Abstract
Aggression is frequently comorbid with neuropsychiatric conditions and is a predictor of worse outcomes, yet current pharmacotherapies are insufficient and have debilitating side effects, precluding broad use. Multiple models of aggression across species suggest that the nicotinic acetylcholine receptor (nAChR) agonist nicotine has anti-aggressive (serenic) properties. Here we demonstrate dose-dependent serenic effects of acute nicotine administration in three distinct mouse strains: C57BL/6, BALB/c, and CD1. While acute nicotine administration (0.25 mg/kg) modestly reduced solitary homecage locomotion, this could not account for nicotine’s serenic effects since social encounters eliminated the hypolocomotor effect, and nicotine did not alter social interaction times. Pretreatment with the homomeric (α7 subunit) nAChR antagonist methyllycaconitine (5 mg/kg), but not the heteromeric (β2 or β4 subunit-containing) nAChR antagonist dihydro-β-erythroidine (DHβE, 3 mg/kg), blocked the serenic effects of nicotine. By contrast, pretreatment with DHβE blocked the effect of acute nicotine administration on locomotion, uncoupling nicotine’s serenic and hypolocomotor effects. Finally, the α7 nAChR partial agonist GTS-21 reduced aggression in C57BL/6 mice. These results support the idea that acute nicotine administration has serenic effects and provide evidence for specificity of this effect distinct from effects on locomotion. Furthermore, pharmacological studies suggest that activation of α7 nAChRs underlies the serenic effects of nicotine. Further studies of nAChRs could enhance understanding of the neurobiology of aggression and may lead to the development of novel, more specific treatments for pathological aggression.
Keywords: aggression, nicotinic acetylcholine receptor, nicotine, GTS-21, DMXB, α7
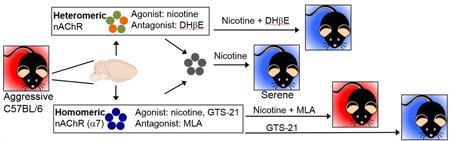
Graphical abstract
1. Introduction
Aggressive behavior is commonly comorbid with neuropsychiatric conditions. While pharmacotherapy with neuroleptics and mood stabilizing agents such as anti-epileptics and lithium can be highly effective for certain individuals, it is incompletely effective in many cases [1], persistent use of these agents frequently results in metabolic and movement disorders, and in geriatric patients with dementia, confers increased risk of death [2]. Further, the exact mechanism of action of these agents as they relate to anti-aggressive, or “serenic” activity, is incompletely understood [3]. Investigation of pharmacological mechanisms that reduce aggression but are distinct from currently available agents may lead to novel, increasingly specific treatments for aggression with more favorable side effect profiles, and provide further understanding of the molecular mechanisms regulating aggression.
Nicotinic acetylcholine receptor (nAChR) modulation by the endogenous neurotransmitter acetylcholine or exogenous agents such as nicotine is one potential mechanism to influence aggressive behavior. Nicotinic AChRs are heterogeneous, widely expressed throughout the brain, influence the release of several neurotransmitters known to be involved in aggression including serotonin, GABA, and dopamine [4, 5], and can modulate higher-order cognitive processes such as attention, mood, anxiety, and impulsivity, all of which may subsequently influence the likelihood of aggressive behavior [6]. Acute nicotine administration reduces aggression in animal models across multiple species, including cats in rat-biting attack assays [7], rats in shock-induced fighting and muricide models [8–12], and mice in resident-intruder paradigms [13]. Furthermore, case studies in non-smoking humans with aggression secondary to severe dementia [14, 15] or autism spectrum disorder [16] report that transdermal nicotine reduces persistent aggression. The convergence of these findings across species and aggression models provide strong rationale to study nAChR modulation as a pharmacological target for the treatment of aggression.
The mechanisms underlying the ability of nicotine to reduce aggression are not known. The serenic effect of nicotine is not blocked by pretreatment with the peripherally active non-specific nAChR antagonist hexamethonium [7, 12], which suggests a central site of action; however, the distinct subset of nAChRs that mediate the effect are unknown. Furthermore, altered locomotor activity can significantly confound the interpretation of behavioral assays following acute nicotine administration in rodents. In the current study we investigated the serenic effect of nicotine in three distinct mouse strains to determine whether this behavioral effect is robust across genetic backgrounds. We used pharmacological tools, including nAChR agonists and antagonists, to identify the nAChR subtypes mediating nicotine’s serenic effect. We also determined whether these effects were influenced by any changes in activity and sociability. These results suggest that pharmacological agents targeting specific subsets of nAChRs should be studied as therapeutic agents to treat persistent aggression.
2. Materials and Methods
2.1 Animals
Male C57BL/6 (B6) and BALB/c mice (age at start of single housing: 10–16 weeks) were purchased from Jackson Laboratory or Charles River. Male retired breeder CD1 mice were purchased from Charles River. Resident mice were single-housed for at least two weeks prior to aggression testing. Intruder mice were group housed (4–5 mice per cage). All mice were housed under standard conditions (temperature 21 ± 2 °C, 12 hour light-dark cycle with food and water available ad libitum). The Yale University Animal Care and Use Committee approved all procedures.
2.2 Drugs
Nicotine hydrogen bitartrate (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in 0.9% normal saline (vehicle). pH was adjusted to 7.0 ± 0.5 with 1M sodium hydroxide (J.T. Baker, Center Valley, PA, USA), and solutions were prepared fresh daily and kept in the dark with aluminum foil. Nicotine doses are expressed as free-base [17] and were injected intraperitoneally (i.p.) 10 minutes prior to testing. Dihydro-β-erythroidine (DHβE) hydrobromide (Tocris, Ellisville, MO, USA) and methyllycaconitine (MLA) citrate salt (Sigma-Aldrich) were dissolved in 0.9% normal saline vehicle, and for pretreatment experiments were injected i.p. 15 minutes prior to nicotine or vehicle i.p. injection, with behavioral testing commencing 10 minutes after last injection. DHβE and MLA doses are expressed as their salts. GTS-21 (Sigma-Aldrich) was dissolved in 0.9% normal saline vehicle and injected i.p. 10 minutes prior to resident-intruder testing. Volumes for i.p. injections were 10 mL/kg for B6 and BALB/c mice, and 5 mL/kg for CD1 mice due to increased body mass.
2.3 Behavioral tests
2.3.1 General testing conditions
Resident-intruder tests were performed within the animal housing facility, whereas sociability and locomotor tests were performed in a dedicated behavioral testing room and animals were allowed at least 30 minutes to habituate to the behavior room prior to testing. All behavioral testing was conducted between 0800 and 1800.
2.3.2 Resident-intruder test
Resident mice were single-housed, with bedding unchanged, for at least two weeks prior to testing. Intruder mice were always group-housed B6 mice of comparable age to resident B6 or BALB/c mice. After the single-housing period, B6 and BALB/c mice underwent three training bouts constituting a 10-minute interaction with an intruder mouse in the resident home cage with at least a one-day interval between training bouts. This training paradigm served to reduce variability in aggressive behavior (data not shown) and allowed animals to reach a stable baseline [18]. Because of modest baseline aggression, the B6 cohorts used for within-subject tests were selected for aggressive behavior based on training trials, whereas the B6 cohorts for the between-subjects experiments were randomly distributed to treatment groups without removing any subjects, to measure the entire range of behavior. CD1 mice were trained in a similar manner with exposure to a B6 intruder in seven training bouts spaced by one day. The resident-intruder test was conducted in the animal facility to maximize expression of aggressive behavior in the resident’s home environment by minimizing novelty in the environment aside from the presence of the intruder. The tail of the intruder mouse was marked to allow for differentiation from the resident. To begin the test, the home cage food tray was removed and the intruder was gently placed in the resident’s home cage at the opposite end of the cage, then the cage replaced on the rack. Time to first resident attack on the intruder was recorded, with a ceiling of 10 minutes. An attack constituted an offensive aggressive behavior such as lunging with contact, boxing, or biting such that the intruder assumes a defensive posture. Care was taken to avoid physical harm to the intruder. If any aggressive behavior resulted in visible wounds, the bout would be stopped, although no fight wounds were observed in any of these experiments. Aggressive grooming or close inspection was not scored as offensive aggression. Likewise, offensive aggression that resulted in a prolonged chase within the cage was only counted as a single attack until the chase ceased and another offensive aggressive maneuver occurred. Once the first attack occurred, the number of subsequent attacks over the next 10 minutes was counted. In a small number of cases, the first attack was made by the intruder on the resident. In this instance, the bout was ended and the resident was scored as non-aggressive (attack latency = 600 seconds). Residents were tested in the resident-intruder test only once daily. Intruders participated in at most two bouts per test day, and care was taken to avoid interactions of residents with the same intruder. Tests were scored in real time by a trained observer. For nicotine, dose ordering was first done by vehicle administration, then highest to lowest nicotine dose and ending with vehicle to determine whether mice return to baseline (Fig. 1). These results were then followed up by a counterbalanced dosing order in the case of nicotine 0.25 mg/kg vs. vehicle in a novel cohort of B6 mice, and similar results were found.
