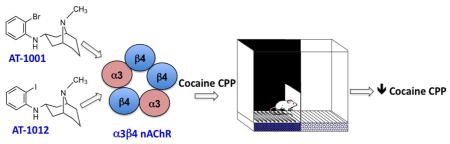
Graphical abstract
1. Introduction
Cocaine remains one of the most abused illicit drugs worldwide, but currently there are no approved medications to treat cocaine addiction. Cocaine increases extracellular dopamine levels in the nucleus accumbens (NAc) by blocking the dopamine transporter (DAT) on the nerve terminals of dopaminergic neurons in the NAc and inhibiting dopamine reuptake. Drug discovery efforts focused on either targeting DAT or reducing dopamine in the NAc have not led to effective treatments for cocaine addiction. Among other neurotransmitter systems and circuitries that have been shown to directly or indirectly modulate cocaine-induced rewarding effects, the cholinergic system is of special interest. Activation of the nicotinic acetylcholine receptors (nAChRs), which are present throughout the mesocorticolimbic system, has been shown to play a major role in enhancing dopamine release in the mesolimbic reward circuitry [1–3], in neuroadaptive changes associated with cocaine abuse [4, 5] and in the rewarding and reinforcing effects of psychostimulants, including cocaine and amphetamines [1, 6, 7].
Several nAChR subtypes present on the cell bodies of dopaminergic neurons in the VTA and on terminals in the NAc have been linked to the reinforcing actions of psychostimulants. For instance, cocaine-induced conditioned place preference, a model of the rewarding effects of drugs, is significantly reduced in mice lacking the β2 nAChR subunit [8], whereas the β2-selective nAChR antagonist dihydro-betaerythroidine (DHβE) but not the α7-selective antagonist methyllycaconitine (MLA), prevents cocaine-induced behavioral sensitization [4, 9, 10]. The nonselective nAChR antagonist mecamylamine has also been shown to decrease cocaine CPP in mice [8] as well as cocaine self-administration in rats [11, 12]. More recently, it was shown that nAChRs containing the α6-subunit, but not the α4 subunit, are important for the induction of cocaine-conditioned reward [13].
Results from several studies suggest also a role for another nAChR subtype, the α3β4* nAChR (* denotes possible presence of accessory subunits), in the rewarding effects of drugs of abuse.[14] The role of the α3β4 nAChR subtype in drug addiction behaviors is intriguing given its limited distribution in the brain, in particular its low levels in the mesolimbic reward circuitry.[15] The α3β4* nAChR is expressed widely in the peripheral nervous system, but in the brain, it is only highly expressed in the medial habenula (MHb), interpeduncular nucleus (IPN) and fasciculus retroflexus and in far lower densities in the ventral tegmental area, dorsolateral tegmentum, and basolateral amygdala [16–19]. The recent findings from human genetic studies that polymorphisms in genes encoding the α3-α5-β4 nAChR on chromosome 15, are associated with increased risk for tobacco dependence, early onset of smoking and alcohol use, and increased dependence liability, strongly supports the role of the α3β4* nAChR in drug-induced reinforcing behaviors [20–24]. However, little is known about the role of this nAChR in the rewarding effects of psychostimulants such as cocaine.
We recently reported a series of small-molecule compounds that have high affinity and selectivity for the α3β4 nAChR [25–27]. Two compounds from this series, AT-1001 and AT-1012 have single-digit nanomolar potency for the α3β4 nAChR, and 80–100-fold selectivity for the α3β4 versus the α4β2 nAChRs [26, 27]. Both compounds are low efficacy partial agonists at the α3β4 nAChR [28] but potently inhibit epibatidine-induced Ca+2 flux in HEK cells transfected with α3β4 nAChRs, with an IC50 of approximately 30–60 nM [27, 29]. In vitro electrophysiology experiments with human α3β4 nAChR-transfected oocytes showed that AT-1001 blocks acetylcholine (ACh) response by rapid desensitization of the receptor at the same concentration at which it exhibits partial agonist response, thereby acting as a functional antagonist at the α3β4 nAChR [28]. In vitro autoradiography in rat brain slices with [125I]-AT-1012 shows marked selective and localized labeling of the MHb, IPN and the fasciculus retroflexus, areas that contain the highest level of α3β4 nAChR [27]. In vivo, AT-1001 inhibits the self-administration of nicotine and cigarette smoke extract in rats, but does not alter food self-administration [26, 30], consistent with the suggested role of the α3-α5-β4 nAChR cluster on nicotine dependence found in the human genetic studies.
The aim of the present study is to examine the role of α3β4* nAChR in cocaine-induced behaviors, by investigating whether pharmacological antagonism of α3β4* nAChR with the α3β4 nAChR-selective ligands AT-1001 and AT-1012 would alter acquisition of cocaine-induced CPP and behavioral sensitization in mice.
2. Materials and Methods
2.1. Animals
Male ICR mice (Charles River) approximately 6 weeks old, and weighing 25–30g at the start of the experiment were used. Animals were group-housed under standard laboratory conditions and were kept on a 12:12-hr day/night cycle (lights on at 7:00am). Animals were handled for 3–4 days before the experiments were conducted. On behavioral test days, animals were transported to the testing room and acclimated to the environment for 1 hr. Testing was conducted between 9:00am–1:00pm daily. Mice were maintained in accordance with the guidelines of SRI International and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003) [31].
2.2. Test Compounds
AT-1001 (N-(2-bromophenyl)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine) hydrochloride [26] and AT-1012 (N-(2-iodophenyl)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine) hydrochloride [32] were synthesized at Astraea Therapeutics. For behavioral experiments, AT-1001 and AT-1012 were dissolved in 1–2% DMSO and 0.5% aqueous hydroxypropylcellulose. Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in water. Test compounds were injected in a volume of 0.1 ml/25g subcutaneously (s.c.). Controls received 0.1 ml/25g of the appropriate vehicles.
