Abstract
Over the past two decades, the biomechanical properties of cells have emerged as key players in a broad range of cellular functions, including migration, proliferation, and differentiation. Although much of the attention has focused on the cytoskeletal networks and the cell’s microenvironment, relatively little is known about the contribution of the cell nucleus. Here, we present an overview of the structural elements that determine the physical properties of the nucleus and discuss how changes in the expression of nuclear components or mutations in nuclear proteins can affect not only nuclear mechanics but also modulate cytoskeletal organization and diverse cellular functions. These findings illustrate that the nucleus is tightly integrated into the surrounding cellular structure. Consequently, changes in nuclear structure and composition are highly relevant to normal development and physiology and can contribute to many human diseases, such as muscular dystrophy, dilated cardiomyopathy, (premature) aging, and cancer.
Keywords: nucleus, biophysics, cancer, muscular dystrophy, lamins, migration
INTRODUCTION
The nucleus is the hallmark of eukaryotic cells. It is generally the largest subcellular organelle (~5–20 µm in diameter) and houses the genetic information that directs the activity of the entire cell. The structural organization of the nucleus and its mechanical properties are critical for a variety of cellular functions and processes. The nuclear envelope, in particular the nuclear lamina, protects the nuclear interior in cells subjected to physical stress (e.g., in muscle tissues) (1, 2); the nuclear envelope can also modulate important signaling pathways by interacting with transcription factors that shuttle between the nucleus and the cytoplasm (3–7). In the nuclear interior, DNA and chromatin occupy well-defined chromosome territories that are often conserved over several generations (8, 9), and the localization of genes within the nucleus can determine their transcriptional activity (10). Importantly, the influence of nuclear structure and mechanics extends beyond the nucleus: Nuclear envelope proteins that physically interface with the cytoskeleton can affect cytoskeletal organization, cell polarization, adhesion, and migration (11–13). The importance of nuclear composition and organization has recently received increasing prominence as mutations in nuclear envelope proteins, such as lamins or emerin, were identified as causing a perplexing number of human diseases, including Emery-Dreifuss muscular dystrophy (EDMD), dilated cardiomyopathy, familial partial lipodystrophy (FPLD), and the premature aging disease Hutchinson-Gilford progeria syndrome (HGPS) (14). In the following, we provide a concise overview of normal nuclear structure and mechanics and the physical interplay between the nucleus and the surrounding cytoskeleton. Subsequently, we discuss how changes in nuclear structure and composition, such as those resulting from mutations in nuclear envelope proteins or changes in expression, can contribute to a variety of human diseases and pathological conditions.
NORMAL NUCLEAR STRUCTURE AND MECHANICAL PROPERTIES
The Nuclear Envelope
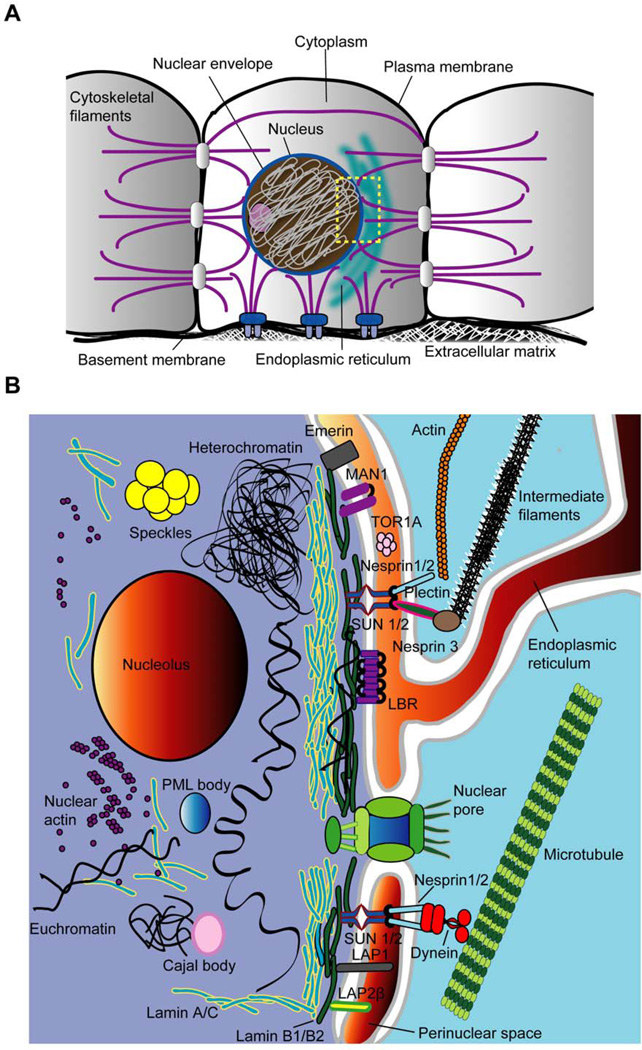
The nucleus can be divided structurally and functionally into two distinct compartments: the nuclear envelope and the nuclear interior (Figure 1). The nuclear envelope physically separates the cell’s genetic material from the cytoplasm. It is composed of two phospholipid bilayers, namely the inner and the outer nuclear membranes, and the underlying nuclear lamina, a dense protein network that dominates the physical properties of the nuclear envelope (1, 15).