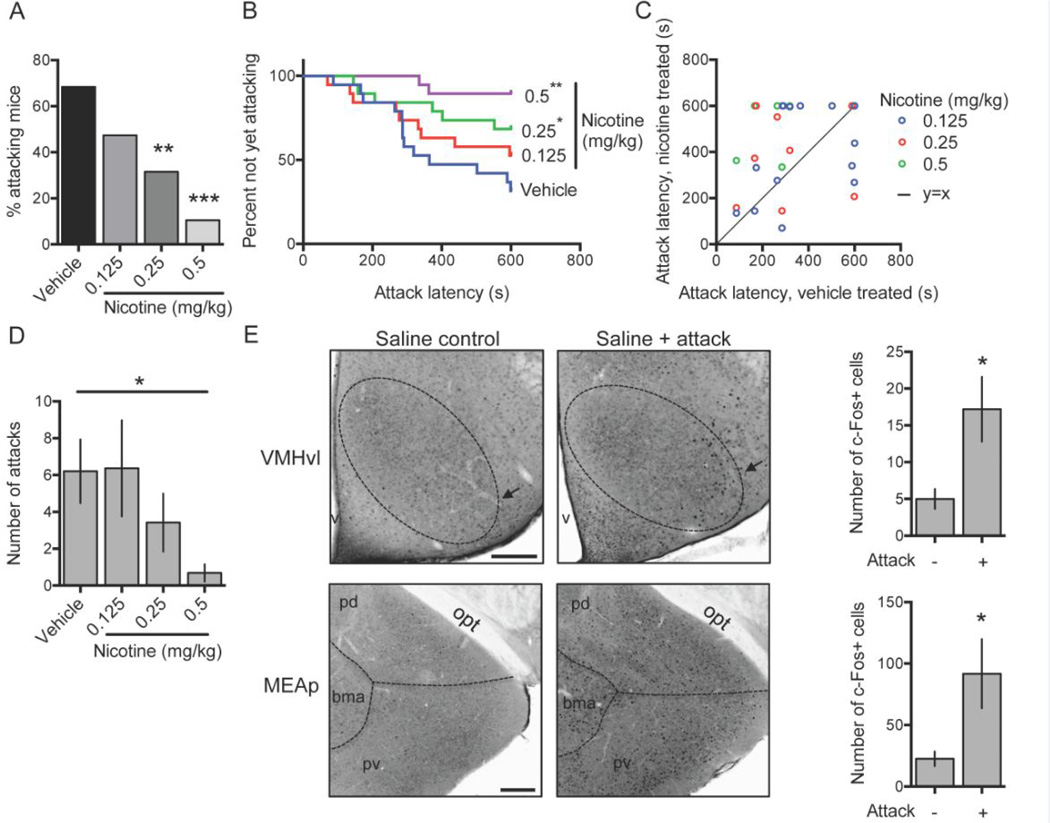
Figure 1.
Single-housed male C57BL/6 mice were tested in resident-intruder assays after vehicle or nicotine administration and percentage of attacking mice (A), latency to first attack (B, C), and number of attacks (D) were measured; N = 19 mice, within-subject testing. Following an aggressive encounter or a control condition without exposure to an intruder mouse, c-Fos positive cells were measured in two regions implicated in aggressive behavior in mice: the ventromedial hypothalamus (VMH, dotted oval) ventrolateral portion (vl, arrow) and the medial amygdala posterior part (MEAp: combination of posterordorsal (pd) and posteroventral (pv)); N = 5 mice per treatment condition (E). Error bars represent S.E.M. Scale bar in E, 200 microns. *P < 0.05; **P < 0.01, ***P < 0.001 vs. vehicle-treated group. bma, basomedial amygdala. opt, optic tract, v, third ventricle.
2.3.3 Locomotor testing
Locomotion was quantified using a Noldus EthovisionXT system. Single-housed B6 mice were used to approximate locomotor properties of resident mice. Mice were injected i.p. with nicotine or vehicle, then returned to their home cage for 10 minutes. After 10 minutes, home cage lids were removed and total distance traveled as well as time spent moving were quantified over the following 10 minutes. Nicotine and vehicle treated mice were recorded side-by-side to eliminate confounding by time of day and were analyzed by paired analysis.
2.3.4 Sociability testing
Sociability testing was measured as the extent to which a mouse approaches and interacts with a novel “stimulus” mouse, as has been reported previously [19]. Tested mice were single-housed B6 mice and stimulus mice were group-housed B6 male mice. In this manner we could determine the sociability properties of socially isolated mice such as those used as residents in resident-intruder tests. The sociability testing apparatus (Noldus) consists of three interconnected chambers with clear Plexiglas walls and white plastic floor. Two identical stimulus mouse cages with Plexiglas bars were placed in the left and right chambers. Tested mice were injected with nicotine or vehicle i.p. 10 minutes prior to the start of the sociability test and were placed back in their home cages until the start of the test. The test began by placing the tested mouse in the center chamber and allowing the mouse to explore the apparatus. Time spent in each chamber, time sniffing each stimulus mouse cage, and locomotor parameters (distance traveled, time spent moving) were recorded for five minutes using the Noldus EthovisionXT behavioral analysis system. After 5 minutes, the tops of both stimulus mouse cages were removed simultaneously, and a stimulus mouse was placed into one cage that had been designated as the “social” side. The hand motion was mimicked at the same time in the stimulus mouse cage on the “nonsocial” side, and both cage tops were replaced simultaneously. The same behavioral parameters were recorded for the next five minutes. Both mice were then removed from the sociability cage, the entire apparatus washed with 30% ethanol followed by multiple water rinses, and another test began. Testing was performed in a counterbalanced manner by dose.
Quantitative analysis was performed as has been reported [19]. Time spent in each chamber (middle, social, and nonsocial), time spent sniffing the social and nonsocial cylinder, distance traveled, and time spent moving were recorded in the absence and presence of the stimulus mouse. The “social approach score” was calculated for each tested mouse in the absence and presence of the stimulus mouse by adding 1 point for each second in the social side, subtracting 1 point for each second in the nonsocial side, and adding 0 points for each second in the center chamber. A similar “cylinder sniffing preference” score was calculated for each tested mouse in the absence and presence of the stimulus mouse by adding 1 point for each second the test mouse sniffed the social cylinder and subtracting 1 point for each second the test mouse sniffed the nonsocial cylinder. Finally, we calculated the “change score” for each tested mouse, which was computed by subtracting the value of the social approach score or the cylinder sniffing preference in the absence of the stimulus mouse from the social approach score or the cylinder sniffing preference in the presence of the stimulus mouse. This method takes into account any intrinsic side preference of the tested mouse. Social approach/cylinder sniffing scores and locomotor data were analyzed by two-way ANOVA with treatment as between-subject factor and presence/absence of stimulus mouse as within-subject factor. Change scores were analyzed by unpaired t-test.
2.4 c-Fos immunohistochemistry, microscopy, and image analysis
B6 mice that had undergone resident-intruder tests were used for immunohistochemistry experiments. Residents were injected i.p. with 0.9% normal saline 10 minutes prior to a standard resident-intruder test of 10 minute duration. Intruder mice were the same group-housed B6 mice used for resident-intruder testing. Control mice were injected with 0.9% normal saline and returned to their home cage without resident-intruder interactions. Resident mice who attacked the intruder (N = 5) and control mice (N = 5) were then anesthetized 90–120 minutes later with an overdose of pentobarbital and perfused intracardially with ice-cold phosphate-buffered saline (PBS, 0.1 M, pH 7.3) followed by ice-cold 4% paraformaldehyde (PFA). Brains were carefully dissected and post-fixed for 24 hours in 4% PFA at 4 °C, then cryoprotected by transfer to PBS containing 30% sucrose at 4 °C. Brains were stored at 4 °C in PBS + 30% sucrose until sectioning.