2.3. CPP Apparatus
The apparatus consisted of rectangular Plexiglas chambers divided into two distinct equal-sized compartments (19cm × 22.8cm × 18cm high, Lafayette Instruments). One compartment had cedar-scented bedding underneath a bar grid floor and all but the front walls were black. The other compartment had pine-scented bedding beneath a mesh floor and all but the front wall were white. The front walls were transparent so that the animal’s behavior could be monitored. A removable partition divided the two compartments. During conditioning, the compartments were divided by a solid partition. On the test day, the solid partition was replaced with a partition that had an opening, allowing the animal free access to both compartments. Previous experiments using this set-up have indicated that the apparatus is unbiased, as untreated animals do not show a preference for one compartment over the other.
2.4. Conditioning Training
CPP training was conducted over three conditioning trials. Each conditioning trial was composed of two sessions conducted over two consecutive days. During the drug session, animals received a s.c. injection of test compound and were confined to one of the compartments for 30 min. On the other day, the vehicle session, animals received an injection of vehicle and were confined to the opposite compartment for 30 min. One group of mice received vehicle in both compartments and served as controls. These two sessions were repeated over six consecutive days such that animals received three drug sessions and three vehicle sessions, during the three conditioning trials. The particular compartment paired with the drug and the order of placement into the drug-paired versus saline-paired compartment was counterbalanced across groups.
2.5. CPP Test Day
24 hr after the last conditioning session, animals were given access to both compartments simultaneously for 15 min and the amount of time the animals spent in each compartment was recorded.
2.6. Assessment of Global Activity
During conditioning, overall activity of the animals following drug and vehicle injections was recorded on all days. These data were captured by the Spontaneous Motor Recording and Tracking software system (SMART; Panlab, Barcelona, Spain), a color image-capturing system that works in real time and tracks all the movements of the animal for a given amount of time via a video camera connected to the computer. This system tracks and records all behavior that results in any movement/positional changes. We term this as “global activity,” because it encompasses all movement detected including fine movement, movement due to rearing, grooming, sniffing, and locomotor activity.
2.7. Dosing Regimen
2.7.1
Initial experiments examined the effects of AT-1001 and AT-1012 alone. Animals received s.c. injections of AT-1001 (0.3–10 mg/kg; N = 8–10/group) or AT-1012 (1–10 mg/kg; N = 8/group). During the drug session, animals received a dose of the test compound and immediately placed in the drug-paired compartment for 30 min, whereas during the vehicle sessions, animals were confined to the opposite compartment following a vehicle injection. The two sessions were repeated over six consecutive days such that animals received three drug sessions and three vehicle sessions. For testing each compound, separate groups of mice (N = 9/group) received vehicle injections before placement into either compartment and served as controls.
2.7.2
The dose-dependent effects of cocaine on CPP and global activity were examined by testing groups of animals after s.c. injections of 1, 5, 10, 20 and 30 mg/kg cocaine (N = 8–10/group) as described above.
2.7.3
To examine the effects of AT-1001 and AT-1012 on cocaine-induced behavior, separate groups of animals received pretreatment with AT-1001 (0.3, 1, 3 or 10 mg/kg, s.c., N = 8–16/group) or AT-1012 (1, 3 or 10 mg/kg, s.c.; N = 8/group) 10 minutes prior to receiving an injection of 5 mg/kg cocaine during the drug sessions. Immediately following the cocaine injection, animals were placed into their drug-paired compartment and tested using the same procedure as above.
2.7.4
The effect of AT-1001 (0.3, 1, 3 or 10 mg/kg, s.c. N = 8–16/group) on behaviors produced by the 30 mg/kg dose of cocaine was also examined. Using two doses of cocaine, 5 mg/kg (the lowest dose producing CPP) and 30 mg/kg (produces CPP and robust behavioral sensitization), allowed us to examine separately the effects of the α3β4*–selective ligands on cocaine-induced CPP and behavioral sensitization. Separate groups of mice received vehicle injections (two injections 10-min apart similar to experimental groups) before placement into either compartment and served as controls.
2.8. Statistical Analyses
Global activity was analyzed using a mixed ANOVA design with drug treatments (AT-1001, AT-1012, cocaine) as between subjects measures and injection day (first versus third) as a repeated measure. Significant interactions were further analyzed with one-way ANOVAs and Bonferroni post-hoc tests. To examine sensitization effects, following a significant overall ANOVA, t-tests were used to compare data from the third injection to the first injection. Sensitization was defined as a within-subject increase in a behavioral response following the third injection relative to the first injection. For the CPP test day data, a difference score was calculated, as time spent in the drug-paired compartment minus time spent in the vehicle-paired compartment. As shown by the first white bars in the figures 2A, 4A, 4B and 6A, vehicle animals spent the same amount of time in both compartments such that the difference score is not significantly different from 0 sec. Difference scores were analyzed using ANOVAs and significant effects were further analyzed with post-hoc tests. A conditioned place preference (CPP) was evident if animals spent significantly more time in their drug-paired compartment, resulting in a positive difference score relative to control animals. A conditioned place aversion (CPA) was evident if animals spent significantly less time in their drug-paired compartment resulting in a negative difference score compared to vehicle controls. The level of significance was set at P< 0.05.
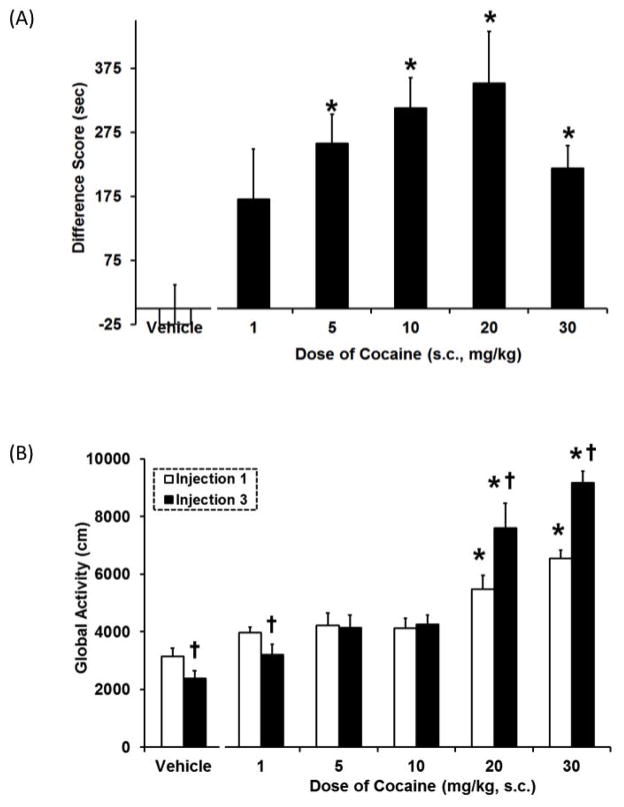
Figure 2. Dose response curve for cocaine CPP (A) and global activity (B).