Figure 1. Overview of nuclear structure and its interplay with the cytoplasm.
(A) Outline of a cell depicting the nucleus and its physical connections to cell-cell junctions and focal adhesions via cytoskeletal filaments. The rectangle marks the area depicted in the second panel. (B) Close up of the nuclear envelope and nuclear interior. The nuclear envelope is composed of the inner and outer nuclear membranes, nuclear pore complexes, and the nuclear lamina. Depicted is only a small subset of nuclear membrane proteins, which are described in further detail in the manuscript. These include the inner nuclear membrane proteins emerin, MAN1, SUN1 and SUN2, Lamin B receptor (LBR), and various lamina-associated polypeptide (LAP) isoforms. On the outer nuclear membrane, nesprins-1 and −2 interact with actin microfilaments and microtubules (via motor proteins such as dynein) whereas nesprin-3 binds to intermediate filaments via the adaptor protein plectin. The nuclear lamina consists of A-type lamins (which are also found in the nuclear interior) and B-type lamins, which form separate but overlapping networks. Chromatin, present as condensed heterochromatin or more loosely organized euchromatin, is the major constituent of the nuclear interior, which also includes other intranuclear structures such as the nucleolus, Cajal bodies and Promyelocytic Leukaemia (PML) bodies. Nuclear actin exists predominantly in monomeric, globular form, but may also organize into short, phalloidin-negative oligomers.
The nuclear membranes
The inner and outer nuclear membranes encapsulate the approximately 30- to 50-nm-wide perinuclear space, which is contiguous with the endoplasmic reticulum. The nuclear envelope is punctuated by nuclear pore complexes, which provide means of nucleocytoplasmic trafficking of macromolecules above the nuclear size exclusion limit. Small molecules less than 40 kDa in size can passively diffuse through the nuclear pores, but macromolecules of more than ~40 to 60 kDa require energy- and signal-dependent transport processes, which are mediated by nuclear import and export proteins (16). The nuclear membranes contain at least 50 to 100 specific membrane proteins, many of which remain uncharacterized (17). Importantly, inner and outer nuclear membranes are joined at nuclear pores, so that small membrane proteins can shuttle between the inner and outer nuclear membrane and into the endoplasmic reticulum, provided that they can pass the nuclear pore. Therefore, inner nuclear membrane proteins, such as emerin, lamin B receptor (LBR), lamina associated proteins (LAPs) and Sad1p/UNC-84 (SUN) proteins (18), are thought to be retained at the inner nuclear membrane by interacting with lamins, chromatin, or other proteins in the nuclear interior (7, 18). Similarly, outer nuclear membrane proteins, e.g., large nesprin isoforms, are prevented from diffusing laterally into the endoplasmic reticulum by interacting with specific inner nuclear membrane proteins. Consequently, loss of protein interaction at the nuclear envelope can lead to functional loss of the associated proteins. For example, loss of lamins A/C also causes mislocalization of emerin into the endoplasmic reticulum, and saturation or depletion of available binding sites of SUN proteins at the inner nuclear membrane results in functional loss of nesprins (19–21).