Sections (40 µm) were cut with a freezing microtome and stored free-floating in PBS containing 0.02 % sodium azide. Two sections from each mouse containing the ventromedial hypothalamus (VMH) or the medial amygdala posterior part (MEAp) were selected, blocked in PBS containing 3% normal donkey serum and 0.3 % Triton X-100 at room temperature for 1 hour, then incubated with rabbit anti-c-Fos diluted in blocking solution (1:500, Santa Cruz Biotechnology) at 4 °C for 48 hours, rinsed in PBS, then incubated in donkey anti-rabbit Alexa Fluor 555 (1:500, Life Technologies) diluted in PBS containing 0.3% Triton X-100 at room temperature for 2 hours. Slices were rinsed, mounted on Superfrost Plus microscope slides (Fisher Scientific), covered with mounting media (Vectashield, Vector Laboratories), and topped with a cover slip sealed with nail polish.
Imaging was performed using an Olympus FV10i confocal microscope at 40X magnification using a single Z-slice. For each mouse, two sections were imaged bilaterally when possible, typically resulting in four images per mouse. Images were analyzed blinded to condition using Fiji [20]. Anatomical areas of interest were outlined, intensity thresholded, and counted using the “analyze particles” macro (size filter: 20–200 pixels2; circularity filter: 0.5–1.0). The total number of c-Fos positive cells in each anatomical region was then averaged (typically four data points per mouse: right and left side X two sections) to obtain a single value per mouse.
2.5 Statistical analysis
Unless otherwise stated, all statistical comparisons are to vehicle-treated subjects. To compare the percentage of attacking residents, McNemar’s test (computed using the binomial distribution) was used for within-subject experiments, and χ2-test for between-subject experiments. The χ2-test for trend or the log-rank test for trend was used to compute the significance of trends in percentage of attacking residents and latency to attack, respectively, in evenly ordered dose-response experiments. Hazard ratios for latency to attack were computed using parametric survival models with adaptive Gauss-Hermite quadrature integration, specifying an exponential distribution for latency. Mice that did not attack by 600 seconds were censored in analyses. When data were generated from repeated measurements, models were analyzed in a multi-level framework specifying an exchangeable variance-covariance matrix in order to account for clustering within mice. Sensitivity analyses demonstrated survival model fit and results were robust to varying specification of the latency distribution and variance-covariance matrix (results not shown). To analyze c-Fos labeling, number of attacks, locomotion, and sociability data, we used the Student t-test to compare two groups (paired or unpaired, as appropriate), and ANOVA (one- or two-way, as appropriate) to compare three or more groups. Repeated measures analysis was used for experiments designated as “within-subjects”. Post hoc comparisons were made using the Holm-Sidak method. All tests were two-sided/tailed. Values of P < 0.05 were considered to be significant. Bar graphs depict mean ± standard error of the mean (S.E.M.). Survival analyses were conducted in Stata version 13.1, all other analyses were conducted using Graphpad Prism6 for Mac.
3. Results
3.1 Acute nicotine administration reduces aggressive behavior in multiple mouse strains in a dose-dependent manner
In B6 mice there was a significant trend in percent of attacking intruders after acute injection of nicotine across a dose range of 0 mg/kg to 0.5 mg/kg [Chi-square test for trend: χ2(1, N = 19) = 14.27, P < 0.001], and doses of 0.25 mg/kg and 0.5 mg/kg significantly reduced the percentage of residents attacking within 10 minutes [N = 19; vehicle: 68%, nicotine 0.25 mg/kg: 32%, nicotine 0.5 mg/kg: 11%; vehicle vs. nicotine 0.25 mg/kg: P = 0.02; vehicle vs. nicotine 0.50 mg/kg, P = 0.001; McNemar test] (Fig. 1A). Latency to first attack was significantly increased by nicotine at both 0.25 mg/kg [HR = 0.37, 95% CI: 0.14–0.98, P = 0.045] and 0.5 mg/kg [HR = 0.09, 95% CI: 0.02–0.40, P = 0.002] (Fig. 1B–C). Nicotine also reduced the total number of attacks [F(3,54) = 3.68, P = 0.02], and post hoc analysis revealed this effect reached significance at the 0.5 mg/kg dose (P < 0.05) (Fig. 1D). The 0.25 mg/kg nicotine dose reduced total number of attacks by ~45%, but this effect did not reach set statistical significance. Dose ordering for this experiment began with vehicle followed by highest to lowest nicotine doses, which was chosen to minimize false positive findings due to resident sensitization to fighting (which would result in significant differences only to the highest doses of nicotine) or habituation to fighting (aggressive behavior that did not return to baseline would suggest habituation). To confirm that order of dosing did not influence results, we repeated this paradigm with a new cohort of mice (N = 16, within-subject) using counterbalanced dosing and an independent observer blinded to treatment condition and compared 0.25 mg/kg nicotine with its vehicle control. We again found that latency to attack was significantly increased by this dose of nicotine in a manner essentially identical to the previous dosing order [HR = 0.36, 95% CI: 0.14–0.88, P = 0.03]. Finally, to confirm the aggression levels we observed in our paradigm were comparable to those previously published in B6 resident-intruder tests, we performed immunohistochemistry for the immediate early gene c-Fos and quantified its expression in the ventrolateral portion (vl) of the VMH, and the MEAp [21] (Fig. 1E). Encounters culminating in offensive aggression after saline injection significantly upregulated c-Fos expression in both brain regions compared to saline injection alone without an aggressive encounter [VMHvl: T(8) = 2.65, P = 0.03; MEAp: T(8) = 2.41, P = 0.04; unpaired t-test], suggesting the offensive expression in this animal model is comparable to previous reports.
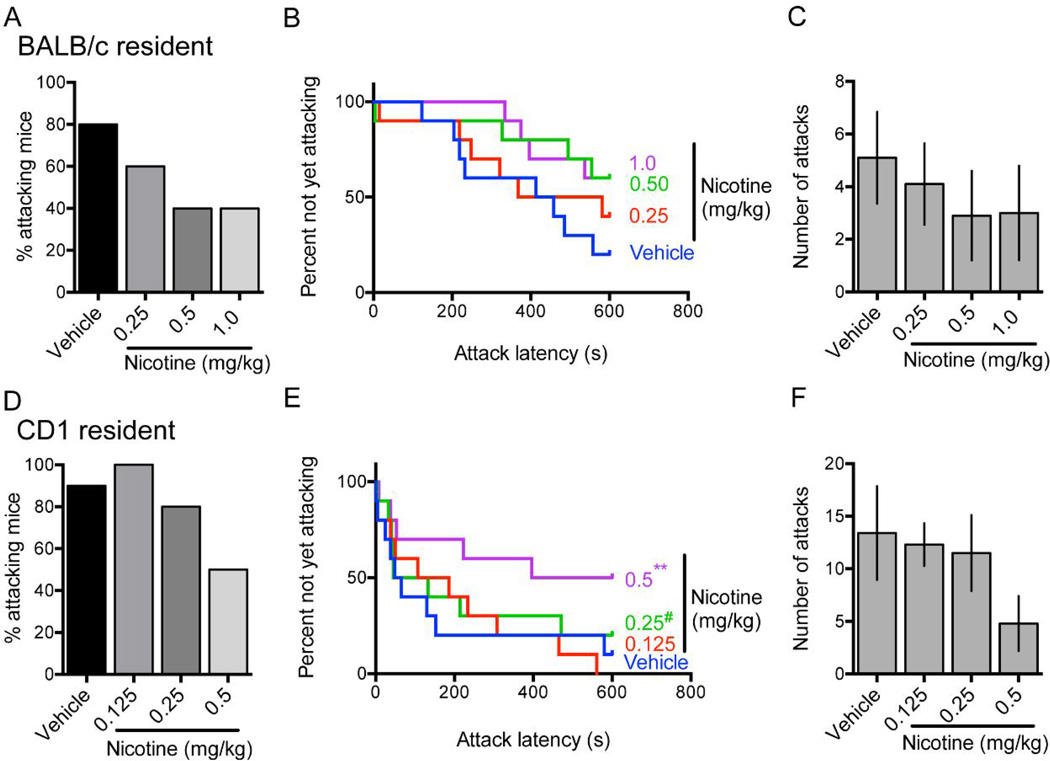
BALB/c and CD1 mice demonstrate different levels of innate aggression than B6 and have differing locomotor effects after acute nicotine administration [22–24]. In BALB/c residents, we observed a significant effect of increasing nicotine dose between 0 mg/kg and 1.0 mg/kg on percent of residents attacking [Chi-square test for trend: χ2(1, N = 10) = 3.96, P = .047] and latency to attack [Log-rank test for trend: χ2(1, N = 10) = 4.51, P = 0.03] (Fig. 2A, B). At both 0.5 mg/kg and 1.0 mg/kg nicotine increased latency to attack compared to vehicle, but this effect did not reach statistical significance at either dose [0.5 mg/kg: HR = 0.39, 95% CI: 0.12–1.30, P = 0.13; 1.0 mg/kg: HR = 0.37, 95% CI: 0.11–1.23, P = 0.11] (Fig. 2B). Nicotine did not significantly reduce the number of attacks at any dose [F(3,27) = 0.54, P = 0.66] (Fig. 2C). In CD1 residents, we observed a significant effect of increasing nicotine dose between 0 mg/kg and 0.5 mg/kg on percent attacking residents [Chi-square test for trend: χ2(1, N = 10) = 6.13, P = .01] and latency to attack [Log-rank test for trend: χ2(1, N = 10) = 5.04, P = 0.02] (Fig. 2D, E). Nicotine administered at 0.5 mg/kg significantly increased latency to attack compared to vehicle [HR = 0.14, 95% CI: 0.04 – 0.47, P = 0.001] (Fig. 2E). As with BALB/c mice, nicotine did not significantly reduce the number of attacks at any dose [F(3,27) = 1.67, P = 0.20] (Fig. 2F). Taken together, our results are consistent with the idea that nicotine can reduce aggressive behavior and suggest the generalizability of this effect across three mouse strains with different levels of innate aggressive behavior.
