Abstract
Rationale
Endothelial cells have the ability to undergo endothelial-mesenchymal transitions (EndMTs), by which they acquire a mesenchymal phenotype and stem-cell like characteristics. We previously found that EndMTs ocurred in the endothelium deficient in matrix Gla protein (MGP) enabling endothelial cells to contribute cells to vascular calcification. However, the mechanism responsible for initiating EndMTs is not fully understood.
Objective
To determine the role of specific serine proteases and sex determining region Y-box 2 (Sox2) in the initiation of EndMTs.
Methods and Results
In this study, we used in vivo and in vitro models of vascular calcification to demonstrate that serine proteases and Sox2 are essential for the initiation of EndMTs in MGP-deficient endothelium. We showed that expression of a group of specific serine proteases was highly induced in endothelial cells at sites of vascular calcification in Mgp null aortas. Treatment with serine protease inhibitors decreased both stem-cell marker expression and vascular calcification. In human aortic endothelial cells, this group of serine proteases also induced EndMTs, and the activation of proteases was mediated by Sox2. Knockdown of the serine proteases or Sox2 diminished EndMTs and calcification. Endothelial-specific deletion of Sox2 decreased expression of stem-cell markers and aortic calcification in MGP-deficient mice.
Conclusions
Our results suggest that Sox2-mediated activation of specific serine proteases is essential for initiating EndMTs, and thus, may provide new therapeutic targets for treating vascular calcification.
Keywords: Endothelium, progenitor cell, calcification, matrix Gla protein, serine protease
INTRODUCTION
Transition of endothelial cells into mesenchymal cells (endothelial-mesenchymal transition, EndMT) allows endothelial cells (ECs) to acquire plasticity and to participate in embryonic development and disease progression1. During embryonic development, the microenvironment or specific factors induce EndMT, driving the endothelium to engage in normal tissue differentiation2–5. In disease, the occurrence of EndMTs may convert endothelium into a multipotent state that contributes cells toward undesirable differentiation such as cardiac and renal fibrosis, cancer progression, fibrodysplasia ossificans progressiva (FOP), pulmonary hypertension, and vascular calcification6–13.
Vascular calcification is a frequent and severe complication of vascular disease, which increases morbidity and mortality in diabetes and atherosclerosis, and is associated with an increased risk of congestive heart failure, myocardial infarction, systemic hypertension, and chronic kidney disease14–18. Previously considered to be a passive process of mineral precipitation, vascular calcification is now known to be an active process that involves ectopic bone formation18–20.
Mice lacking matrix Gla protein (MGP), an inhibitor of bone morphogenetic protein (BMP)-2, 4 and 7, is a well-known model for vascular calcification21. The lack of MGP activates BMP signaling throughout the vascular wall22 and is associated with ectopic osteochondrogenic differentiation, vascular calcification, and EndMTs11, 21. However, the mechanism by which EndMT is initiated in the calcifying arteries has not been determined. Here, we show critical roles for endothelial specific serine proteases and sex determining region Y-box 2 (Sox2) in the activation of EndMTs. Reduction of proteases activity or Sox2 levels limits EndMTs and vascular calcification.
METHODS
See Online Data Supplement for detailed Methods section.
Animals
Mgp+/− mice on the C57BL/6J background23 were obtained from Dr. Cecilia Giachelli, University of Washington, with the permission of Dr. Gerard Karsenty, Columbia University. Cdh5Cre (B6.Cg-Tg(Cdh5-cre)7Mlia/J) and Sox2flox/flox (Sox2tm1.1Lan/J) mice were obtained from the Jackson Laboratory. Genotypes were confirmed by PCR21, 24, 25, and experiments were performed with generations F4–F6. Littermates were used as wild type controls. All mice were fed a standard chow diet (Diet 8604, HarlanTeklad Laboratory). The studies were reviewed and approved by the Institutional Review Board and conducted in accordance with the animal care guideline set by the University of California, Los Angeles. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011). Diisopropylfluorophosphate (DFP) (Sigma-Aldrich) and serpina1 (Origene) were injected via tail vein or retro-orbital injection (20–50 ng/g, daily) as in previous studies26, 27. Injections in Mgp−/− mice were started at 2 weeks of age, and continued for 2–4 weeks.
Tissue culture and siRNA transfections
Human aortic endothelial cells (HAECs) were cultured as previously described28. For treatment of HAECs, BMP-4 (40 ng/ml, R&D system), glucose (22 nmol/L, Sigma-Aldrich), DFP (300 ng/ml), serpina1 (300 ng/ml), elastase 1 (50 ng/ml, Abnova), elastase 2 (50 ng/ml, Abcam), and kallikrein 1, 5 and 6 (all 10 ng/ml, Abnova) were added as indicated in the text. Transient transfections of HAECs with siRNA (Silencer® predesigned siRNA, Ambion) were performed with Lipofectamine™2000 (Invitrogen) using 60 nM siRNA. The amount of siRNA was optimized per the manufacturer's instructions. Three separate siRNAs and scrambled siRNA with the same nucleotide content were tested. When compared with unrelated control siRNA and scrambled siRNA, the specific siRNAs resulted in an 80–95% decrease in mRNA and protein levels as determined by real-time PCR and immunoblotting, respectively. The siRNA that provided the most efficient inhibition (90–95%) was used for all experiments. Silencer® predesigned siRNAs were obtained for MGP, SMAD1, SMAD5, SMAD8, Sox2, elastase 1 and 2, and kallikrein 1, 5 and 6. The same total amount of siRNA was added when transfections with multiple siRNAs were performed.
RNA analysis
Real-time PCR analysis was performed as previously described29. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control gene29. Primers and probes for mouse Sox2, Kruppel-like factor 4 (Klf4), snail family zinc finger 2 (Slug or Snail2), spinocerebellar ataxia type 1 (Sca1), cluster of differentiation (CD)10, CD44, CD71, CD90, c-kit (or CD117), N-cadherin, and all elastases and kallikreins were obtained from Applied Biosystems as part of Taqman® Gene Expression Assays.
Immunoblotting
Immunoblotting was performed as previously described30. Equal amounts of cellular protein or tissue lysates were used. Blots were incubated with specific antibodies to elastase 1 (200ng/ml; Santa Cruz Biotechnology), elastase 2 (200 ng/ml; Abgent), kallikrein 1 and 6 (both 200 ng/ml; Sigma-Aldrich), kallikrein 5 (300 ng/ml; Acris Antibodies), c-kit (200 ng/ml; Cell Signaling Technology), Sca1 (200 ng/ml; Merck Millipore), CD10 (1:100; ThermoFisher), CD44 and CD90 (both 200 ng/ml; Abcam), CD71 (1:200; ThermoFisher), pSMAD1/5/8 (200ng/ml; Santa Cruz Biotechnology), Sox2, Klf4, Slug and pSMAD2/3 (all 400 ng/ml; Cell Signaling Technology) and total SMAD (400 ng/ml; Santa Cruz Biotechnology). β-Actin (1:5000 dilution; Sigma-Aldrich) was used as loading control.
Immunofluorescence
Tissue sections were fixed in 4% paraformaldehyde and processed as previously described31. Immunofluorescence was performed as previously described11. We used specific antibodies for CD31 (Merck Millipore), vWF (Dako), Cbfa1, Osterix and elastase 1 (all from Santa Cruz Biotechnology), elastase 2 (Abgent), kallikrein 1 and 6 (Sigma-Aldrich), kallikrein 5 (Acris Antibodies), Sox2, Klf4, c-kit, Slug (all from Cell Signaling Technology), Sca1 (Merck Millipore), CD10 and CD71 (both from ThermoFisher), N-cadherin (Life technology), CD44 and CD90 (both from Abcam). Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich).
Flow cytometric analysis
Fluorescence-activated cell sorting (FACS) analysis was performed as described11. The cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or Alexa Fluor 488 (AF-488)-conjugated antibodies against Sox2, VE-cadherin, elastase 1 and 2, kallikrein 1, 5, and 6. Nonspecific fluorochrome- and isotype-matched IgGs (BD Pharmingen) served as controls.
Expression profile
The total mRNA was extracted from aortic tissue and examined by Mouse Ref-8v 2.0 Expressin Bead Chip kit (Illumina). The array data was analyzed by using GenomeStudio software (Illumina). The expression profiles of all serine proteases were extracted from array data, and the heat map was generated by using GenomeStudio.