The nuclear lamina
The nuclear lamina is mainly comprised of lamins, i.e., type V intermediate filaments specific to the nucleus. Mammalian somatic cells express two types of lamins, A-type (lamins A and C, resulting from alternative splicing of the LMNA gene) and B-type lamins (lamins B1 and B2/B3, encoded by LMNB1 and LMNB2, respectively). Although the expression of A-type lamins is developmentally regulated and mostly restricted to differentiated cells, at least one B-type lamin is constitutively expressed in all cells (22). Mutations in A-type lamins cause a broad spectrum of human diseases, collectively referred to as laminopathies (14). These diseases include EDMD, dilated cardiomyopathy, Dunnigan-type FPLD, Charcot-Marie-Tooth disorder (Type II), and HGPS (reviewed in 14), which are discussed in more detail below. Mice lacking lamins A and C develop severe muscular dystrophy and dilated cardiomyopathy and die prematurely at 4 to 8 weeks of age (23). In contrast to A-type lamins, no human mutations have been identified in B-type lamins, except for duplications of LMNB1 associated with leukodystrophy (24) and leukoencephalopathy (25). Because lamin B1- and B2-deficient mice die at birth (26–28) and knockdown of B-type lamins is lethal in cultured cells (29), these findings suggest that B-type lamins are essential. A- and B-type lamins form distinct but overlapping networks at the nuclear envelope (30). While B-type lamins have permanent farnesylation which anchors them tothe nuclear envelope, lamins A and C exist in dynamic exchange between the nuclear lamina and the nuclear interior (31, 32). Electron micrographs reveal a regular lattice structure of lamins in Xenopus oocytes (33, 34); however, in somatic cells, the structural configuration of lamins remains unclear, and electron micrographs often show only a dense, poorly defined protein network. Nonetheless, it is now well established that A-type lamins play a key role in the maintenance of nuclear shape (35–37), structure (13, 37, 38), and stability (1, 37, 38). In addition to their structural role, A-type lamins have been linked to changes in gene expression, as they can interact with numerous transcriptional regulators, including retinoblastoma protein (Rb), c-Fos, or sterol response element binding protein-1 (SREBP-1) (5, 7, 39–41).
The Nuclear Interior
Chromatin and chromosome territories
Encapsulated within the nucleus, DNA is wrapped around histones and other chromosomal proteins to form nucleosomes, which are then assembled into ~30-nm-thick chromatin fibers. In interphase nuclei, chromatin can be found in two configurations: heterochromatin and euchromatin. The highly condensed heterochromatin is predominantly silent and, in the case of constitutive heterochromatin, often consists of gene-poor regions of DNA. Conversely, euchromatin is typically gene rich, transcriptionally active, and exists in a more relaxed and accessible configuration. These two forms of chromatin occupy distinct regions inside the nucleus. Euchromatin is found toward the nuclear interior and near nuclear pores, whereas heterochromatin is often found at the nuclear periphery, possibly because of direct interaction with the nuclear lamina. (10). Chromosomes occupy discrete and nonrandomly distributed regions, so-called chromosome territories, in the interphase nucleus, and the positions of these territories are, in part, heritable through mitosis (9). Recent computational and experimental analysis suggests that chromatin organization resembles a fractal, knot-free globule that enables dense packing (in humans, ~2 m of total DNA are packaged into each nucleus), while allowing rapid folding and unfolding of specific genomic loci (42).
Nuclear bodies and intranuclear structures
In addition to chromatin, the nuclear interior contains several distinct structures and features. The most prominent, and visible even by phase contrast microscopy, are nucleoli, which are the sites of ribosome biogenesis. Although nucleoli are distinct structures within the nucleoplasm, nucleolar proteins are constantly exchanged with the nucleoplasm, with transition times on the order of seconds (43). Nuclear speckles, named after the irregular and punctuate appearance in the nucleoplasm when fluorescently labeled, are dynamic structures enriched in premessenger RNA splicing factors. On electron micrographs, nuclear speckles appear as clusters of interchromatin granules (44). As described for nucleoli, the protein and RNA components of nuclear speckles continuously cycle between the speckles and the nucleoplasm. Cajal bodies, also known as coiled bodies, are associated with small nuclear ribonucleoproteins and nucleoli (45). These dynamic structures are regulated by cellular stress conditions, such as heat shock and DNA damage (46, 47).
Promyelocytic leukemia bodies
Promyelocytic leukemia (PML) bodies are thought to be associated with transcriptional regulation (48) and changes in chromatin structure (e.g., during senescence) (49). In cells exposed to mechanical stress, PML bodies respond by an increase in number and size (46, 50). For most of these intranuclear structures, their precise function and organization remains the focus of intense research.
The nucleoskeleton
Although the structural and functional aspects of the cytoskeleton are well established, the existence and configuration of a stable nucleoskeleton is still a subject of scientific debate. Numerous structural proteins commonly associated with the cytoskeleton have been found in the nuclear interior, including actin, myosin, spectrin, and titin (51–56). Most nuclear actin is found in the form of G-actin monomers; however, it can also form (presumably short) oligomers (57–59). Together with nuclear myosin, nuclear actin could contribute to dynamic rearrangements of chromatin (60), and recent experiments suggest that nuclear actin may also be involved in DNA transcription (61).