Figure 2.
Single-housed male BALB/c (A–C) or CD1 (D–F) residents were tested in resident-intruder assays after vehicle or nicotine administration and percentage of attacking mice (A, D), latency to first attack (B, E), and number of attacks (C, F) were measured; N = 10 mice per strain, within-subject testing. Error bars represent S.E.M. #P = 0.067; **P < 0.01 vs. vehicle-treated group.
3.2 The serenic effect of nicotine is likely not due to reduced locomotor activity or altered sociability
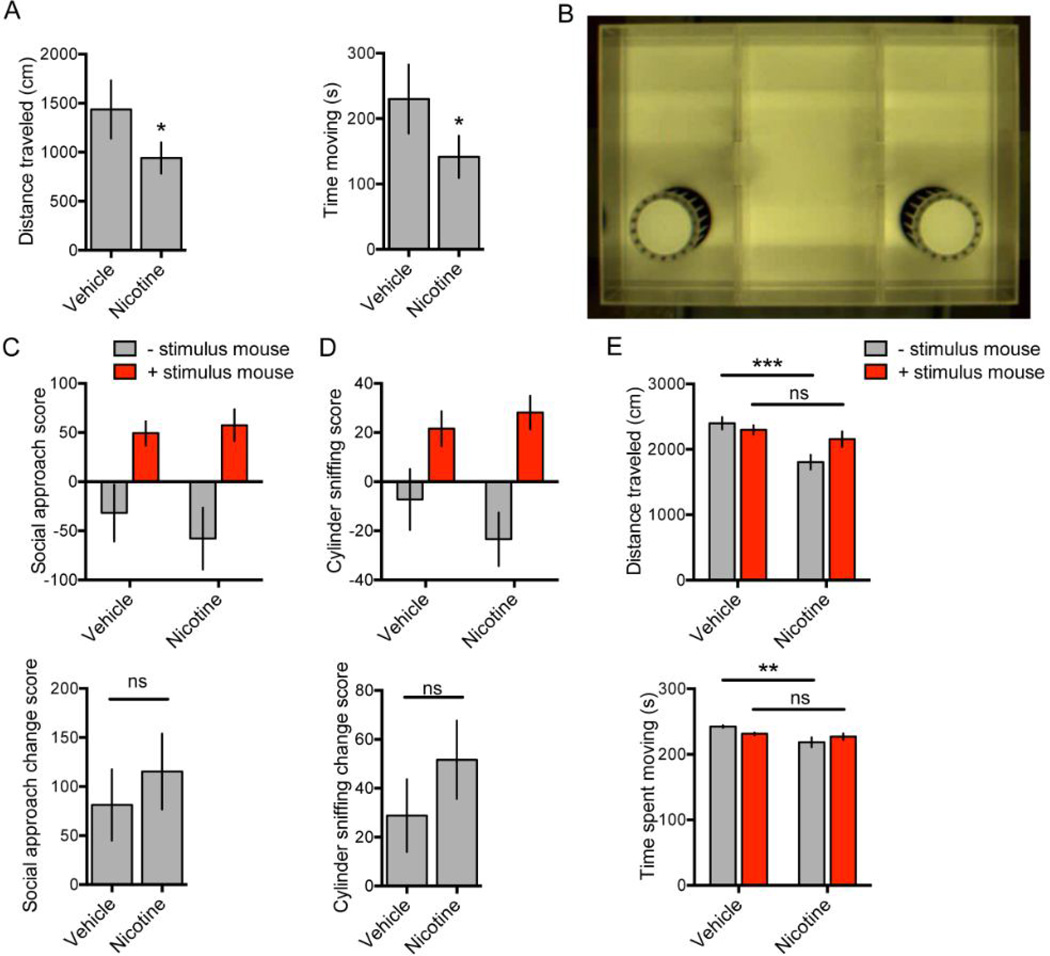
The finding that acute administration of nicotine reduces measures of aggression in mouse resident-intruder tests may be secondary to confounding behavioral effects that reduce the probability of attack but are independent of a change in primary aggressive motivation. Reduced locomotor activity or reduced interaction with another mouse or with novel objects might be measured as reduced aggression yet would not be informative about nicotine’s specific effect on aggression. We thus examined home cage locomotion, sociability measures, and locomotion in a social context in B6 mice that were socially isolated and administered serenic doses of nicotine. Home cage locomotion was modestly (~30%), but significantly, reduced by 0.25 mg/kg nicotine administration compared to vehicle injection 10 minutes prior to locomotor recording for a single 10-minute epoch [Time spent moving: T(4) = 3.42, P = 0.03; Total distance traveled: T(4) = 3.04, P = 0.04; paired t-test] (Fig. 3A).
Figure 3.
Nicotine (0.25 mg/kg) or vehicle was administered to single-housed male C57BL/6 resident mice. Ten minutes after injection, home cage total distance traveled (A, left) and total time spent moving (A, right) were recorded for an additional 10 minutes; between-subjects testing, N = 5 mice per treatment condition. A sociability apparatus (B) was also used to test time of interaction and locomotor properties in social encounters with a stimulus mouse. 10 minutes after nicotine (0.25 mg/kg) or vehicle administration, a single-housed C57BL/6 mouse was placed in the sociability apparatus. After 5 minutes, a stimulus mouse was added to one cage for another 5 minutes and social approach and cylinder sniffing scores (C, D, upper panels), change scores (C, D, bottom panels), distance traveled (E, upper panel), and time spent moving (E, lower panel) were measured for each 5 minute time block; between-subjects testing, N = 12 mice per treatment condition. Error bars represent S.E.M. *P < 0.05; **P < 0.01; ns, not significant.
To test sociability, socially isolated B6 mice were administered 0.25 mg/kg nicotine or vehicle and placed in a sociability chamber (Fig. 3B), and location and locomotor data were recorded before and after the addition of a male group housed B6 “stimulus” mouse. Analysis of variance of the social approach scores (see methods) revealed a main effect of stimulus mouse addition [F(1,22) = 13.76, P = 0.001] but no significant main effect of treatment [F(1,22) = 0.20, P = 0.66] and no significant stimulus mouse X treatment interaction [F(1,22) = 0.41, P = 0.53] (Fig. 3C, top). Similarly, analysis of variance of the cylinder sniffing scores revealed a main effect of stimulus mouse addition [F(1,22) = 13.62, P = 0.001] but no significant main effect of treatment [F(1,22) = 0.35, P = 0.56] and no significant stimulus mouse X treatment interaction [F(1,22) = 1.09, P = 0.31] (Fig. 3D, top). Change scores, which represent the difference in the social approach or cylinder sniffing score in the presence of the stimulus mouse vs. the absence of the stimulus mouse, were also calculated for both social approach and cylinder sniffing, neither of which significantly differed by treatment [Social approach change score: T(22) = 0.64, P = 0.53; Cylinder sniffing change score: T(22) = 1.05, P = 0.31, unpaired t-test] (Fig. 3C–D, bottom). Finally, analysis of variance of the distance traveled during the sociability testing revealed significant main effects of treatment [F(1,22) = 7.87, P = 0.01] and stimulus mouse [F(1,22) = 5.40, P = 0.03], and a significant stimulus mouse X treatment interaction [F(1,22) = 17.62, P = 0.0004]. Post hoc analysis revealed a significant difference between vehicle and nicotine treated mice only in the absence of the stimulus mouse (P < 0.001) (Fig. 3E, top). Analysis of time spent moving revealed a significant main effect of treatment [F(1,22) = 5.90, P = 0.02], no main effect of stimulus mouse [F(1,22) = 0.15, P = 0.70], and a significant stimulus mouse X treatment [F(1,22) = 8.49, P = 0.008] (Fig. 3E, bottom). Post hoc analysis again revealed a significant difference of time spent moving only in the absence of the stimulus mouse (P < 0.01). Taken together, these results show that while acute injection of nicotine 0.25 mg/kg reduces home cage locomotion of a solitary mouse, nicotine treated mice do not display deficits in time spent in social interaction, and the presence of a social mouse negates the modest hypolocomotor effect, suggesting that the serenic activity of nicotine in resident-intruder tests cannot be entirely explained by locomotor or sociability changes.