Data are mean (± SEM) Difference score calculated as time spent in the drug-paired compartment minus time spent in the vehicle-paired compartment (A) or activity (cm) following first and last drug injection (B). For the conditioning sessions, animals received an s.c. injection of their assigned dose of cocaine or vehicle and were immediately placed into their designated compartment. *, P<0.05, significant difference from vehicle controls. †, P<0.05, significant difference from the first injection.
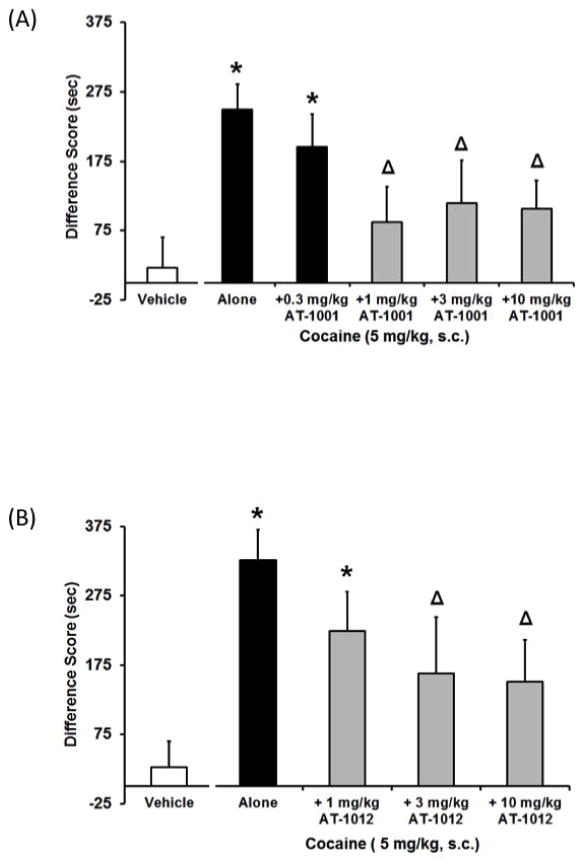
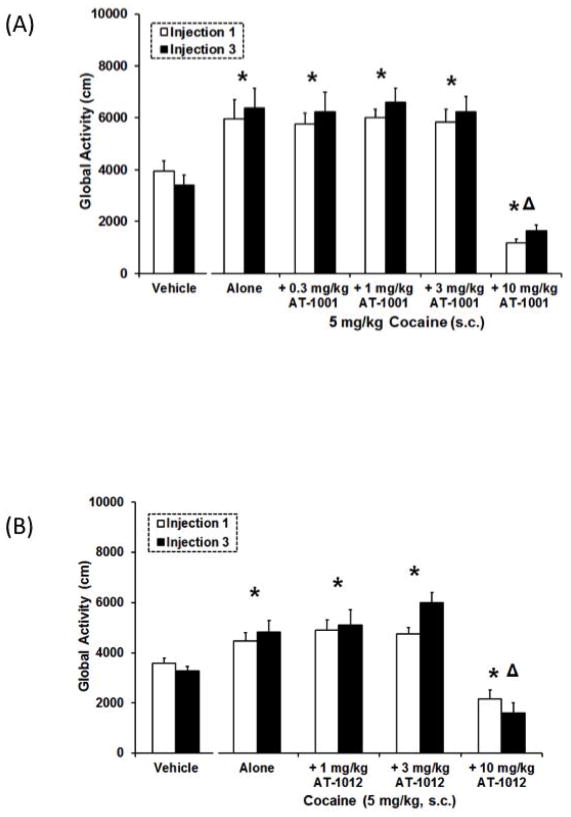
Figure 4. Effects of AT-1001 (A; 0.3–10mg/kg) and AT-1012 (B; 1–10 mg/kg) on CPP induced by 5 mg/kg cocaine.
Data are mean (± SEM) Difference score calculated as time spent in the drug-paired compartment minus time spent in the vehicle-paired compartment. *, P<0.05, significant difference from vehicle controls. △, P<0.05, significant difference from cocaine alone.
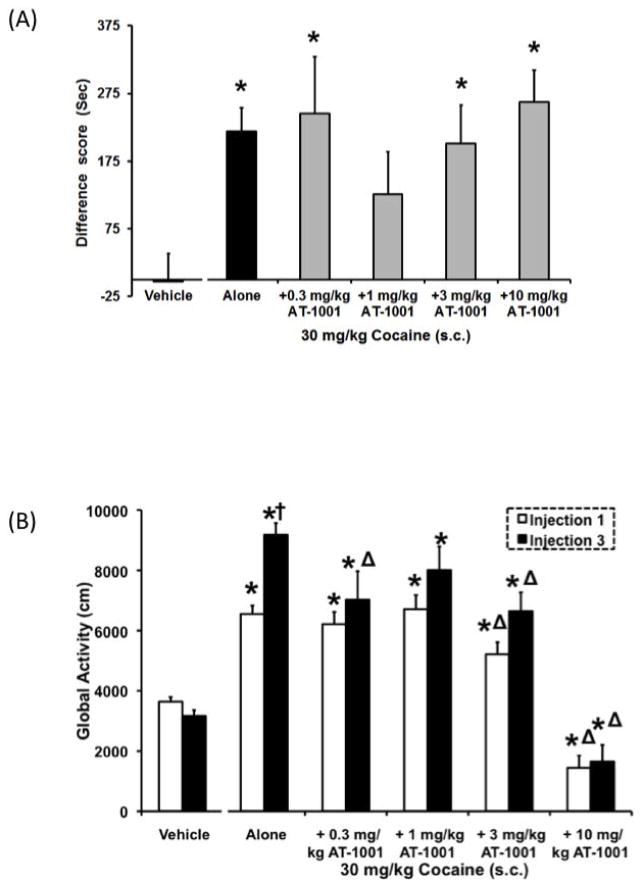
Figure 6. Effects of AT-1001 (0.3–10mg/kg) on CPP (A) and global activity (B) induced by 30 mg/kg cocaine.