Quantification of aortic calcium
The aortic calcium was measured by using calcium assay kit (Bioassay) as previous described32.
Enzyme-linked immunosorbent assay (ELISA)
The levels of elastases and kallikreins in plasma were examined using ELISA kits, elastase 1 (Antibodies-online), elastase 2 (MyBiosource), kallikrein 1 (Abcam), kallikrein 5 (Biocompare) and kallikrein 6 (Enzo). The assays were performed as per the manufacturer’s protocols.
Lentivirus infection
Lentiviral vectors containing Sox2 open reading frame (ORF) under the cytomegalovirus (CMV) promoter were obtained from Applied Biological Materials Inc. The cell infection was performed as per the manufacturer’s protocol.
Transmission electron microscopy and scanning electron microscopy
Transmission electron microscopy and scanning electron microscopy were performed as described11.
Statistical analysis
Data were analyzed for statistical significance by ANOVA with post hoc Tukey’s analysis. The analyses were performed using GraphPad Instat®, version 3.0 (GraphPad Software). Data represent mean ± SD. P<0.05 was considered significant, and experiments were performed a minimum of three times.
RESULTS
Lack of MGP causes endothelial-mesenchymal transition
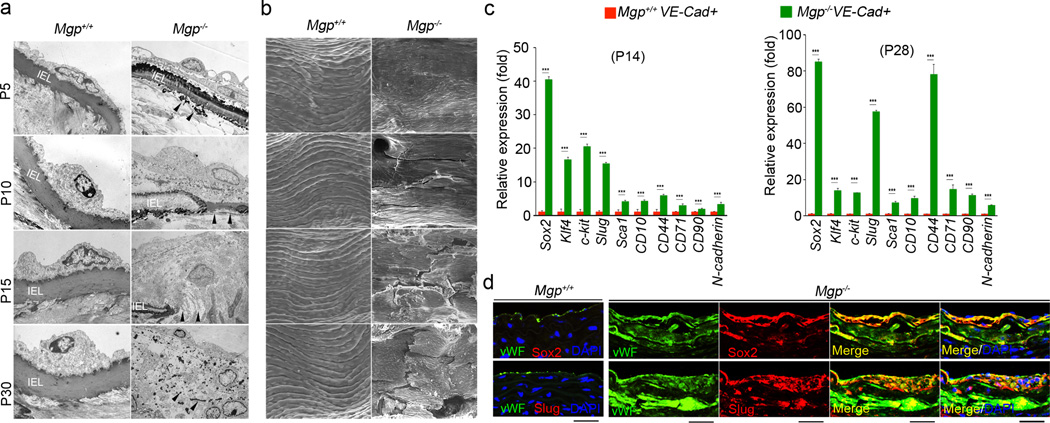
To investigate EndMTs in MGP deficient endothelium, we examined lesions of aortic calcification in 4-week-old Mgp−/− mice. We observed multiple layers of EC-like cells that appeared to have penetrated into the medial tissues and proliferated in the areas adjacent to an osteochondrogenic zone (Online Figure I). The internal elastic lamina (IEL) was undetectable and EC-like cells were oriented as if migrating from the luminal side toward the calcifying lesion (Online Figure I). We further examined the lesions using transmission electron microscopy (TEM) and scanning electron microscopy (SEM) at different time points from postnatal day 5 (P5) to P30. TEM indicated that degradation of IEL, which is in close contact with endothelium, began in the Mgp−/− aorta as early as P5 (Figure 1a). At P15, fractures in the IEL were apparent and Mgp−/− aortic ECs lost their normal morphology. At P30, the IEL was completely degraded, and a mixture of cells had replaced the normal aortic endothelium (Figure 1a). Abnormalities in the Mgp−/− aortic endothelium were also observed by SEM, which showed ruptures in the endothelium at P10 that became progressively worse at P15 and P30 (Figure 1b).
Figure 1. Lack of MGP causes EndMTs in aortic tissue.
(a–b) Mgp+/+ and Mgp−/− aortic endothelium was examined by transmission electron microscopy (TEM) (a) and scanning electron microscopy (SEM) (b). Magnification for TEM, 3.7×103. Magnification for SEM, 5×102. IEL: Internal elastic lamina. Areas of IEL degradation are indicated by arrowheads. (c) Expression of stem-cell and mesenchymal markers in VE-cadherin-positive and CD45-negative presorted cells from Mgp+/+ and Mgp−/− aortas at postnatal day (P) 14 and 28, as determined by real-time PCR. ***p<0.001. (d) Co-expression of the endothelial marker vWF with the stem-cell marker Sox2 and the mesenchymal marker Slug in calcified lesions of Mgp−/− aortas. Scale bars, 100 µm.
To examine whether EndMTs drove the Mgp−/− endothelium into a multipotent state, we analyzed the expression of the stem-cell markers Sox2, Klf4, c-kit, Sca1, CD10, CD44, CD71, CD90, and N-cadherin, and the typical mesenchymal marker Slug (also known as Snail2) in isolated Mgp−/− aortic ECs at P14 and P28. We found that expression of all of these markers was upregulated (Figure 1c). We also immunostained the calcified aortic tissues using specific antibodies for these markers and for vWF, an endothelial marker. All of the stem-cell and mesenchymal markers were strongly induced in vWF positive cells, which were detected in the aortic medial layer and calcified lesional areas (Figure 1d and Online Figure II) suggesting that EndMTs occurred in the Mgp−/− endothelium during the calcifying process.
Identification of serine proteases in EndMTs and calcification
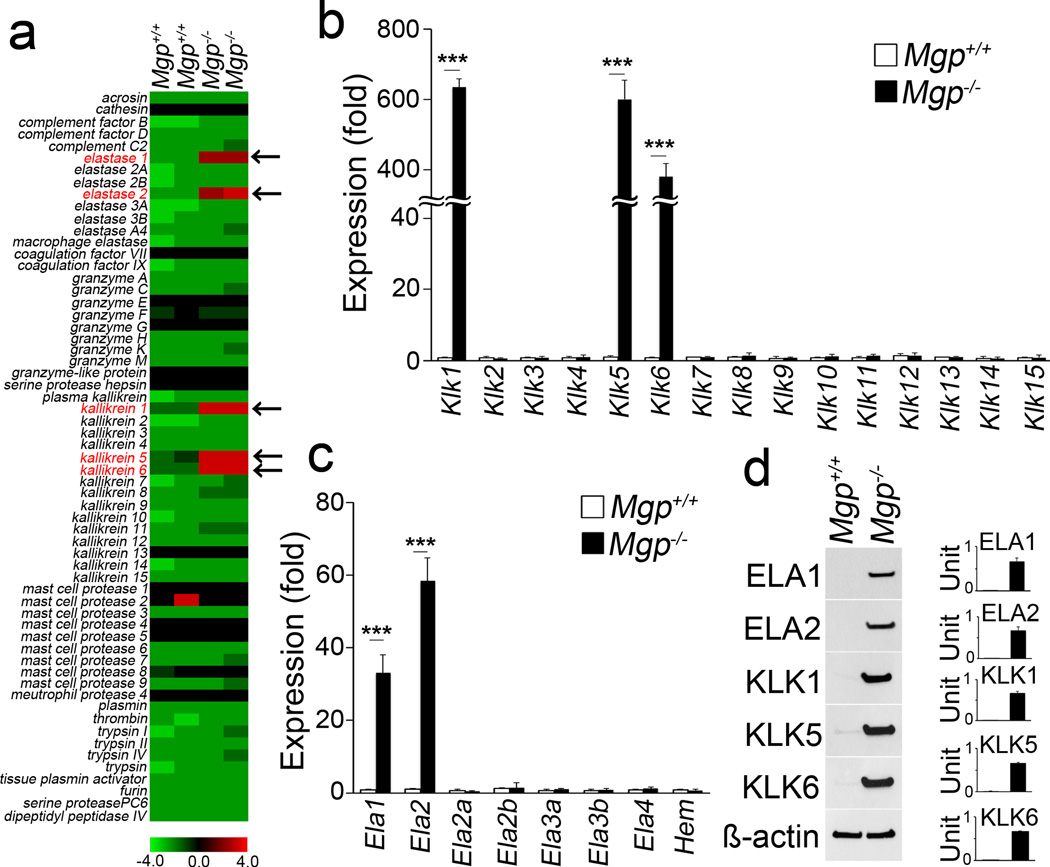
Because of the highly degraded IEL in Mgp−/− aortic tissues, we examined whether serine proteases were induced in these tissues. By comparing gene expression profiles of aortic tissues from wild type and Mgp−/− mice at 4 weeks of age, we identified 5 serine proteases, elastase 1 and 2, and kallikrein 1, 5, and 6, which were highly induced in Mgp−/− aortas (Figure 2a). Real-time PCR and immunoblotting of Mgp−/− aortic tissue confirmed the induction of these 5 serine proteases (Figure 2b–2d).