3.3 Differential effects of nAChR antagonists on serenic effects of nicotine and locomotor depression
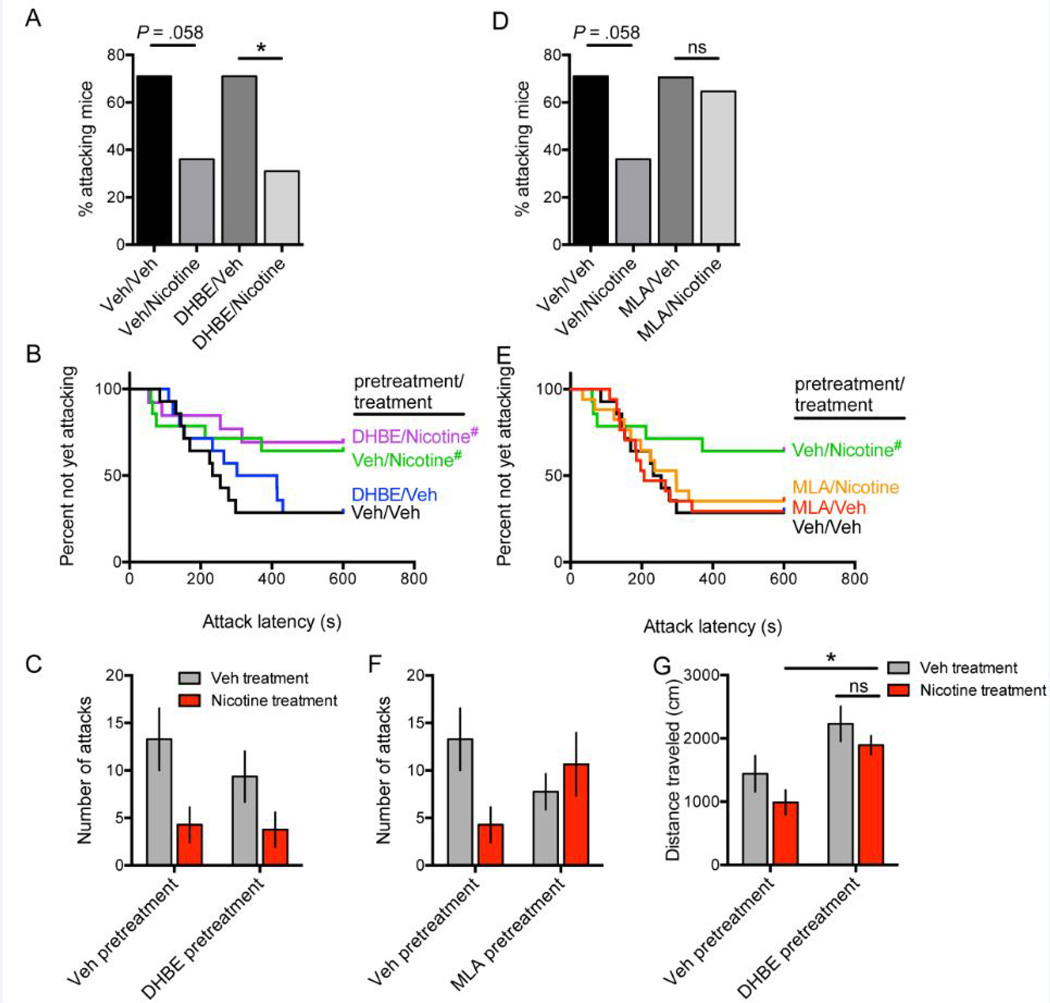
To identify the mechanisms underlying the reduction in aggressive behavior following acute nicotine administration in mice, we used pharmacological agents in a between-subjects approach to antagonize the two major subclasses of nAChR in the brain: heteromeric nAChRs and homomeric α7 nAChRs. The between-subjects approach was used to eliminate confounding by numerous injections to the same animal. We used dihydro-β-erythroidine (DHβE, 3 mg/kg) pretreatment to block heteromeric (primarily β2-containing) nAChRs 15 minutes prior to treatment with nicotine 0.25 mg/kg or vehicle, and then tested aggression in resident-intruder tests 10 minutes later. A control group received vehicle pretreatment followed by vehicle treatment, and a positive control group received vehicle pretreatment followed by nicotine 0.25 mg/kg treatment. Pretreatment with DHβE did not block the serenic effect of nicotine as measured by percent attacking residents [vehicle pretreatment/vehicle treatment vs. vehicle pretreatment/nicotine treatment: χ2(1, N = 28) = 3.59, P = 0.058; DHβE pretreatment/vehicle treatment vs. DHβE pretreatment/nicotine treatment: χ2(1, N = 27) = 4.46, P = 0.035], latency to attack [Vehicle pretreatment, nicotine vs. vehicle treatment: HR = 0.35, 95% CI: 0.11 – 1.06, P = 0.06; DHβE pretreatment, nicotine vs. vehicle treatment: HR = 0.32, 95% CI: 0.10 – 1.06, P = 0.06], or number of attacks as evidenced by a main effect of treatment [F(1,51) = 8.48, P = 0.005] but no significant main effect of pretreatment [F(1,51) = 0.79, P = 0.38] and no significant treatment X pretreatment interaction [F(1,51) = 0.46, P = 0.50] (Fig. 4A–C). We then tested whether nicotine’s serenic effect requires α7 nAChRs, and pretreated mice with the α7-selective antagonist methyllycaconitine (MLA, 5 mg/kg) 15 minutes prior to nicotine (0.25 mg/kg) or vehicle administration in a similar between-subjects approach with B6 mice. We found that pretreatment with MLA eliminated the serenic effect of acute nicotine injection, as we observed no difference in percent of attacking mice [χ2(1, N = 34) = 0.13, P = .71], latency to attack [MLA pretreatment, nicotine vs. vehicle treatment: HR = 0.86, 95% CI: 0.35–2.09, P = 0.73], or number of attacks as evidenced by no significant main effect of treatment [F(1,58) = 1.27, P = 0.26] or pretreatment [F(1 ,58) = 0.024, P = 0.88], but a significant treatment X pretreatment interaction [F(1,58) = 4.80, P = 0.033] (Fig. 4D–F). Furthermore, MLA pretreatment, regardless of subsequent nicotine or vehicle treatment, resulted in mice with aggressive properties similar to vehicle pretreatment/vehicle treatment controls, suggesting blockade of nicotine’s effect by MLA rather than a serenic effect of MLA itself and no additive effect by nicotine. These data suggest that nAChRs sensitive to MLA, likely the α7 receptor, are required to mediate the serenic effect of acute nicotine injection at 0.25 mg/kg.
Figure 4.
Single-housed male C57BL/6 mice were pretreated with the heteromeric nAChR antagonist dihydro-β-erythroidine (DHβE, 3 mg/kg) (A–C) or the homomeric nAChR antagonist methyllycaconitine (MLA, 5 mg/kg) (D–F) 15 minutes prior to injection with nicotine (0.25 mg/kg) or vehicle. Control groups were pretreated with vehicle followed by treatment with vehicle or nicotine. Percentage of attacking mice (A, D), latency to attack (B, E), and total number of attacks (C, F) were recorded; between-subjects testing: N = 14 for vehicle/vehicle, vehicle/nicotine, DHβE/vehicle; N = 13 for DHβE/nicotine; N = 17 for MLA/vehicle and MLA/nicotine. Note the vehicle/vehicle and vehicle/nicotine data shown in A-C and D-F are from one experiment that is duplicated for comparison to both antagonist groups. Home cage distance traveled was recorded 10 minutes after pretreatment with either vehicle or DHβE followed by treatment with either vehicle or nicotine (0.25 mg/kg) (G); between-subjects testing: N = 5 per treatment condition. Error bars represent S.E.M. *P < 0.05. #P = 0.06 vs. respective vehicle-treated group; ns, not significant.