Data are mean (± SEM) Difference score calculated as time spent in the drug-paired compartment minus time spent in the vehicle-paired compartment (A) or activity (cm) following the first and last drug injection (B). For drug-paired sessions during conditioning, animals received an s.c. injection of AT-1001 10 minutes prior to receiving an injection of 30 mg/kg cocaine and were placed immediately into their designated compartment. Vehicle controls received two injections 10-min apart and animals were placed into test compartments immediately following the second injection. *, P<0.05, significant difference from vehicle controls. △, P<0.05, significant difference from cocaine alone.†, P<0.05, significant difference from the first injection.
3. Results
3.1. Cocaine dose-response curve for CPP and sensitization to global activity in ICR mice
Cocaine induced a dose-dependent CPP (F5,53=7.6, P<0.05, Figure 2A). All doses except 1 mg/kg produced a significant CPP (P<0.05). Cocaine administration also caused dose-dependent changes in global activity and behavioral sensitization (Figure 2B). The overall ANOVA indicated a significant dose × injection day interaction (F5,53=12.8, P<0.05). Following the first and third injections, 20 and 30 mg/kg cocaine produced an increase in global activity compared to vehicle controls. Furthermore, at the 20 and 30 mg/kg cocaine dose, sensitization of cocaine-induced global activity was also evident, as a significant increase in activity after the third injection compared to the first injection (P<0.05). In contrast, vehicle controls and 1 mg/kg cocaine produced a significant decrease in global activity after the third injection relative to the first injection (P<0.05), indicative of the development of familiarity with the environment.
3.2. Effect of AT-1001 and AT-1012 alone on CPP and Global Activity
The effect of repeated injections of AT-1001 and AT-1012 alone on place conditioning is shown in Table 1. The overall ANOVAs showed no significant effects for both AT-1001 and AT-1012 indicating they did not produce CPP or CPA (n.s.).
TABLE 1.
Potential rewarding/aversive effects of AT-1001, AT-1012, and their respective controls as measured by the CPP paradigm. Data are mean (± SEM) difference score calculated as time spent in the drug-paired compartment minus time spent in the vehicle-paired compartment.
| Compound | Dose (mg/kg, s.c) | Difference Score (s) |
|---|---|---|
| AT-1001 | 0.0 | 11.1 ± 37.2 |
| 0.3 | 125 ± 50.0 | |
| 1.0 | −48.0 ± 76.2 | |
| 3.0 | −76.0 ± 66.9 | |
| 10.0 | 0.4 ± 55.4 | |
| AT-1012 | 0.0 | 27.22 ± 37.94 |
| 1.0 | −62.1 ± 38.0 | |
| 3.0 | −28.9 ± 63.7 | |
| 10.0 | −68.1 ± 63.6 |
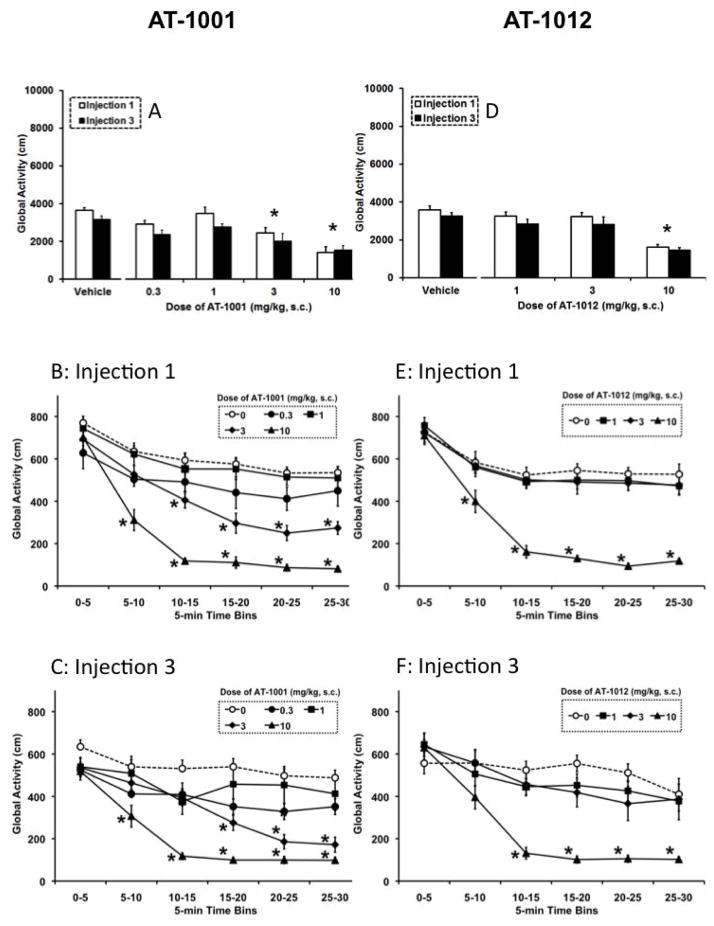
During conditioning, overall activity was assessed following the first and third injections of AT-1001 (Figure 3A) and AT-1012 (Figure 3D). For AT-1001, the overall ANOVA indicated that there was a significant main effect of dose (F4, 40=11.3, P<0.05). The two highest doses (3 and 10 mg/kg) of AT-1001 produced a decrease in global activity following the first and third injections relative to vehicle controls (P<0.05). These animals were curled up in a ball with their eyes closed but without any motor deficits as muscles were not rigid or flaccid. The mice were easily aroused when nudged and removed from the conditioning compartments. The time course of the decrease in global activity was plotted in 5-min bins across the 30-min conditioning session, after the first and third injections (Figures 3B and 3C). The 0.3 and 1.0 mg/kg doses of AT-1001 were not statistically different from vehicle controls throughout the 30-min test session following acute (first) and repeated (third) injections. However, the 3 mg/kg dose produced a decrease in activity that was evident after the first 10-mins following the first injection and after 15-mins following the third injection. The 10 mg/kg dose produced a decrease in activity that was significantly different compared to controls within the second 5-min bin following the first and third injections. Similarly, the overall ANOVA for AT-1012 alone indicated a significant main effect of dose (F3, 29=16.5, P<0.05). The highest dose (10 mg/kg) of AT-1012 produced a decrease in global activity following the first and third injections relative to vehicle controls (P<0.05) (Figure 3D). Looking at the time course of global activity across the 30-min session post-injection, 1 and 3 mg/kg AT-1012 produced no differences from vehicle controls at any time point, whereas the decrease in activity produced by the 10 mg/kg dose was evident after the first 5-mins following the first injection and after 10-mins following the third injection (Figures 3E and 3F).