Figure 2. Activation of serine proteases in EndMTs and calcification.
(a) Global gene expression profile of serine proteases in aortas from wild type (Mgp+/+) and Mgp−/− mice. (b–d) Expression of elastases (ELA) and kallikreins (KLK) in Mgp+/+ and Mgp−/− aortas, determined by real-time PCR and immunoblotting with densitometry. ***p<0.001.
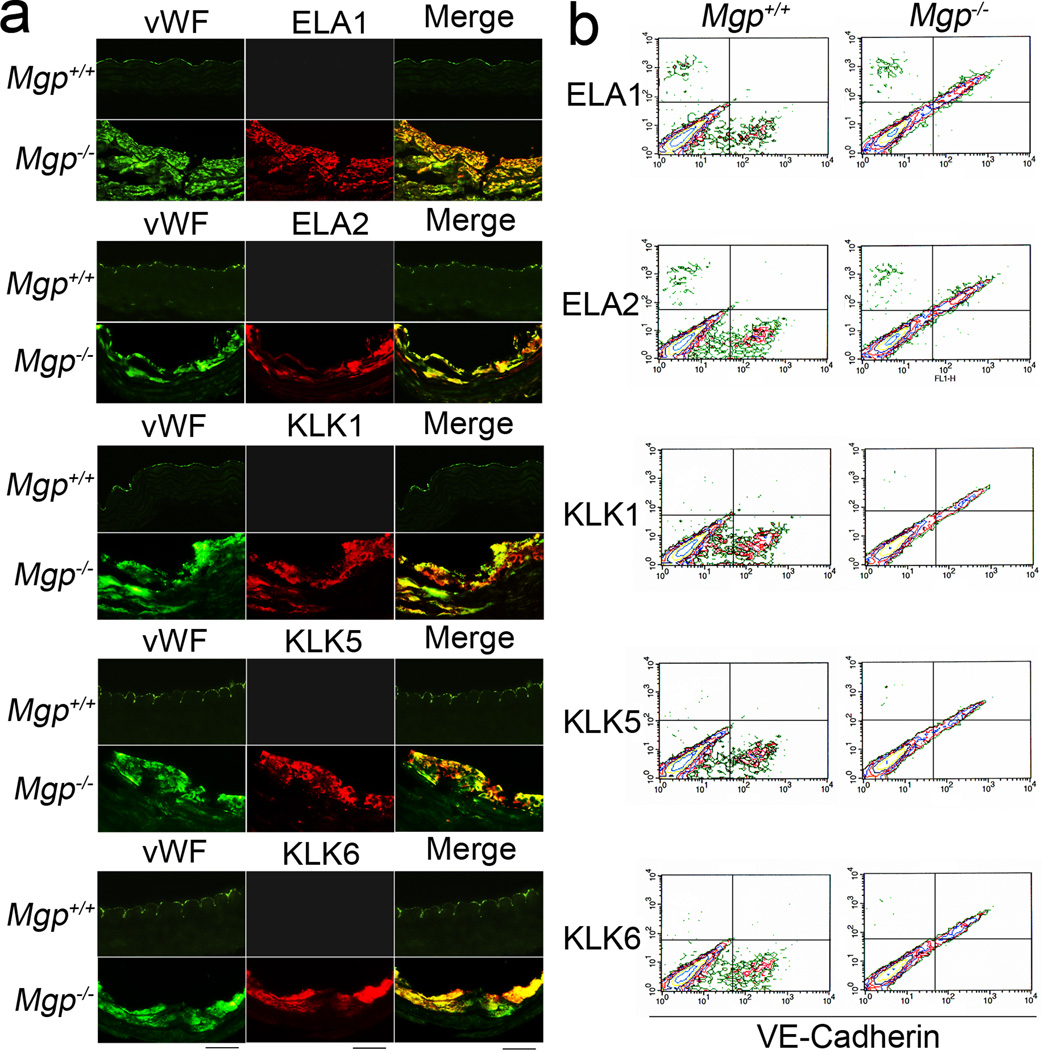
We also analyzed elastases and kallikreins in plasma by ELISA, which showed similar protease levels in Mgp−/− and Mgp+/+ mice (data not shown). Among isolated Mgp−/− aortic cells, the fraction of elastase- and kallikrein-positive cells was 11–14% (Online Figure III). We performed immunohistochemistry to localize the elastase- and kallikrein-positive cells in Mgp−/− aortic tissue. Elastase- and kallikrein-positive cells co-localized with the endothelial marker vWF (Figure 3a). The flow cytometric analysis also showed co-expression of elastases and kallikreins with the endothelial marker VE-cadherin in isolated Mgp−/− aortic cells (Figure 3b), providing evidence that elastases and kallikreins were induced in Mgp−/− aortic endothelium. By way of confirmation, co-localization was not observed between kallikrein 5 and smooth muscle myosin heavy chain, nor between elastase 1 and alpha-smooth muscle actin (SMA) (Online Figure IV).
Figure 3. Induction of elastases and kallikreins in Mgp−/− aortic endothelium.
(a) Immunostaining to detect co-localization of ELA 1, 2 and KLK 1, 5, 6 with the EC marker vWF. Scale bar: 100 µm. (b) Flow cytometric analysis of co-expression of KLK 1, 5, 6 and VE-Cadherin in Mgp−/− aortic cells.
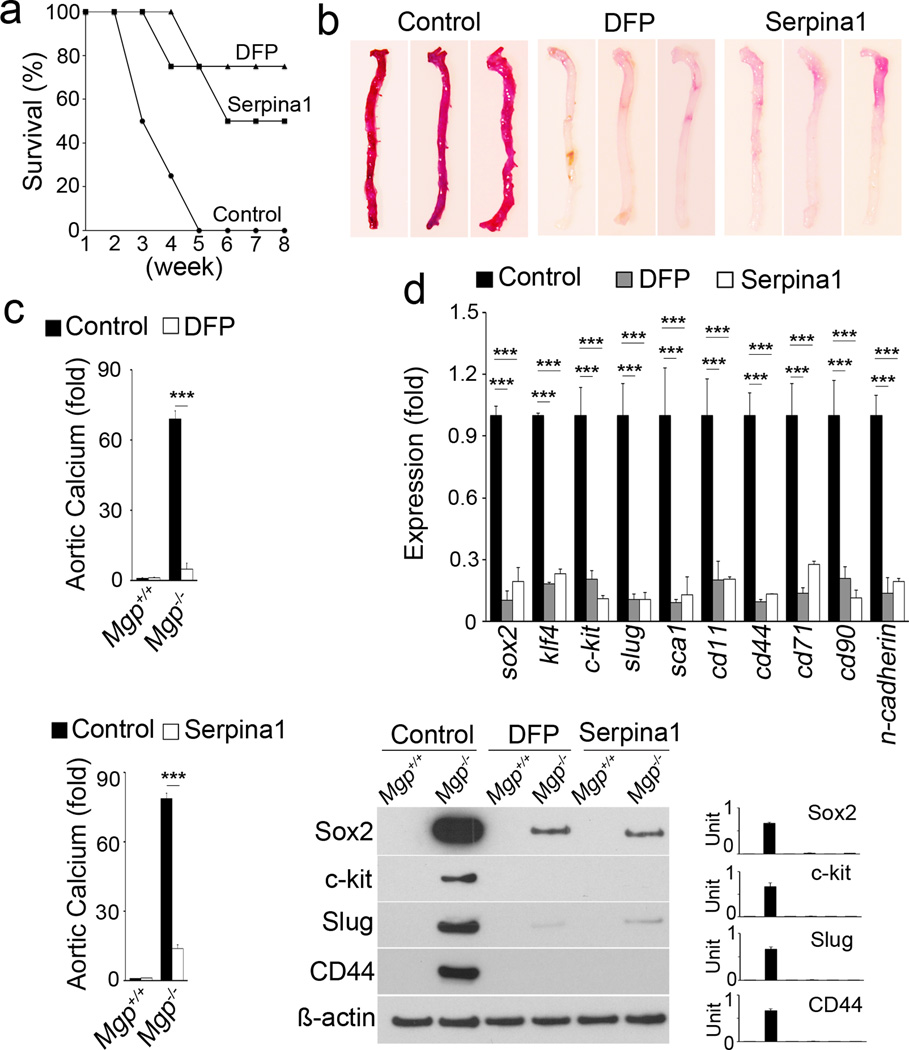
We treated Mgp−/− mice with the serine protease inhibitors DFP and serpina1 for two weeks starting at 2 weeks of age. We found that 80% and 50% of Mgp−/− mice were rescued by DFP and serpina1, respectively, after 6 weeks of treatment (Figure 4a). Aortic calcification in Mgp−/− mice, as visualized by Alizarin Red staining, was dramatically decreased after the DFP and serpina1 treatments (Figure 4b). Total aortic calcium was similarly decreased (Figure 4c), suggesting that serine protease inhibitors limited the calcifying process. To determine whether DFP and serpina1 affected EndMTs, we analyzed the expression of stem-cell and mesenchymal markers in aortic tissues by real-time PCR and immunoblotting. We found a significant decrease in these markers in Mgp−/− aortic tissues after DFP and serpina1 treatments (Figure 4d), suggesting that serine protease inhibitors also limited EndMTs. Together, our results suggest that serine proteases play important roles in transitioning ECs to a multipotent state allowing for osteochondrogenic differentiation.