Finally, as a positive pharmacological control for DHβE, we performed the same pretreatment paradigm with DHβE (3 mg/kg) or vehicle 15 minutes prior to nicotine 0.25 mg/kg or vehicle treatment, and then 10 minutes later recorded the home cage distance traveled and time spent moving for 10 minutes as an assessment of locomotor activity. Analysis of variance revealed a main effect of pretreatment with DHβE on distance traveled [F(1,16) = 13.30, P = 0.002] (Fig. 4G). Post hoc analysis demonstrated pretreatment with DHβE blocked the hypolocomotor effect of acute nicotine treatment on distance traveled (DHβE pretreatment, vehicle vs. nicotine treatment: P > 0.05; DHβE vs. vehicle pretreatment, nicotine treatment: P < 0.05). Similar findings were obtained when time spent moving was analyzed (data not shown). These data serve as a positive control for DHβE’s pharmacological effects as our findings were similar to previous data reporting that genetic deletion of the β2 subunit in B6 reduced acute nicotine’s hypolocomotor effect [25]. Furthermore, because DHβE reduced nicotine hypolocomotion but did not reduce nicotine’s serenic effect, these results provide complementary evidence that the serenic effect of nicotine is likely not secondary to general hypolocomotor effects.
3.4 Acute administration of an α7 nAChR partial agonist reduces aggressive behavior in mice
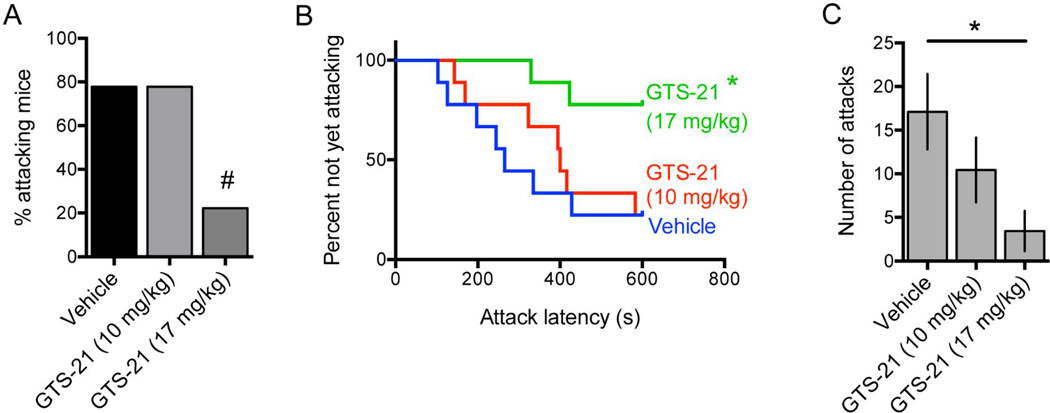
To determine whether activation of α7 nAChRs was sufficient to reduce aggression, we administered the α7 partial agonist GTS-21 (also known as DMXB), which has been shown to enter the brain rapidly following peripheral administration and to normalize sensory inhibition in DBA/2 mice [26]. Using a within-subjects design, administration of GTS-21 10 min prior to initiation of the resident-intruder test reduced the percentage of residents attacking within 10 minutes [N = 9, vehicle: 78%, GTS-21 (10 mg/kg): 78%, GTS-21 (17 mg/kg): 22%; vehicle vs. GTS-21 17 mg/kg: P = 0.06; McNemar test] (Fig. 5A). GTS-21 at 17 mg/kg also increased latency to first attack significantly [HR = 0.20, 95% CI: 0.04 – 0.97, P = 0.045] (Fig. 5B). Finally, GTS-21 treatment reduced the total number of attacks [F(2,16) = 4.23, P = 0.03], and post hoc analysis revealed a significant effect at the 17 mg/kg dose (P < 0.05) (Fig. 5C). GTS-21 at 10 mg/kg reduced the number of attacks by ~40%, however this effect did not reach statistical significance. These results provide additional evidence that activation of α7 nAChRs can decrease aggression in mice.
Figure 5.
Single-housed male C57BL/6 residents were tested in resident-intruder assays after vehicle or GTS-21 administration and percentage of attacking mice (A), latency to first attack (B), and number of attacks (C) were measured; N = 9, within-subject testing. Error bars represent S.E.M. #P = 0.06; *P < 0.05 vs. vehicle-treated group.
4. Discussion
The current study shows that acute systemic administration of nicotine at low to moderate doses reduced indices of aggression in three diverse mouse strains, that the serenic effect of nicotine in B6 mice is specific for aggression rather than secondary to hypolocomotion or altered sociability, and that activation of α7 nAChRs is sufficient to reduce aggression whereas an α7-selective nAChR antagonist blocks the serenic effect of nicotine.
The current results are consistent with previous work showing that acute nicotine administration reduced aggression in cats [7] as well as in rat pair encounters, shock-induced fighting, and muricide assays [8–12]. Previous studies of the effect of nicotine on aggression in mice have shown that nicotine can reduce offensive aggression measures in socially-isolated Swiss Webster mice in home cage resident-intruder encounters [13], however this study did not evaluate the contribution of locomotor or sociability changes due to nicotine. In neutral cage encounters of socially-isolated OF1 mice with anosmic opponents, nicotine administered either acutely or chronically did not reduce aggression, whereas lobeline, a less specific nAChR agonist that also interacts with the dopamine transporter and vesicular monoamine transporter [27] reduced aggression even at doses that did not affect locomotion [28, 29]. Acute nicotine administration also did not influence sociability significantly in socially isolated OF1 mice [30]. In contrast with other studies, male albino mice administered nicotine i.p. or intraventricularly demonstrated potentiated aggression in a footshock-induced aggression paradigm [31]. It is important to note that the current study used socially isolated resident mice in resident-intruder assays, similar to those performed in Swiss Webster mice [13], while the studies finding no effects of nicotine used neutral cage encounters [28, 29]. It is possible that the neural mechanism of territorial aggression expressed in the resident-intruder assay is not entirely identical to that engaged during neutral cage encounters, and that in mice acute administration of nicotine may preferentially act on the former. Finally, it is possible that the differing effects of nicotinic agents in previous mouse studies is due to variability in the nAChR subtype selectivity across agents or to differential desensitization of various subtypes. The identification of the α7 nAChR as a critical target for the serenic effects of nicotine in the current study will allow for more selective activation of this subtype going forward.
The current study identified parallel serenic effects across mouse strains with differing baseline levels of aggressive behavior, and measured the extent that potentially confounding factors, such as changes in locomotor activity and non-aggressive social interaction, might influence the assay’s measure of aggression. We assessed the influence of nicotine across three diverse mouse strains: B6, BALB/c, and CD1, and found that in all three nicotine reduced measures of aggressive behavior in social isolation-induced aggression and resident-intruder assays. This conserved action of nicotine provides strong evidence for its role as a serenic agent, as acute administration of nicotine to each strain has different locomotor consequences [22–24], and the three strains of mice differ in innate aggression levels [32] and sociability measures [19]. We also found that acute nicotine administration does not alter sociability, in agreement with previous studies in mice [28, 30, 33]. Interestingly, while acute administration of nicotine (0.25 mg/kg) to solitary B6 mice predictably reduced locomotion in both home cage and sociability apparatus environments by ~30% [22], we found that the addition of a social “stimulus” mouse rescued this locomotor deficit, providing evidence that locomotor deficits cannot fully explain the serenic effect of nicotine in our assay. This is consistent with the detailed ethological analysis performed previously in OF1 mice acutely dosed with nicotine (0 – 0.6 mg/kg) that found no changes in nonsocial exploration, arguing against a general sedative effect [28]. Finally, the finding that B6 mice pretreated with DHβE do not demonstrate acute hypolocomotion after acute nicotine administration is consistent with previous findings showing that pretreatment with DHβE significantly reduces nicotine’s hypolocomotor effect [34], and deletion of nAChR β2-containing subunits render B6 mice less susceptible to acute hypolocomotor effects of nicotine as well as increases baseline locomotion in open-field tests [25, 35], and provides complementary evidence that hypolocomotion cannot fully explain the serenic effects of nicotine.
The use of pharmacological agents targeting specific nAChR subtypes serves as a starting point from which to explore the molecular mechanisms underlying the serenic action of nicotine, and by extension, the effects of nAChRs on aggressive behavior. We find that the heteromeric nAChR antagonist DHβE (3 mg/kg), a dose that blocks nicotine’s enhancement of contextual fear conditioning in B6 [36], does not influence the serenic effect of nicotine or have any significant effects on aggression by itself. Further, blockade of the acute nicotine-induced hypolocomotor response by DHβE pretreatment at the dose that did not influence aggression provides evidence that this dose penetrated and acted on the central nervous system, as previous work has shown that the hypolocomotor effect of nicotine in mice is centrally mediated [34]. These experiments therefore suggest that β2 subunit-containing nAChRs do not mediate the serenic effect of nicotine. This is consistent with a study using knockout mice lacking β2 subunit-containing nAChRs (β2−/−) which found no difference in baseline aggression measures between β2−/− mice and wild type B6 controls in a social interaction task following 4-weeks of social isolation [37], despite exaggerated social interactions of β2−/− mice with a conspecific [38].