Figure 3. Effects of AT-1001 (A–C) and AT-1012 (D–F) on global activity.
Figures 3A and 3D are mean (± SEM) total activity (cm) during the 30-min conditioning session following the first drug injection (first conditioning trial) and last drug injection (third conditioning trial). Figures 3B, 3C, 3E, and 3F are the time course data of activity (cm ± SEM) in 5-min bins during the 30-min session. Throughout conditioning, animals received a s.c. injection of the test compound and were immediately placed in the drug-paired compartment for 30 min. Separate groups of mice received vehicle injections before placement into either compartment and served as controls. *, P<0.05, significant difference from vehicle controls.
3.3. Effect of AT-1001 and AT-1012 on CPP induced by 5 mg/kg cocaine
AT-1001 (0.3–10 mg/kg) was tested for its ability to attenuate CPP induced by 5 mg/kg cocaine. AT-1001 pretreatment dose-dependently blocked CPP induced by 5 mg/kg cocaine (F5,73=2.7, p<0.05, Figure 4A). Animals that received 5 mg/kg cocaine alone or with 0.3 mg/kg AT-1001 pretreatment spent significantly more time in their drug-paired compartment resulting in a positive difference score (difference score of 250 s and 196 s, respectively) relative to vehicle controls (21 s). However, animals that received a pretreatment of 1–10 mg/kg AT-1001 prior to 5 mg/kg cocaine during conditioning spent a similar amount of time in their drug-paired compartment relative to vehicle controls (n.s.) and spent significantly less time in the drug-paired compartment relative to cocaine alone (5 mg/kg; P<0.05) demonstrating a significant attenuation of cocaine CPP for these dose groups.
Similarly, AT-1012 also blocked CPP induced by 5 mg/kg cocaine (F4,37=4.0, p<0.05; Figure 4B). Pretreatment with 1 mg/kg AT-1012 did not attenuate cocaine-induced CPP, whereas 3 and 10 mg/kg AT-1012 attenuated cocaine CPP compared to 5 mg/kg cocaine alone (P<0.05).
3.4. Effect of AT-1001 and AT-1012 on Global Activity induced 5 mg/kg cocaine
At 0.3–3 mg/kg, AT-1001 did not alter the increase in global activity induced by 5 mg/kg cocaine following acute or repeated administration (Figure 5A). The overall ANOVA indicated a significant main effect of dose (F5,73=24.1, P<0.05) and 5 mg/kg cocaine alone produced a significant increase in global activity relative to vehicle controls (P<0.05) that was not altered by pretreatment with 0.3–3 mg/kg AT-1001. However, 10 mg/kg AT-1001 suppressed cocaine-induced hyperactivity and resulted in a significant decrease in global activity relative to vehicle controls (P<0.05). Levels of global activity observed in cocaine-treated animals pretreated with 10 mg/kg AT-1001 were similar to those observed after 10 mg/kg AT-1001 was given alone (see above; Figure 3A).
Figure 5. Effects of AT-1001 (A; 0.3–10 mg/kg) and AT-1012 (B; 1–10mg/kg) on global activity induced by 5 mg/kg cocaine.
Data are mean (± SEM) activity (cm) following the first and last drug injection. For drug-paired sessions during conditioning, animals received an s.c. injection of AT-1001 or AT-1012 10 minutes prior to receiving an injection of 5 mg/kg cocaine and were placed immediately into their designated compartment. Vehicle controls received two injections 10-min apart and animals were placed into test compartments immediately following the second injection. *, P<0.05, significant difference from vehicle controls. △, P<0.05, significant difference from cocaine alone.
Similarly, only the highest dose of 10 mg/kg AT-1012 significantly decreased cocaine-induced global activity induced by 5 mg/kg cocaine (F4,37=19.1, P<0.05; Figure 5B) as pretreatment with 1 and 3 mg/kg AT-1012 did not alter cocaine-induced hyperactivity following acute and repeated administration.
3.5. Effect of AT-1001 on CPP and Global Activity induced by 30 mg/kg cocaine
The effect of AT-1001 pretreatment on CPP produced by 30 mg/kg cocaine is shown in Figure 6A. The overall ANOVA indicated that there was a significant main effect of dose when AT-1001 was given as a pretreatment 10 minutes prior to 30 mg/kg cocaine (F5,99=4.5, P<0.05). As expected, 30 mg/kg cocaine alone produced a robust CPP and pretreatment with 0.3, 3, and 10 mg/kg AT-1001 did not affect cocaine-induced CPP. Although pretreatment with 1 mg/kg AT-1001 did not produce CPP, the effect was not significantly different than that of cocaine alone. Taken together, the data indicate that at 30 mg/kg cocaine, AT-1001 did not consistently or significantly attenuate cocaine-induced CPP.