Figure 4. Inhibition of serine proteases decreases EndMTs and vascular calcification.
(a) Survival rate of Mgp−/− mice after treatment with DFP and serpina1 (n=8 per group). (b) Alizarin Red staining of Mgp−/− aortas after 2 weeks of treatment with DFP or serpina1 injections. (c) Aortic calcium in Mgp−/− aortas after injection of DFP (top) or serpina1 (bottom). ***p<0.001. (d) Expression of stem-cell markers in Mgp−/− aortas after treatment with DFP or serpina1 as determined by real-time PCR (top) and immunoblotting with densitometry (bottom). ***p<0.001.
Complexes of serine proteases induce EndMTs
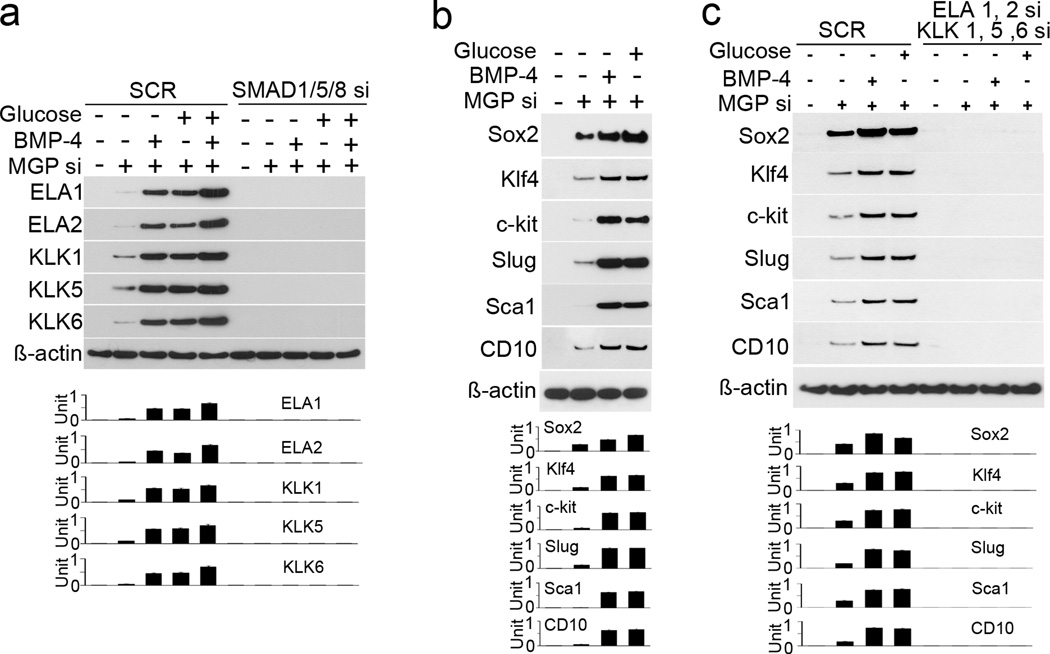
To determine if the complexes of elastases and kallikreins induce EndMTs, we used HAECs, more than 99.5% of which were CD31 positive11. We depleted MGP by siRNA transfection, which decreased MGP expression to <10% of normal levels after 20 to 24 hours30. We combined siRNA transfection with treatment of the cells with BMP-4 (40 ng/mL) or high glucose (22 nmol/L), which strongly induces BMP expression in ECs11, 22. The results showed that expression of elastase 1, 2 and kallikrein 1, 5, 6 increased with both MGP depletion and the two treatments (Online Figure V). Combined transfection of siRNA to SMAD1, 5 and 8, however, abolished the induction (Figure 5a), suggesting that elastases and kallikreins were induced by BMP signaling. We also found that expression of stem-cell and mesenchymal markers were induced in MGP-depleted HAECs (Figure 5b). We then transfected MGP-depleted HAECs treated with BMP-4 and glucose with siRNA to elastase 1, 2 and kallikrein 1, 5, 6. Depletion of the elastases and kallikreins abolished the induction of stem-cell and mesenchymal markers in MGP-depleted HAECs, and addition of BMP-4 or glucose failed to restore the expression (Figure 5c). Addition of all of elastase 1, 2 and kallikrein 1, 5, 6 induced the expression of stem-cell and mesenchymal markers in HAECs after 24 hours of treatment (Online Figure VI). We tested the addition of individual elastases, kallikreins and various combinations; only the combination of all five proteases affected the marker expression (data not shown). We examined the progenitor capacity of HAECs undergoing EndMTs. We transfected HAECs with MGP siRNA and treated with glucose and BMP-4, or treated with serine proteases. We examined the proliferative activity, and found increased proliferation in MGP-depleted HAECs. The increase was enhanced by BMP-4 and glucose (Online Figure VIIa). We examined colony-forming unit fibroblast (CFU-F) ability by alkaline phosphatase staining, and found that the CFU-F was higher in MGP-depleted or serine proteases treated HAECs (Online Figure VIIb). We also treated cells with adipogenic, cardiomyogenic or neurogenic medium. After 7 days of treatment, we examined the expression of adipogenic markers (peroxisome proliferator-activated receptor gamma, PPARgamma and CCAAT/enhancer-binding protein beta, C/EBPβ), cardiomyocyte markers (NK2 homeobox 5, Nkx2.5, and Troponin I), and neuronal markers (nestin and neuroD) by real-time PCR. All these markers were increased in the MGP-depleted or serine proteases treated HAECs (Online Figure VIIc–d). The results suggested that HAECs gained multipotency after MGP-depletion or treatment with serine proteases. Together, the results suggested that EndMTs are activated by complexes of elastases and kallikreins induced by BMP signaling in the endothelium.
Figure 5. Elastases and kallikreins induce EndMTs in HAECs.
(a) Elastase (ELA) 1, 2 and kallikrein (KLK) 1, 5, 6 were induced after depletion of MGP by siRNA (MGP si) in HAEC. The induction was enhanced by BMP-4 or high glucose, and abolished by transfection of siRNA to SMAD1/5/8 as shown by immunoblotting with densitometry. (b) Stem-cell and mesenchymal marker were induced after MGP si depletion in HAECs. The induction was enhanced by BMP-4 or high glucose as shown by immunoblotting with densitometry. (c) Depletion of elastase 1, 2 and kallikrein 1, 5, 6 by siRNA (si) abolished the induction of stem-cell markers in MGP-depleted HAECs as shown by immunoblotting with densitometry. SCR: scrambled siRNA.