Interestingly, β2−/− mice do exhibit increased measures of “social dominance”, which suggests that the two closely related but distinct behavioral repertoires can be uncoupled [37]. Future experiments will test the serenic effects of nAChR-selective agents in mice with genetic deletion of the β2 or α7 nAChR subunits to complement these pharmacological findings.
The ability of MLA (5 mg/kg) to block the effects of nicotine on aggressive behavior suggests that α7 nAChRs are necessary to mediate its serenic action. It should be noted that MLA is also an antagonist at α6-containing nAChRs [39, 40], so this finding cannot rule out a contribution of α6-containing nAChRs to the serenic effects of nicotine. This dose of MLA was chosen because a higher dose (7.5 mg/kg) can precipitate nicotine withdrawal in mice with a genetic deletion of α7 nAChRs, suggesting higher doses lack subtype-specificity [41]. Since DHβE did not influence nicotine’s serenic effect, it is unlikely that non-specific actions of MLA at β2 subunit-containing receptors could explain the effect of MLA. Like β2 subunit-containing nAChRs, α7 nAChRs are widely expressed in mouse brain, including in regions that regulate aggression and related behaviors, such as cortex, hypothalamus, hippocampus, and amygdala [42, 43]. α7 nAChRs are ionotropic receptors that generate an excitatory current but are also prone to desensitization by nicotine [44, 45]. If nicotine’s serenic action were due to desensitization of α7 nAChRs, we might expect MLA on its own to be serenic, which is not supported by the current data. Rather, following administration of MLA, nicotine-treated mice attack in a similar manner to control mice. Activation of α7 nAChRs can increase the release of serotonin [46–48], GABA [49], and dopamine [5, 50, 51], all of which are neurotransmitters classically involved in regulation of aggressive behavior, providing potential mechanisms for serenic effects of nicotine. It is unknown whether the expression profile of nAChR subunits differs in increasingly aggressive mice in brain regions that govern aggression such as VMHvl. Future experiments will address this question as such changes may provide rationale to design increasingly selective pharmacological agents to target pathological aggression.
Our finding that acute administration of GTS-21, a partial agonist selective for α7 nAChRs [52–54], reduces aggression supports our hypothesis that activation of α7 nAChRs decreases aggressive behavior. This finding also supports the sufficiency of α7 nAChR activation for this behavioral effect, which could not be concluded from MLA alone given its activity at α6-containing nAChRs, although. It should be noted that the effect of GTS-21 on α6-containing nAChRs is not well studied. Future experiments in knockout mice will further clarify this issue. In DBA/2 mice, GTS-21 normalizes abnormal inhibition of auditory responses [26] that are believed to be secondary to reduced hippocampal α7 expression [55]. Similar deficits in auditory evoked responses are observed in humans with schizophrenia, and DMBX-A (GTS-21) can correct such deficits along with improvements in certain neurocognitive tasks [56]. It is important to note that our experiments found effects of GTS-21 on aggression only at higher doses than those required to normalize auditory inhibition in DBA/2 mice [26], which may be secondary to pharmacokinetic differences between mouse strains, need for greater receptor activation to reduce aggression, or other parameters. Previous studies in CD1 mice, however, show that GTS-21 does not alter locomotion at doses less than 62 µmol/kg (~24 mg/kg), suggesting that the serenic effect observed at 17 mg/kg was likely not secondary to hypolocomotion [53]. It should also be noted that a metabolite of GTS-21 has weak activity at 5-HT3 receptors [57], so the current study cannot rule out off-target effects. α7 nAChRs are implicated in multiple neuropsychiatric disorders that can feature comorbidity with aggressive behavior, including autism spectrum disorder [58–60], 15q13.3 microdeletion syndrome that frequently presents with autism and aggression [61–64], schizophrenia [65], and attention deficit hyperactivity disorder [66]. Pharmacological agents with activity at α7 nAChRs have been studied in humans with some of these disorders [67, 68], focusing primarily on cognitive function and in some cases, irritability or mood symptoms. The current data suggest that further study of the role of nAChRs, especially α7 nAChRs, may contribute to understanding neurobiological mechanisms underlying aggressive behavior, and could represent a novel mechanism of action for serenic agents that would meet a great clinical need.
Acknowledgements
This work was supported by National Institutes of Health grants MH19961 and MH014276 (ASL), and DA14221 and MH07895 (MRP). We thank Samantha Sheppard and Nadia Jordan-Spasov for excellent technical assistance, and Tenna Mose for assistance with mouse training.
Non-standard abbreviations
- DHβE
dihydro-beta-erythroidine
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article
(-) Nicotine hydrogen bitartrate (PubChem CID: 11957628); Dihydro-β-erythroidine hydrobromide (PubChem CID: 11957537); methyllycaconitine citrate (PubChem CID: 16219626); GTS-21 (PubChem CID: 6438361)
Conflict of interest: The authors declare no conflicts of interest.
Contributor Information
Alan S. Lewis, Email: alan.lewis@yale.edu.
Yann S. Mineur, Email: yann.mineur@yale.edu.
Philip H. Smith, Email: philip.h.smith@yale.edu.
Emma L. M. Cahuzac, Email: emma.cahuzac@yale.edu.
Marina R. Picciotto, Email: marina.picciotto@yale.edu.
References
- 1.Adler BA, Wink LK, Early M, Shaffer R, Minshawi N, McDougle CJ, et al. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: A chart review study. Autism : the international journal of research and practice. 2014 doi: 10.1177/1362361314524641. [DOI] [PubMed] [Google Scholar]
- 2.Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA psychiatry. 2015;72:438–445. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citrome L, Volavka J. Pharmacological management of acute and persistent aggression in forensic psychiatry settings. CNS Drugs. 2011;25:1009–1021. doi: 10.2165/11596930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Advances in genetics. 2011;75:151–169. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. Journal of molecular neuroscience : MN. 2006;30:137–140. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- 6.Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berntson GG, Beattie MS, Walker JM. Effects of nicotinic and muscarinic compounds on biting attack in the cat. Pharmacol Biochem Behav. 1976;5:235–239. doi: 10.1016/0091-3057(76)90072-1. [DOI] [PubMed] [Google Scholar]
- 8.Silverman AP. Behaviour of rats given a "smoking dose" of nicotine. Animal behaviour. 1971;19:67–74. doi: 10.1016/s0003-3472(71)80136-7. [DOI] [PubMed] [Google Scholar]
- 9.Schechter MD. Effect of nicotine on response to frustrative non-reward in the rat. European journal of pharmacology. 1974;29:312–315. doi: 10.1016/0014-2999(74)90032-6. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers RJ. Effects of nicotine, mecamylamine, and hexamethonium on shock-induced fighting, pain reactivity, and locomotor behaviour in rats. Psychopharmacology. 1979;66:93–98. doi: 10.1007/BF00431996. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll P, Baettig K. Selective inhibition by nicotine of shock-induced fighting in the rat. Pharmacol Biochem Behav. 1981;14:175–179. doi: 10.1016/0091-3057(81)90240-9. [DOI] [PubMed] [Google Scholar]
- 12.Waldbillig RJ. Suppressive effects of intraperitoneal and intraventricular injections of nicotine on muricide and shock-induced attack on conspecifics. Pharmacol Biochem Behav. 1980;12:619–623. doi: 10.1016/0091-3057(80)90198-7. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SK, Carlson KM, Lee J, Burr LE, Wagner GC. Effects of nicotine on target biting and resident-intruder attack. Life sciences. 2003;73:311–317. doi: 10.1016/s0024-3205(03)00289-3. [DOI] [PubMed] [Google Scholar]
- 14.Carmel H, Sheitman BB. Adjunctive transdermal nicotine reduced behavioral agitation in severe dementia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:449. doi: 10.1097/01.JGP.0000235688.05709.e2. [DOI] [PubMed] [Google Scholar]
- 15.Rosin RA, Levine MD, Peskind E. Transdermal nicotine for agitation in dementia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2001;9:443–444. [PubMed] [Google Scholar]
- 16.Van Schalkwyk GI, Lewis AS, Qayyum Z, Koslosky K, Picciotto MR, Volkmar FR. Reduction of Aggressive Episodes After Repeated Transdermal Nicotine Administration in a Hospitalized Adolescent with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2015 doi: 10.1007/s10803-015-2471-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 18.Winslow JT, Miczek KA. Habituation of Aggressive-Behavior in Mice - a Parametric Study. Aggressive Behav. 1984;10:103–113. [Google Scholar]