However, AT-1001 dose-dependently decreased sensitization of global activity induced by 30 mg/kg cocaine (F5,99=6.6, P<0.05; Figure 6B). Cocaine (30 mg/kg) alone produced an increase in global activity relative to controls following the first injection (P<0.05). After the third injection, animals that received cocaine alone showed a further increase in activity relative to the first injection, indicating behavioral sensitization. Pretreatment with 0.3–3 mg/kg AT-1001 prior to 30 mg/kg cocaine also increased global activity, following the first injection, similar to that produced by cocaine alone. After the third injection, animals pretreated with 0.3 and 1 mg/kg AT-1001 still exhibited an increase in global activity, however the increase in activity was similar to that observed after the first injection. Since the 0.3 and 1 mg/kg doses of AT-1001 alone did not decrease global activity (Figure 3A), it is possible that at these two lower doses, AT-1001 blocked the acquisition of cocaine-induced behavioral sensitization of global activity, without blocking the locomotor stimulant effect of cocaine per se.
However, animals receiving a pretreatment of 3 mg/kg AT-1001 prior to cocaine exhibited a lower level of hyperactivity compared to that with cocaine alone. With the highest dose of AT-1001 (10 mg/kg), cocaine-induced hyperactivity was blocked after the first and third injections. Since the higher doses of AT-1001 (3 and 10 mg/kg) produce a nonspecific decrease in activity (Figure 3A), both the stimulant and behavioral sensitization effects of cocaine are blocked at these doses (Figure 6B).
4. Discussion
The α3β4-selective nAChR ligands AT-1001 and AT-1012, which do not induce a place preference or aversion in mice per se (Table 1) significantly and dose-dependently suppressed acquisition of cocaine CPP induced by a low (5 mg/kg) dose of cocaine. The minimal effective doses for decreasing preference for the cocaine-paired compartment (reduction of the difference score) were 1 mg/kg (AT-1001, Fig. 4A) and 3 mg/kg (AT-1012, Fig. 4B). This effect of AT-1001 and AT-1012 is specific at doses below 10 mg/kg, since at these doses, the compounds either given alone or combined with cocaine, do not decrease overall activity of the animals (Fig. 3 and Fig. 5). These results suggest that the α3β4* nAChR, which play a role in nicotine addiction and smoking behavior as shown by human genetic studies [20–22] and animal knockout experiments [33–35] may also be involved in the rewarding effects of cocaine and possibly other psychostimulants.
Previous studies with the nonselective nAChR antagonist mecamylamine had provided pharmacological evidence that the cholinergic system is involved in psychostimulant reward, and that nAChR antagonism could be an approach to reduce psychostimulant addiction. Mecamylamine attenuated CPP induced by a 5 mg/kg dose of cocaine in mice [8] and decreased cocaine self-administration in rats [11, 36]. Several nAChR subtypes are thought to mediate the cholinergic mechanisms that underlie cocaine dependence. Mice null for the β2 nAChR subunit show decreased cocaine CPP [8] whereas α6 knockout mice do not develop cocaine CPP [13]. Interestingly, Sanjakdar and colleagues also reported that the α6β2* nAChR-selective antagonist, α-conotoxin MII blocked cocaine CPP both in α4 knockout and wild-type mice, further corroborating the importance of the β2 and the α6 nAChR subunit in mediating cocaine’s rewarding effects. Our pharmacological approach, using α3β4 nAChR-selective functional antagonists adds to this body of data and implicates a contribution of the α3β4 nAChR subtype to the rewarding effects of cocaine. These results are consistent with reports that 18-methoxycoronaridine (18-MC), a semisynthetic ibogaine derivative that is an α3β4 nAChR antagonist [37] decreases acquisition of cocaine CPP [38] and cocaine self-administration [39]. However, 18-MC is not very selective for the α3β4 nAChR [40], and it cannot be ruled out that other neurotransmitter systems contribute to the anti-addictive effects of 18-MC. In contrast, AT-1001 and AT-1012 have nanomolar affinity for the human and rat α3β4 nAChR, are >80-fold selectivity for the α3β4 nAChR compared to the α4β2 and α7 nAChR [26, 27, 29] and do not significantly bind to other non-nicotinic receptors [26]. We recently showed that AT-1001 has low efficacy partial agonist activity at the human α3β4 nAChR transfected into oocytes, showing approximately 30% of the efficacy of acetylcholine [28]. However, at concentrations at which it produces a partial agonist effect, AT-1001 causes significant desensitization of the ACh response, thus effectively antagonizing any further agonist-induced activation [28]. Therefore, although AT-1001 and closely-related compounds like AT-1012 have partial agonist activity at the α3β4 nAChR, they can act as potent ‘functional antagonists’ of human α3β4 nAChRs, and prevent the agonist (ACh or nicotine)-induced response.
Taken together, the inhibition of cocaine-induced CPP by AT-1001 and AT-1012 observed in the present study suggests that cholinergic activation via the α3β4 nAChR is important for producing rewarding effects of cocaine (and in cocaine-induced behavioral sensitization, as discussed below), and that antagonism of the ACh response via the α3β4 nAChR is sufficient to elicit a reduction in these rewarding effects, as observed in the CPP paradigm. Future studies with β4 nAChR knockout animals similar to the CPP studies with β2 nAChR knockout mice [8] would be useful to confirm the involvement of the β4 nAChR-containing subtype in cocaine-induced reward.