Induction of Sox2-mediated mesenchymal transitions
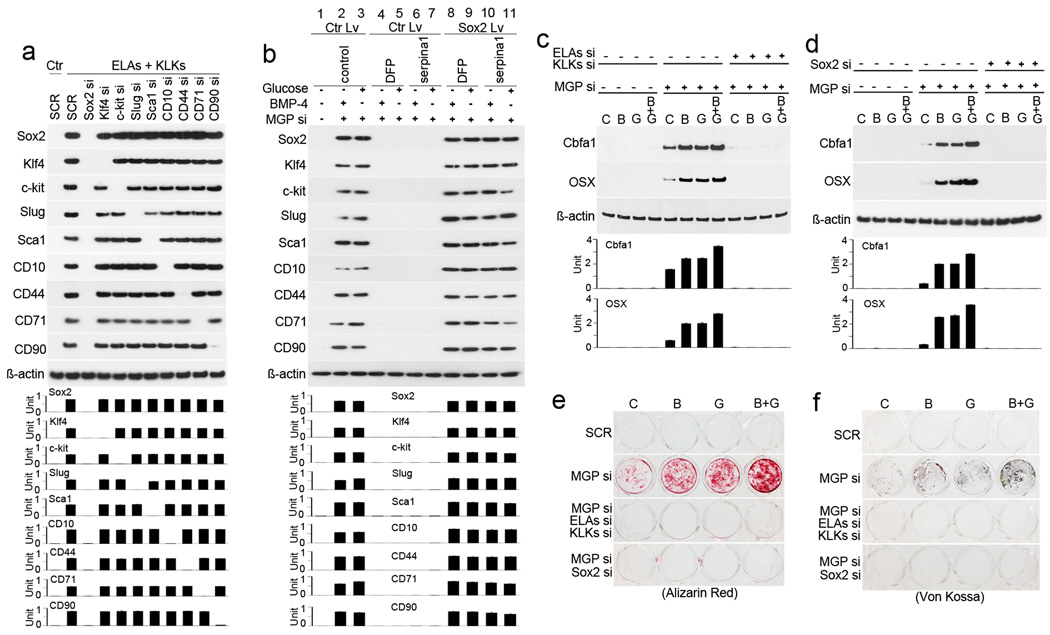
To identify factors that mediate activation of EndMTs by elastases and kallikreins, we focused on the highly induced stem-cell markers that included both transcription factors and cell surface receptors. We depleted the expression of Sox2, Klf4, c-kit, Slug, Sca1, CD10, CD44, CD71 and CD90 individually in HAECs (using specific siRNA), and treated with elastase and kallikrein. Immunoblotting showed that depletion of Sox2 abolished the induction of all other markers (Figure 6a). We then used Sox2-expressing lentivirus to restore Sox2 in HAECs depleted of MGP, elastases, and kallikreins, and treated the cells with BMP-4 and high glucose. Immunoblotting showed a re-emergence of stem-cell and mesenchymal markers in HAECs after restoration of Sox2 (Online Figure VIII). We also restored Sox2 in MGP-depleted HAECs treated with DFP and serpina1, which similarly led to the re-emergence of stem-cell and mesenchymal markers (Figure 6b). These results suggested that Sox2 mediated EndMTs after depletion of MGP.
Figure 6. Sox2 mediates activation of elastases and kallikreins in the induction of EndMTs.
(a) The expression of stem-cell markers was examined by immunoblotting with densitometry in HAECs treated with medium containing elastase 1, 2 (ELAs) and kallikrein 1, 5, 6 (KLKs), and transfected with siRNA (si) to each of the stem-cell markers individually. Ctr: control. SCR: scrambled siRNA. (b) HAECs were depleted of MGP by siRNA (si) and treated with high glucose and BMP-4, then treated with control (lane 1–3), DFP (lane 4–5), serpina1 (lane 6–7), or a combination of DFP, serpina1, and lentivirus (Lv) expressing Sox2 (lane 8–11). Expression of stem-cell markers was examined by immunoblotting with densitometry. (c–f) MGP-depleted HAECs were transfected with siRNAs (si) to elastase (ELA) 1, 2 and kallikrein (KLK) 1, 5, 6, or Sox2, and then treated with control (C), BMP-2 (B), glucose (G), or a combination. Expression of Cbfa1 and Osterix (OSX) was determined by immunoblotting with densitometry (c and d). Calcium accumulation was determined by Alizarin Red and Von Kossa staining (e and f). SCR: Scrambled siRNA.
To examine the effects of elastases, kallikreins, and Sox2 on osteogenic differentiation in MGP-depleted HAECs, we depleted MGP, elastases, and kallikreins, and then treated the cells for 4 days with osteogenic medium alone, osteogenic medium with BMP-2 (200 ng/mL) and high glucose (22 nmol/L), or a combination of osteogenic medium with either BMP-2 or glucose starting the day after siRNA transfection. Immunoblotting showed that with all four treatments, expression of the osteogenic markers Cbfa1 and Osterix was increased when MGP was depleted, but when elastases and kallikreins were knocked down, the expression of osteogenic markers was abolished (Figure 6c). Depletion of Sox2 also inhibited induction of Cbfa1 and Osterix in MGP-depleted HAECs in the same type of experiment (Figure 6d). Mineral staining (Alizarin Red and Von Kossa) confirmed that depletion of elastases and kallikreins or Sox2 limited calcium accumulation in MGP-depleted HAECs after 7 and 14 days of treatment (Figure 5e–f).
Endothelial-specific deletion of Sox2 decreases aortic calcification
We examined if Sox2 co-localized with proteases and Cbfa1 in vivo using immunofluorescence. Elastase 1, 2 and kallikrein 1, 5, 6 all co-localized with Sox2 and Cbfa1 in aortic endothelium of Mgp−/− mice at 4 weeks of age (Figure 7a and Online Figure IX). We pre-sorted VE-cadherin+CD45− aortic ECs from aortic tissues; CD45+ cells, which may represent CD31+ leukocyte populations, were excluded. Flow cytometric analysis showed that more than 30% of aortic ECs co-expressed elastases and kallikreins together with Sox2 (Online Figure X). These results suggested that the induction of Sox2 expression in ECs is caused by induction of elastases and kallikreins.
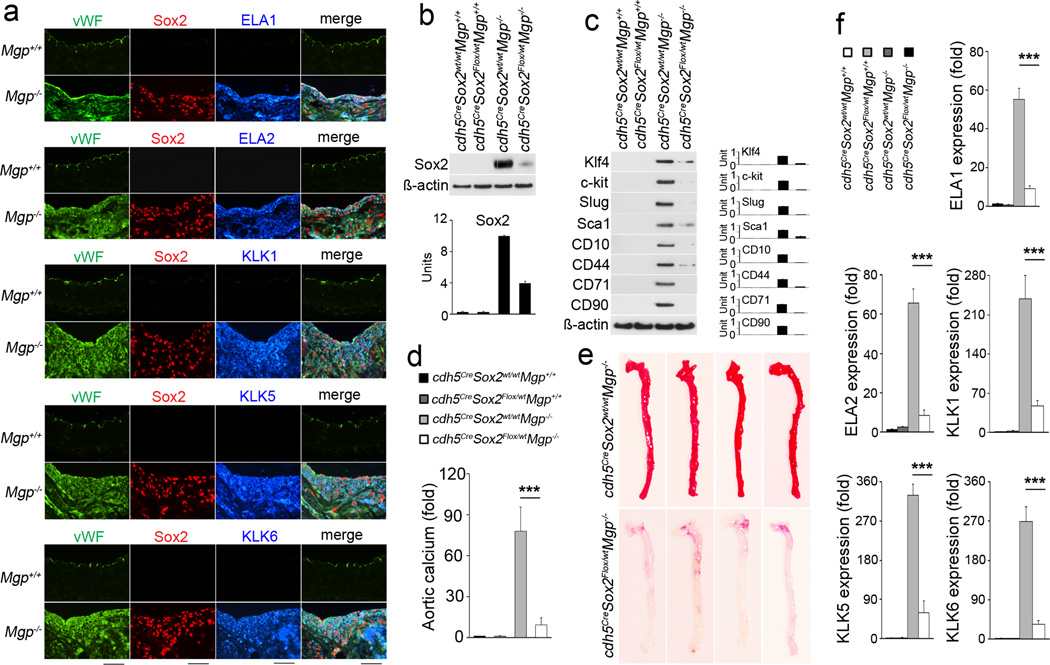
Figure 7. Limiting Sox2 in endothelium decreases EndMTs and calcification in Mgp−/− aortas.