- 19.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226:291–302. [PubMed] [Google Scholar]
- 23.Brioni JD, O'Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. European journal of pharmacology. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- 24.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 25.Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, et al. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- 26.Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelm CJ, Johnson RA, Eshleman AJ, Janowsky A. Lobeline effects on tonic and methamphetamine-induced dopamine release. Biochem Pharmacol. 2008;75:1411–1415. doi: 10.1016/j.bcp.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redolat R, Oterino MC, Carrasco MC, Berry MS, Brain PF. Effects of acute administration of nicotine and lobeline on agonistic encounters in male mice. Aggressive Behav. 2000;26:376–385. [Google Scholar]
- 29.Redolat R, Oterino MC, Carrasco MC, Brain PF. A specific anti-aggressive effect of repeatedly administered lobeline. Addiction biology. 2002;7:301–306. doi: 10.1080/13556210220139514. [DOI] [PubMed] [Google Scholar]
- 30.Gomez MC, Carrasco MC, Redolat R. Differential sensitivity to the effects of nicotine and bupropion in adolescent and adult male OF1 mice during social interaction tests. Aggress Behav. 2008;34:369–379. doi: 10.1002/ab.20255. [DOI] [PubMed] [Google Scholar]
- 31.Rolinski Z, Herbut M. The importance of central nicotinic receptors in footshock-induced aggression in mice. Polish journal of pharmacology and pharmacy. 1981;33:569–576. [PubMed] [Google Scholar]
- 32.Schneider R, Hoffmann HJ, Schicknick H, Moutier R. Genetic analysis of isolation-induced aggression. I. Comparison between closely related inbred mouse strains. Behav Neural Biol. 1992;57:198–204. doi: 10.1016/0163-1047(92)90150-3. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Kurasawa M. Anxiolytic effects of aniracetam in three different mouse models of anxiety and the underlying mechanism. European journal of pharmacology. 2001;420:33–43. doi: 10.1016/s0014-2999(01)01005-6. [DOI] [PubMed] [Google Scholar]
- 34.Damaj MI, Welch SP, Martin BR. In vivo pharmacological effects of dihydro-beta-erythroidine, a nicotinic antagonist, in mice. Psychopharmacology. 1995;117:67–73. doi: 10.1007/BF02245100. [DOI] [PubMed] [Google Scholar]
- 35.Avale ME, Faure P, Pons S, Robledo P, Deltheil T, David DJ, et al. Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc Natl Acad Sci U S A. 2008;105:15991–15996. doi: 10.1073/pnas.0807635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- 37.Coura RS, Cressant A, Xia J, de Chaumont F, Olivo-Marin JC, Pelloux Y, et al. Nonaggressive and adapted social cognition is controlled by the interplay between noradrenergic and nicotinic receptor mechanisms in the prefrontal cortex. FASEB J. 2013;27:4343–4354. doi: 10.1096/fj.13-231084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granon S, Faure P, Changeux JP. Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci U S A. 2003;100:9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fucile S, Matter JM, Erkman L, Ragozzino D, Barabino B, Grassi F, et al. The neuronal alpha6 subunit forms functional heteromeric acetylcholine receptors in human transfected cells. Eur J Neurosci. 1998;10:172–178. doi: 10.1046/j.1460-9568.1998.00001.x. [DOI] [PubMed] [Google Scholar]
- 40.Vailati S, Hanke W, Bejan A, Barabino B, Longhi R, Balestra B, et al. Functional alpha6-containing nicotinic receptors are present in chick retina. Mol Pharmacol. 1999;56:11–19. doi: 10.1124/mol.56.1.11. [DOI] [PubMed] [Google Scholar]
- 41.Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morganstern I, Lukatskaya O, Moon SH, Guo WR, Shaji J, Karatayev O, et al. Stimulation of nicotine reward and central cholinergic activity in Sprague-Dawley rats exposed perinatally to a fat-rich diet. Psychopharmacology. 2013;230:509–524. doi: 10.1007/s00213-013-3178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 44.Briggs CA, McKenna DG. Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology. 1998;37:1095–1102. doi: 10.1016/s0028-3908(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 45.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seth P, Cheeta S, Tucci S, File SE. Nicotinic--serotonergic interactions in brain and behaviour. Pharmacol Biochem Behav. 2002;71:795–805. doi: 10.1016/s0091-3057(01)00715-8. [DOI] [PubMed] [Google Scholar]
- 47.Aznar S, Kostova V, Christiansen SH, Knudsen GM. Alpha 7 nicotinic receptor subunit is present on serotonin neurons projecting to hippocampus and septum. Synapse. 2005;55:196–200. doi: 10.1002/syn.20108. [DOI] [PubMed] [Google Scholar]
- 48.Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduno J, et al. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse. 2008;62:601–615. doi: 10.1002/syn.20526. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez-Vazquez F, Chavarria K, Garduno J, Hernandez-Lopez S, Mihailescu SP. Nicotine increases GABAergic input on rat dorsal raphe serotonergic neurons through alpha7 nicotinic acetylcholine receptor. J Neurophysiol. 2014;112:3154–3163. doi: 10.1152/jn.00223.2014. [DOI] [PubMed] [Google Scholar]
- 50.Huang M, Felix AR, Flood DG, Bhuvaneswaran C, Hilt D, Koenig G, et al. The novel alpha7 nicotinic acetylcholine receptor agonist EVP-6124 enhances dopamine, acetylcholine, and glutamate efflux in rat cortex and nucleus accumbens. Psychopharmacology. 2014;231:4541–4551. doi: 10.1007/s00213-014-3596-0. [DOI] [PubMed] [Google Scholar]
- 51.Seipel AT, Yakel JL. The frequency-dependence of the nicotine-induced inhibition of dopamine is controlled by the alpha7 nicotinic receptor. J Neurochem. 2010;114:1659–1666. doi: 10.1111/j.1471-4159.2010.06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, Papke RL. Characterization of a series of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative is a selective agonist at neuronal nicotinic alpha 7/125I–alpha-bungarotoxin receptor subtypes. Mol Pharmacol. 1995;47:164–171. [PubMed] [Google Scholar]
- 53.Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, et al. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997;57:231–241. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- 54.Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-hydroxy, 2-Methoxybenzylidene)anabaseine selectivity and activity at human and rat alpha-7 nicotinic receptors. J Pharmacol Exp Ther. 1998;287:918–925. [PubMed] [Google Scholar]
- 55.Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, et al. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- 56.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 57.Machu TK, Hamilton ME, Frye TF, Shanklin CL, Harris MC, Sun H, et al. Benzylidene analogs of anabaseine display partial agonist and antagonist properties at the mouse 5-hydroxytryptamine(3A) receptor. J Pharmacol Exp Ther. 2001;299:1112–1119. [PubMed] [Google Scholar]
- 58.Yasui DH, Scoles HA, Horike S, Meguro-Horike M, Dunaway KW, Schroeder DI, et al. 15q11.2–13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Human molecular genetics. 2011;20:4311–4323. doi: 10.1093/hmg/ddr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS genetics. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mikhail FM, Lose EJ, Robin NH, Descartes MD, Rutledge KD, Rutledge SL, et al. Clinically relevant single gene or intragenic deletions encompassing critical neurodevelopmental genes in patients with developmental delay, mental retardation, and/or autism spectrum disorders. American journal of medical genetics Part A. 2011;155A:2386–2396. doi: 10.1002/ajmg.a.34177. [DOI] [PubMed] [Google Scholar]
- 61.Hoppman-Chaney N, Wain K, Seger PR, Superneau DW, Hodge JC. Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. Clinical genetics. 2013;83:345–351. doi: 10.1111/j.1399-0004.2012.01925.x. [DOI] [PubMed] [Google Scholar]
- 62.Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A, et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharp AJ, Mefford HC, Li K, Baker C, Skinner C, Stevenson RE, et al. A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet. 2008;40:322–328. doi: 10.1038/ng.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB, et al. A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry. 2014;76:128–137. doi: 10.1016/j.biopsych.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. International review of neurobiology. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 66.Stergiakouli E, Hamshere M, Holmans P, Langley K, Zaharieva I, de CG, et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry. 2012;169:186–194. doi: 10.1176/appi.ajp.2011.11040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freedman R. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annual review of medicine. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- 68.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36:96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]