The effects of α3β4 nAChR partial agonists on cocaine-induced CPP warrants a discussion on the possible neurotransmitter systems and circuitries involved. It is thought that the main neurochemical correlate of the rewarding effect of cocaine is elevated extracellular dopamine (DA) concentrations in the NAc via inhibition of DA reuptake. But direct injections of of cocaine into the NAc fail to produce CPP in mice [41] whereas systemic cocaine injections elicit robust CPP (this study and others). Therefore, other brain regions have to be involved in the development of cocaine CPP and increased NAc DA levels. There is a large body of data showing that glutamatergic (see [42]) and cholinergic activation (see [3]) of the VTA play important roles in activating DA neurons terminating in the NAc. However, the cholinergic neurotransmission modulating cocaine-induced activation of the DA pathways, and thus the rewarding effects, is quite complex, because of the extensive distribution of different nAChRs subtypes that modulate DA function in the mesolimbic DA pathways. For example, β2* nAChR are expressed on cell bodies of DA and GABA neurons originating in the VTA and in the terminals of these neurons in the NAc. While there seems to be a direct neuronal circuit linking the β2* nAChR expressing neurons to NAc DA release, the circuitry involved in the α3β4* nAChR’s modulation of the rewarding effects of cocaine is not clear, particularly given the limited and specific localization of this nAChR subtype in the brain. By far the highest concentrations of the α3β4* nAChRs in the brain are in the MHb, IPN and the fasciculus retroflexus (fr) linking these two brain structures. We have shown, using in vitro autoradiography in rat brain slices, that [125I]-AT-1012 only labels the MHb, IPN and fr, with low to no labeling in other brain regions [27], confirming the specificity of this ligand for the α3β4 nAChR. While there is significant evidence that a direct pathway exists between the lateral habenula and the VTA, it is not known if the medial habenula or the IPN circuitry can directly alter the functioning of midbrain dopaminergic pathways leading to the VTA. There is, however, a small subpopulation of MHb neurons that have been shown to innervate the LHb [43], suggesting the possibility of the MHb regulating the VTA via the LHb. However, this has not yet been established, and further studies are needed to understand the signaling from the MHb/IPN to other brain regions involved in various aspects of cocaine and nicotine drug addiction.
Though cocaine elicits a robust CPP in doses ranging from a low dose of 5 mg/kg to higher doses of 30 mg/kg (Fig. 2), the locomotor and behavioral sensitization to repeated cocaine injections is only observed at the higher doses of 20 mg/kg and above (Fig. 2). These results are similar to those from other laboratories that reported behavioral sensitization in mice only observed with doses of cocaine 20 mg/kg or higher [4, 8, 44]. Interestingly, however, AT-1001, at the low doses of 0.3 and 1 mg/kg (at which it does not decrease overall activity), selectively decreased the behavioral sensitization observed with the third injection of 30 mg/kg cocaine, without attenuating the cocaine-induced increase in global activity post-injection (Fig. 6B). These effects of AT-1001 are distinctly unlike the effects of antipsychotics such as haloperidol, which block cocaine-induced locomotor sensitization but also attenuate cocaine-induced locomotor activity at the same doses [45, 46].
Previous studies have shown that psychostimulant reward and locomotor stimulation, as measured by the CPP paradigm, can occur through independent pathways [47]. Behavioral sensitization to pscyhostimulant and nicotine administration, defined as the progressive increase in locomotor activity upon repeated administration of the drug, is considered to be indicative of neuroadaptations that maintain the addicted state [48, 49]. These neuroadaptations primarily occur in the VTA [50] where nAChRs are abundantly expressed both dopaminergic and non-dopaminergic cell bodies, as well as on the terminals of glutamatergic afferents from the prefrontal cortex. Indeed, sensitization of the cocaine response was shown to correlated to upregulation of α4β2 nAChR in the VTA, but not those in the NAc [44]. The specific involvement of the VTA nAChR but not the accumbal nAChR was further corroborated by studies that showed that intra-accumbal injections of the β4 nAChR antagonist DHβE had no effect on cocaine-induced behavioral sensitization [9] whereas systemic DHβE injections suppressed the expression of behavioral sensitization after chronic cocaine administration [4]. The same study also showed that mecamylamine pretreatment completely prevented cocaine-induced behavioral sensitization, further confirming that nicotinic cholinergic antagonists can inhibit the development and expression of behavioral sensitization to cocaine. In this context, our results showing that the α3β4 nAChR-selective antagonist AT-1001 inhibits behavioral sensitization to repeated injections of cocaine are in line with the above observations with other nicotinic antagonists. However, the α3β4* nAChR have limited and specific neuroanatomical localization in the MHb-fr-IPN, which are not directly or functionally connected with VTA [15, 51]. Our results therefore suggest that there may be cholinergic pathways other than those converging into the VTA that are activated during the development of behavioral sensitization to cocaine. These remain to be determined and would indeed expand the understanding of role of cholinergic activation in psychostimulant drug effects.
We have previously shown that AT-1001 blocks nicotine self-administration [26] and nicotine-induced reinstatement in rats [29], at doses similar to those in this study, at which it showed a decrease in acquisition of cocaine CPP and blockade of cocaine-induced behavioral sensitization. Nicotine and cocaine addiction often occur as co-morbid addictions, and human clinical and epidemiological reports show that cocaine-dependent individuals exhibit higher rates of cigarette smoking [52, 53], while cigarette smoking can predispose adolescent individuals to other forms of drug abuse [54]. Our results with the AT-1001 class of potent and selective α3β4 nAChR antagonists, in this and previous studies, suggest that the α3β4 nAChR are involved in the cholinergic activation of drug addiction, and that α3β4 nAChR are a valuable target for medications to treat nicotine and cocaine addiction. The AT-1001 class of α3β4 functional antagonists are promising for development as smoking cessation medications and cocaine/drug abuse pharmacotherapy.
Figure 1.
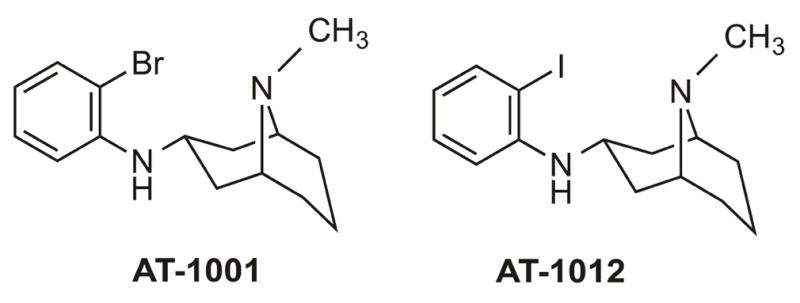
Chemical structures of α3β4 nAChR ligands AT-1001 and AT-1012
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse R43DA033744 (NZ), R44DA033744 (NZ) and R01DA020811 (LT).