(a) Co-expression of elastase (ELA) 1, 2 and kallikrein (KLK) 1, 5, 6 with EC marker vWF and multipotency marker Sox2 in Mgp−/− aortas as shown by immunostaining. Scale bars: 100 µm. (b) Decreased expression of Sox2 in aortas of Cdh5CreSox2Flox/wtMgp−/−, as shown by immunoblotting with densitometry. (c) Decreased expression of stem-cell and mesenchymal markers in aortas of Cdh5CreSox2Flox/wtMgp−/−, as shown by immunoblotting with densitometry. (d) Aortic calcium in Cdh5CreSox2wt/wtMgp−/− and Cdh5CreSox2Flox/wtMgp−/− mice. ***p<0.001. (e) Alizarin Red staining of Cdh5CreSox2wt/wtMgp−/− and Cdh5CreSox2Flox/wtMgp−/− aortas. (f) Decrease of expression of elastase (ELA) 1, 2 and kallikrein (KLK) 1, 5, 6 in the in aortas of Cdh5CreSox2Flox/wtMgp−/−, as shown by real-time PCR. ***p<0.001.
To determine if a cell-specific decrease of Sox2 in endothelium would limit EndMTs and vascular calcification, we crossed Cdh5Cre and Sox2Flox/Flox mice with Mgp+/− mice to selectively reduce Sox2 by Cre-mediated deletion in ECs by Cdh5 (VE-cadherin)-driven Cre expression. We examined the aortas of Cdh5CreSox2Flox/wtMgp−/− mice at 4 weeks of age and found that expression of Sox2 was decreased in the aortic endothelium (Figure 7b). Immunoblotting showed that expression of stem-cell and mesenchymal markers were significantly decreased in the aortic endothelium of Cdh5CreSox2Flox/wtMgp−/− mice (Figure 7c). Total aortic calcium was significantly reduced (Figure 7d) and Alizarin Red staining of whole aortic tissues showed a decrease in aortic mineralization in Cdh5CreSox2Flox/wtMgp−/− mice (Figure 7e). This suggested that EndMTs and vascular calcification are limited by endothelial Sox2 reduction.
We further examined the expression of elastase 1, 2 and kallikrein 1, 5, 6 in aortas of Cdh5CreSox2Flox/wtMgp−/− mice by real-time PCR. We found decreased expression of all the five serine proteases (Figure 7f), suggesting that reducing Sox2 in Mgp−/− endothelium decreased the induction of serine proteases.
Mutual regulation of Sox2 and Twist1 in EndMTs in vascular calcification
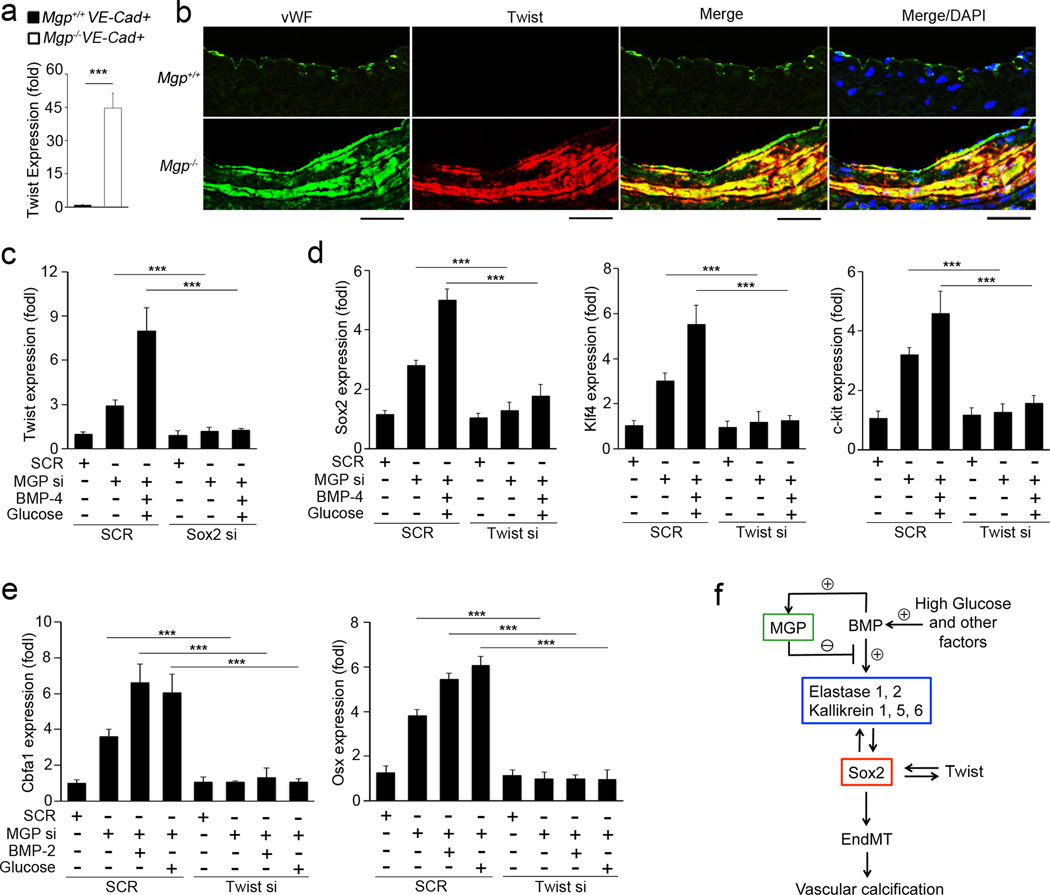
Twist1 is known to induce osteogenesis and play important roles in EndMTs and in epithelial-mesenchymal transitions33–35, where Twist1 and Sox2 were also shown to regulate each other36. To determine if Twist1 plays a role in EndMTs in Mgp−/− aortas, we examined the expression of Twist1 in presorted VE-cadherin+CD45− aortic ECs from 4-week-old Mgp−/− mice by real-time PCR, and found it to be increased (Figure 8a). We co-stained Twist1 with the endothelial marker vWF, which showed that Twist1 co-localized with vWF in Mgp−/− aortas (Figure 8b). We also examined expression of Twist1 in MGP-depleted HAECs, and similarly found it to be increased. The induction of Twist1 was enhanced by treatment of BMP-4 and glucose, and abolished by depletion of Sox2 (Figure 8c). We then depleted Twist1 in MGP-depleted HAECs, treated with BMP-4 and glucose, and examined the stem-cell markers Sox2, Klf4 and c-kit. The depletion of Twist1 decreased induction of Sox2, Klf4 and c-kit (figure 8d), suggesting that Twist1 and Sox2 are able to mutually regulate each other during the initiation of EndMTs in MGP-depleted HAECs.
Figure 8. Mutual regulation of Sox2 and Twist1 in EndMTs in vascular calcification.
(a) Increase of Twist1 expression in VE-cadherin+CD45− presorted cells from Mgp−/− aortas, as shown by real-time PCR. ***p<0.001. (b) Co-localization of Twist1 with the EC marker vWF in Mgp−/− aortas, as shown by immunostaining. Scale bars: 100 µm. (c) MGP-depleted HAECs were transfected with siRNAs (si) to Sox2, and then treated with combinations of BMP-4 and glucose. Expression of Twist1 was examined by real-time PCR. ***p<0.001. (d) MGP-depleted HAECs were transfected with siRNAs (si) to Twist1, and then treated with combination of BMP-4 and glucose. Expression of Sox2, Klf4 and c-kit was examined by real-time PCR. ***p<0.001. (e) MGP-depleted HAECs were transfected with siRNAs (si) to Twist1, and then treated with BMP-2 or glucose for 4 days. Expression of Sox2, Klf4 and c-kit was examined by real-time PCR. ***p<0.001. (f) Schematic working model for the induction of serine proteases and Sox2 in EndMTs in vascular calcification.
Furthermore, we depleted MGP and Twist1, and treated the cells for 4 days with osteogenic medium with either BMP-2 or glucose starting the day after siRNA transfection. The results showed that depletion of Twist1 abolished the induction of Cbfa1 and Osterix in MGP-depleted HAECs (Figure 8e). Altogether, the results suggested that Twist1 cooperated with Sox2 and played a role in initiating EndMTs leading to calcification.
DISCUSSION
Our results suggest that lack of MGP, a BMP inhibitor, results in the induction of endothelial elastases and kallikreins. The proteolytic activation of the elastases and kallikreins is instrumental in initiating EndMTs via a process mediated by Sox2, and allows for the emergence of multipotency and ultimately vascular calcification. Other studies have shown that elastases and kallikreins play important roles in vascular development and diseases, such as remodeling of the brachial artery and pulmonary hypertension37–39. Our results expand the role of serine proteases to the transition of ECs into stem-cell like cells, suggesting that tight regulation of these enzymes is important for the maintenance of endothelial stability and differentiation.