Footnotes
Preliminary reports of these data were presented at the annual meetings of the Society for Neuroscience and College on Problem of Drug Dependence.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- 2.Mark GP, Kinney AE, Grubb MC, Keys AS. Involvement of acetylcholine in the nucleus accumbens in cocaine reinforcement. Ann N Y Acad Sci. 1999;877:792–795. doi: 10.1111/j.1749-6632.1999.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 3.Mark GP, Shabani S, Dobbs LK, Hansen ST. Cholinergic modulation of mesolimbic dopamine function and reward. Physiol Behav. 2011;104:76–81. doi: 10.1016/j.physbeh.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You ZB, Wang B, Zitzman D, Wise RA. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28:9021–9029. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciudad-Roberts A, Camarasa J, Pubill D, Escubedo E. Heteromeric nicotinic receptors are involved in the sensitization and addictive properties of MDMA in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;44:201–209. doi: 10.1016/j.pnpbp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 9.Champtiaux N, Kalivas PW, Bardo MT. Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behavioural Brain Research. 2006;168:120–126. doi: 10.1016/j.bbr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Zanetti L, de Kerchove D’Exaerde A, Zanardi A, Changeux JP, Picciotto MR, Zoli M. Inhibition of both alpha7* and beta2* nicotinic acetylcholine receptors is necessary to prevent development of sensitization to cocaine-elicited increases in extracellular dopamine levels in the ventral striatum. Psychopharmacology (Berl) 2006;187:181–188. doi: 10.1007/s00213-006-0419-y. [DOI] [PubMed] [Google Scholar]
- 11.Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiology & Behavior. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- 12.Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- 13.Sanjakdar SS, Maldoon PP, Marks MJ, Brunzell DH, Maskos U, McIntosh JM, Bowers MS, Damaj MI. Differential Roles of [alpha]6[beta]2[ast] and [alpha]4[beta]2[ast] Neuronal Nicotinic Receptors in Nicotine- and Cocaine-Conditioned Reward in Mice. Neuropsychopharmacology. 2015;40:350–360. doi: 10.1038/npp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Pharmacol. 2002b;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- 15.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- 18.Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhu PJ, Stewart RR, McIntosh JM, Weight FF. Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. J Neurophysiol. 2005;94:3081–3091. doi: 10.1152/jn.00974.2004. [DOI] [PubMed] [Google Scholar]
- 20.Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, Chen C, Jacobs K, Wheeler W, Landi MT, Ziegler RG, Hunter DJ, Chanock S, Hankinson S, Kraft P, Bergen AW. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. Novel alpha3beta4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. J Med Chem. 2010;53:8187–8191. doi: 10.1021/jm1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toll L, Zaveri NT, Polgar W, Jiang F, Khroyan TV, Zhou W, Xie X, Stauber GB, Costello MR, Leslie FM. AT-1001: A High Affinity and Selective α3β4 Nicotinic Acetylcholine Receptor Antagonist Blocks Nicotine Self-Administration in Rats. Neuropsychopharmacology. 2012;37:1367–1376. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Perry DC, Bupp J, Jiang F, Polgar WE, Toll L, Zaveri NT. [125I]AT-1012, a New High Affinity Radioligand for the α3β4 Nicotinic Acetylcholine Receptors. Neuropharmacology. 2014;77:193–199. doi: 10.1016/j.neuropharm.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaveri NT, Bertrand S, Yasuda D, Bertrand D. Nicotine & Tobacco Research. 2014. Functional Characterization of AT-1001, an α3β4 Nicotinic Acetylcholine Receptor Ligand, at Human α3β4 and α4β2 nAChR. in process. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cippitelli A, Wu J, Gaiolini KA, Mercatelli D, Schoch J, Gorman M, Ramirez A, Ciccocioppo R, Khroyan TV, Yasuda D, Zaveri NT, Pascual C, Xie XS, Toll L. AT-1001: a high-affinity alpha3beta4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol. 2015;172:1834–1845. doi: 10.1111/bph.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 32.Jiang F, Bupp J, Rhee S, Toll L, Zaveri NT. Radiosynthesis of a 125I analog of a highly selective alpha3beta4 nicotinic acetylcholine receptor antagonist ligand for use in autoradiography studies. Journal of Labelled Compounds and Radiopharmaceuticals. 2012;55:177–179. [Google Scholar]
- 33.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. Journal of Neuroscience. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Gorlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD, Ibanez-Tallon I. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc Natl Acad Sci U S A. 2013;110:17077–17082. doi: 10.1073/pnas.1313103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann N Y Acad Sci. 2000b;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- 38.McCallum SE, Glick SD. 18-Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett. 2009;458:57–59. doi: 10.1016/j.neulet.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- 40.Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, Glick SD, Bidlack JM. Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents. J Med Chem. 2003;46:2716–2730. doi: 10.1021/jm020562o. [DOI] [PubMed] [Google Scholar]
- 41.Hemby SE, Jones GH, Justice JB, Jr, Neill DB. Conditioned locomotor activity but not conditioned place preference following intra-accumbens infusions of cocaine. Psychopharmacology. 1992;106:330–336. doi: 10.1007/BF02245413. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- 44.Metaxas A, Keyworth HL, Yoo JH, Chen Y, Kitchen I, Bailey A. The stereotypy-inducing and OCD-like effects of chronic ‘binge’ cocaine are modulated by distinct subtypes of nicotinic acetylcholine receptors. British Journal of Pharmacology. 2012;167:450–464. doi: 10.1111/j.1476-5381.2012.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chausmer AL, Katz JL. The role of D2-like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology (Berl) 2001;155:69–77. doi: 10.1007/s002130000668. [DOI] [PubMed] [Google Scholar]
- 46.Cha SK, Kang UG. Effects of clozapine, haloperidol, and fluoxetine on the reversal of cocaine-induced locomotor sensitization. Psychiatry Investig. 2014;11:454–458. doi: 10.4306/pi.2014.11.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carr GD, Phillips AG, Fibiger HC. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology (Berl) 1988;94:221–226. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- 48.Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- 49.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 51.Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I. The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology. doi: 10.1016/j.neuropharm.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- 53.Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. The American journal of drug and alcohol abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindsay GB, Rainey J. Psychosocial and pharmacologic explanations of nicotine’s “gateway drug” function. J Sch Health. 1997;67:123–126. doi: 10.1111/j.1746-1561.1997.tb03430.x. [DOI] [PubMed] [Google Scholar]