In our experiments, all five serine proteases had to be combined to induce mesenchymal stem-cell markers. Any combination with fewer serine proteases failed to initiate EndMTs. Elastase 1 and 2, and kallikrein 1, 5 and 6 all have chymotrypsin-like specificities, but because of different structures, the cleavage of various protein substrates differs40, 41. Under MGP-deficient conditions, it is possible that these proteases modify the endothelial microenvironment allowing the ECs to adopt a different cell fate.
Vascular calcification is a common complication of diabetic vasculopathy and atherosclerosis, and levels of elastases and kallikreins are elevated in diabetic patients as well as in atherosclerotic lesions42, 43. Our observations point toward new ways of explaining vascular calcification in these conditions and may lead to new strategies for limiting calcification.
Endothelial Sox2 was instrumental in the activation of EndMTs in HAECs. As an essential factor for development and somatic cell reprograming, Sox2 acts as a key factor in cell fate determination44. Previous studies have shown that Sox2 plays a role in epithelial-mesenchymal transition in neural crest development and cancer45, 46. It has also been reported that fibroblasts exhibit mesenchymal phenotypes after infection with Sox2 lentiviral vectors47, 48. Our findings broaden the functions of Sox2 to also include activation of EndMTs in the aortic endothelium.
Our observation that serine proteases induce Sox2 also provides new information applicable to development. Expression of Sox2 is sustained along the ectoderm, where organs emerge from interactions between the epithelium and the mesenchyme49, and proteolytic activity appears to be induced in tissue development50–52. Based on our data, it is possible that serine proteases regulate Sox2 to modulate mutual transitions of the endothelium and the mesenchyme to achieve correct cell differentiation as well as coordination between tissue-specific elements and the vasculature.
In our study, BMP-4 acted as an inductive factor of EndMTs in MGP deficiency, which is consistent with results from other investigators6, 53. The BMPs elicit their activity through ligand-initiated heterodimerization of BMP type II receptors (BMPRII) and type I receptors referred to as activin receptor-like kinase (ALK) 1, 2, 3, and 654. Activation of the type I receptor by BMPRII leads to phosphorylation of a series of SMAD proteins, which mediate the gene regulation54. Our previous studies in HAECs showed that the same glucose treatment used to induce EndMTs also enhanced expression of BMPRII22. Interestingly, it has been shown that EndMTs associated with pulmonary arterial hypertension occur when the BMPRII levels are reduced34, 55. Therefore, we also examined the expression of stem-cell markers in Mgp−/− lungs, and the effect of MGP-deficiency, BMP-4, glucose, and serine proteases on EndMTs in cultured human pulmonary artery ECs (HPAECs). To our surprise, we found no evidence of enhanced stem-cell marker expression or EndMTs in the Mgp−/− lungs or the HPAECs (unpublished observations), strongly suggesting that the pulmonary arteries differ in their response to MGP deficiency. This is further supported by the finding that MGP-deficiency causes arteriovenous malformations (AVMs) rather than vascular calcification in the pulmonary circulation30. Indeed, tissue-specific ECs from various organs have been reported to have unique signatures of gene expression, and to differ in their responses to stimulus56. In addition, we have observed different responses to MGP-deficiency in the pulmonary and cerebral vascular beds. VEGF is enhanced in the pulmonary vasculature, whereas Notch signaling is enhanced in cerebral vasculature, even though in both cases, MGP-deficiency leads to AVMs30, 57.
The response to loss of BMPRII may be difficult to predict, since BMPRII has been shown to be a target of multiple pro-inflammatory factors58, and absence of BMPRII might allow for enhanced BMP6/7 signaling through recruitment of the activin type II receptor59. Another complicating factor is that different BMP and TGFβ ligands affect EndMTs differently. Whereas BMP-4 enhances EndMTs, BMP-2 and -7 have been reported to reverse EndMTs induced by TGFβ155. TGFβ1 elicits its activity through ALK5, which is induced by BMP-4 in HAECs30, suggesting that TGFβ1-mediated EndMTs might still be regulated by BMP-4 depending on the type of endothelium. In addition, excess BMP-2 and -7 might compete with BMP-4 for binding to the same BMP receptors, thereby altering the balance that promotes or reverses EndMTs.
Previous studies have shown increased alpha-SMA expression in ECs undergoing EndMTs in pulmonary hypertension34. In addition, we showed increased expression of alpha-SMA and SM22alpha in EC-like cells in the media of Mgp−/− aortas11, further supporting that these early SMC markers are expressed in transitioning ECs. Speer et al.21 demonstrated by lineage-tracing that SM22alpha positive cells contribute to osteochondrogenic differentiation in Mgp−/− aortas, although it is not clear if a fraction of these cells might have been derived from the endothelium.
Smooth muscle cells (SMCs) reside in the vascular media, which is separated from the endothelium by the IEL. As the IEL is degraded, the SMCs are likely to be exposed to endothelial signaling, matrix alterations, and other effects of increased serine protease activity. This may affect the SMC phenotype and contribute to vascular calcification.
During development, expression of VE-cadherin is detected in ECs as well as in hematopoietic cells that arise from the hemogenic endothelium of the dorsal aortas60. Therefore, we cannot rule out a contribution to the observed phenotype from altered Sox2 signaling in hematopoietic cells. However, we have previously shown that expression of inflammatory and monocyte markers is minimal or undetectable in Mgp−/− aortas61. Thus it is less likely that hematopoietic cells, such as monocytes, would directly transit to or affect osteoprogenitor cells in the Mgp−/− aortas. Furthermore, even if the exclusion of CD45+ cells prior to flow cytometric analysis of the Mgp−/− aortic cells removed CD45+C31+ leukocytic cells, 20–30% of the analyzed ECs still co-expressed CD31 and Sox2. EC lineage tracing and co-staining of EC and multipotency or osteogenic markers in our previous studies further supported the endothelium as a source of osteogenic cells11. Altogether, our results suggest an important role for Sox2 in initiating EndMTs in vascular calcification.
Supplementary Material
Novelty and Significance.
What Is Known?
Vascular calcification is a frequent complication of cardiovascular diseases.
Endothelial-mesenchymal transitions (EndMTs) contribute to vascular calcification.
Lack of matrix Gla protein (MGP), an inhibitor of bone morphogenetic proteins (BMP), induces EndMTs thereby promoting vascular calcification.
What New Information Does This Article Contribute?
Specific serine proteases and sex determining region Y-box 2 (Sox2) are induced in endothelial cells at sites of vascular calcification in Mgp null aortas.
Serine proteases are essential for the initiation of EndMTs by inducing Sox2 in MGP-deficient endothelium.
Inhibition of serine proteases or suppression of Sox2 diminished EndMTs and vascular calcification.
Vascular calcification frequently complicates vascular diseases. EndMTs enable endothelium to contribute cells to vascular calcification. However, the initiation of EndMTs in vascular calcification is poorly understood. We report that Sox2-mediated activation of specific serine proteases is essential for initiating EndMTs. Using mouse models and cultured endothelial cells, we demonstrate that lack of the BMP-inhibitor MGP or enhanced BMP expression activates serine proteases and induces EndMTs. The activation of serine proteases is mediated by Sox2. These findings identify the serine proteases and Sox2 as the factors in EndMTs and vascular calcification and may provide therapeutic targets for treating vascular calcification.
ACKNOWLEDGEMENT
Electron microscopy was performed under supervision of Sirus A. Kohan at the Electron Microscopy Services Center of UCLA Brain Research Institute.
SOURCES OF FUNDING
Funding for this work was provided in part by NIH grants NS79353, HL30568, HL81397, and HL112839, and by a grant from the American Heart Association (Western Affiliate).
Nonstandard Abbreviations and Acronyms
- AVMs
Arteriovenous malformations
- BMP
Bone morphogenetic protein
- Cbfa1
Core binding factor alpha 1
- CD
Cluster of differentiation
- DFP
Diisopropylfluorophosphate
- EndMT
Endothelial-mesenchymal transition
- ELA
Elastase
- ELISA
Enzyme-linked immunosorbent assay
- FOP
Fibrodysplasia ossificans progressiva
- Gla
Gamma-carboxyglutamic acid
- HAEC
Human aortic endothelial cells
- KLF4
Kruppel-like factor 4
- KLK
Kallikrein
- MGP
Matrix Gla protein
- Sox2
Sex determining region Y-box 2
- Sca1
Stem Cell Antigen 1
- SEM
Scanning electron microscopy
- pSMAD
Phosphorylated SMAD
- SMAD
Homolog of the drosophila protein, mothers against decapentaplegic (MAD) and the C. elegans protein SMA
- SMC
Smooth muscle cells
- SMA
alpha-smooth muscle actin
- TEM
Transmission electron microscopy
- Twist1
Twist-related protein 1
- vWF
von Willebrand factor
- wt
wild type
Footnotes
DISCLOSURES
The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation. 2012;125:1795–1808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 3.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 4.Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 6.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: Potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Bostrom KI. A role for the endothelium in vascular calcification. Circ Res. 2013;113:495–504. doi: 10.1161/CIRCRESAHA.113.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wylie-Sears J, Aikawa E, Levine RA, Yang JH, Bischoff J. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Friehs I, Zhong Hu T, Melnychenko I, Tampe B, Alnour F, Iascone M, Kalluri R, Zeisberg M, Del Nido PJ, Zeisberg EM. Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circ Res. 2015;116:857–866. doi: 10.1161/CIRCRESAHA.116.305629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–S85. [PubMed] [Google Scholar]
- 16.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part ii. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 17.Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–249. doi: 10.1016/j.atherosclerosis.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: An early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 20.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix gla protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 24.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. Ve-cadherin-cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 25.Shaham O, Smith AN, Robinson ML, Taketo MM, Lang RA, Ashery-Padan R. Pax6 is essential for lens fiber cell differentiation. Development. 2009;136:2567–2578. doi: 10.1242/dev.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365:2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 27.Lin T, Duek O, Dori A, Kofman O. Differential long term effects of early diisopropylfluorophosphate exposure in balb/c and c57bl/j6 mice. Int J Dev Neurosci. 2012;30:113–120. doi: 10.1016/j.ijdevneu.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix gla protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 29.Bostrom K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix gla protein stimulates vegf expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem. 2004;279:52904–52913. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- 30.Yao Y, Jumabay M, Wang A, Bostrom KI. Matrix gla protein deficiency causes arteriovenous malformations in mice. J Clin Invest. 2011;121:2993–3004. doi: 10.1172/JCI57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jumabay M, Abdmaulen R, Urs S, Heydarkhan-Hagvall S, Chazenbalk GD, Jordan MC, Roos KP, Yao Y, Bostrom KI. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J Mol Cell Cardiol. 2012;53:790–800. doi: 10.1016/j.yjmcc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuen HF, Kwok WK, Chan KK, Chua CW, Chan YP, Chu YY, Wong YC, Wang X, Chan KW. Twist modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis. 2008;29:1509–1518. doi: 10.1093/carcin/bgn105. [DOI] [PubMed] [Google Scholar]
- 34.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Pechoux C, Bogaard HJ, Dorfmuller P, Remy S, Lecerf F, Plante S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 35.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ. Bmi1 is essential in twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 36.Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS. Cord blood stem cells revert glioma stem cell emt by down regulating transcriptional activation of sox2 and twist1. Oncotarget. 2011;2:1028–1042. doi: 10.18632/oncotarget.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yayama K, Kunimatsu N, Teranishi Y, Takano M, Okamoto H. Tissue kallikrein is synthesized and secreted by human vascular endothelial cells. Biochim Biophys Acta. 2003;1593:231–238. doi: 10.1016/s0167-4889(02)00393-2. [DOI] [PubMed] [Google Scholar]
- 38.Aimes RT, Zijlstra A, Hooper JD, Ogbourne SM, Sit ML, Fuchs S, Gotley DC, Quigley JP, Antalis TM. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost. 2003;89:561–572. [PubMed] [Google Scholar]
- 39.Azizi M, Boutouyrie P, Bissery A, Agharazii M, Verbeke F, Stern N, Bura-Riviere A, Laurent S, Alhenc-Gelas F, Jeunemaitre X. Arterial and renal consequences of partial genetic deficiency in tissue kallikrein activity in humans. J Clin Invest. 2005;115:780–787. doi: 10.1172/JCI23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Debela M, Magdolen V, Schechter N, Valachova M, Lottspeich F, Craik CS, Choe Y, Bode W, Goettig P. Specificity profiling of seven human tissue kallikreins reveals individual subsite preferences. J Biol Chem. 2006;281:25678–25688. doi: 10.1074/jbc.M602372200. [DOI] [PubMed] [Google Scholar]
- 41.Paliouras M, Diamandis EP. The kallikrein world: An update on the human tissue kallikreins. Biol Chem. 2006;387:643–652. doi: 10.1515/BC.2006.083. [DOI] [PubMed] [Google Scholar]
- 42.Campbell DJ, Kladis A, Zhang Y, Jenkins AJ, Prior DL, Yii M, Kenny JF, Black MJ, Kelly DJ. Increased tissue kallikrein levels in type 2 diabetes. Diabetologia. 2010;53:779–785. doi: 10.1007/s00125-009-1645-8. [DOI] [PubMed] [Google Scholar]
- 43.Antonicelli F, Bellon G, Debelle L, Hornebeck W. Elastin-elastases and inflamm-aging. Curr Top Dev Biol. 2007;79:99–155. doi: 10.1016/S0070-2153(06)79005-6. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar A, Hochedlinger K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandalos N, Rhinn M, Granchi Z, Karampelas I, Mitsiadis T, Economides AN, Dolle P, Remboutsika E. Sox2 acts as a rheostat of epithelial to mesenchymal transition during neural crest development. Front Physiol. 2014;5:345. doi: 10.3389/fphys.2014.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers sox2, oct4 and nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campolo F, Gori M, Favaro R, Nicolis S, Pellegrini M, Botti F, Rossi P, Jannini EA, Dolci S. Essential role of sox2 for the establishment and maintenance of the germ cell line. Stem Cells. 2013;31:1408–1421. doi: 10.1002/stem.1392. [DOI] [PubMed] [Google Scholar]
- 48.Yang N, Hui L, Wang Y, Yang H, Jiang X. Overexpression of sox2 promotes migration, invasion, and epithelial-mesenchymal transition through the wnt/beta-catenin pathway in laryngeal cancer hep-2 cells. Tumour Biol. 2014;35:7965–7973. doi: 10.1007/s13277-014-2045-3. [DOI] [PubMed] [Google Scholar]
- 49.Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 50.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 51.Yamakoshi Y, Hu JC, Fukae M, Yamakoshi F, Simmer JP. How do enamelysin and kallikrein 4 process the 32-kda enamelin? Eur J Oral Sci. 2006;114(Suppl 1):45–51. doi: 10.1111/j.1600-0722.2006.00281.x. discussion 93-45, 379-380. [DOI] [PubMed] [Google Scholar]
- 52.Bando Y, Ito S, Nagai Y, Terayama R, Kishibe M, Jiang YP, Mitrovic B, Takahashi T, Yoshida S. Implications of protease m/neurosin in myelination during experimental demyelination and remyelination. Neurosci Lett. 2006;405:175–180. doi: 10.1016/j.neulet.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 53.Malhotra R, Burke MF, Martyn T, Shakartzi HR, Thayer TE, O'Rourke C, Li P, Derwall M, Spagnolli E, Kolodziej SA, Hoeft K, Mayeur C, Jiramongkolchai P, Kumar R, Buys ES, Yu PB, Bloch KD, Bloch DB. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix gla protein deficiency. PLoS One. 2015;10:e0117098. doi: 10.1371/journal.pone.0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyazono K, Maeda S, Imamura T. Bmp receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of bmpr2 attenuates pulmonary hypertension. Eur Respir J. 2012;39:329–343. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 56.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, Bostrom KI. Reducing jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix gla protein deficiency. Proc Natl Acad Sci U S A. 2013;110:19071–19076. doi: 10.1073/pnas.1310905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim CW, Song H, Kumar S, Nam D, Kwon HS, Chang KH, Son DJ, Kang DW, Brodie SA, Weiss D, Vega JD, Alberts-Grill N, Griendling K, Taylor WR, Jo H. Anti-inflammatory and antiatherogenic role of bmp receptor ii in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1350–1359. doi: 10.1161/ATVBAHA.112.300287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (bmp) type ii receptor deletion reveals bmp ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 60.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.