Apart from their localization at the nuclear lamina, A-type lamins also exist in the nuclear interior, where they form stable structures (32). The role of these intranuclear lamins is not completely understood; recent reports suggest that they could function in chromatin organization, DNA repair, and transcriptional regulation, for example, by forming a complex with LAP2α and Rb (3, 23, 62–64). This hypothesis is supported by data from lamin A/C-deficient (Lmna−/−) mice and patients with EDMD that reveal abnormal chromatin organization and impaired transcriptional regulation in response to mechanical strain or chemical stimulation (2, 23, 65). Furthermore, lamin A/C speckles in the nuclear interior colocalize with RNA splicing factors (66), and the expression of a dominant negative lamin mutant can inhibit RNA polymerase II-dependent transcription (67), confirming a role of A-type lamins in nuclear organization and DNA transcription.
Mechanical Properties of the Nucleus
Although many biology textbooks depict the nucleus as a static structure containing chromosomes and other intranuclear domains, it is important to realize that in living cells, the nucleus and its contents are continuously subjected to mechanical forces, which result in dynamic nuclear deformations and rearrangements. These forces can originate from the nuclear interior, for example, through transcriptional events and DNA synthesis; from cytoskeletal processes; or from the cellular environment, i.e., neighboring cells or the extracellular matrix. Experiments on - various cells using diverse experimental techniques, such as a micropipette aspiration, atomic force microscopy (AFM), compression with microplates, particle tracking, or cellular strain application, consistently find that the interphase nucleus is approximately 2 to 10 times stiffer than the surrounding cytoplasm, although the reported values for nuclear stiffness vary from 0.1 kPa to 10 kPa, depending on the type of cell and the experimental method employed (68–71). The mechanical properties of the nucleus are primarily determined by the nuclear interior and the nuclear lamina. The relative contribution between nuclear interior, i.e., chromatin, and nuclear lamina were recently determined by micropipette aspiration of nuclei isolated from African green monkey kidney epithelium cells that were either osmotically swollen or condensed (reference #72: Dahl et al. BiophysJ 2005). In swollen nuclei, which resemble the nuclear morphology in intact cells, the nuclear lamina is the major load-bearing element; in the condensed state, overall nuclear stiffness increases 10-fold, and the condensed chromatin becomes the major contributor to nuclear stiffness (ref #72). The nuclear membranes do not contribute significantly to nuclear stiffness or shape stability, regardless of the nuclear condition, as they exhibit fluid-like behavior and deform at energies typical for lipid bilayers (15).
Mechanical contributions from the nuclear lamina
Studies on nuclei from Xenopus oocytes, which are significantly larger than mammalian cells and thus more amendable to physical manipulation, have greatly contributed to our current understanding of the mechanical properties of the nucleus. These studies suggest that the nuclear lamina acts as an elastic, load-bearing element that provides structural integrity to the nucleus, in particular under tensile load application (1, 72, 73). Consequently, nuclei assembled in Xenopus egg extracts depleted for lamin are highly fragile (74), confirming the importance of lamins on nuclear mechanics. In mammalian somatic cells, which express A-type and B-type lamins, lamin A and-to a lesser extent-lamin C are the major contributors to nuclear stiffness: Lmna−/− mouse embryo fibroblasts (MEFs) and myoblasts have significantly more deformable nuclei than wild-type controls; cells expressing only lamin C but not lamin A have an intermediate phenotype (37, 75). In contrast, cells lacking functional lamin B1 have normal nuclear mechanics (37). Consistent with these findings, only expression of lamin A, but not lamins B1, B2, or C, restores nuclear stiffness in Lmna−/− MEFs (37). These observations of distinct mechanical functions of A- and B-type lamins are consistent with recent reports that A- and B-type lamins form separate structures and networks at the nuclear envelope (30, 34, 73). In addition to lamins, other nuclear envelope proteins such as emerin, nesprins, or SUN proteins may contribute toward nuclear stability by forming additional structural networks (e.g., in combination with nuclear actin) or by crosslinking the nuclear lamina to the nuclear membrane and chromatin structures. Furthermore, as the nuclear lamina mediates nucleocytoskeletal coupling (see the following sections), mutations in lamins or other nuclear envelope proteins could alter force transmission between the nucleus and the cytoskeleton.
Mechanical contribution from the nuclear interior
In addition to the nuclear lamina, the nuclear interior also plays a key role in determining the mechanical properties of the nucleus. The major constituent of the nuclear interior is chromatin. As a consequence, modifications in chromatin structure and organization, for example, during differentiation, treatment with histone deacetylase inhibitors, upregulation of heterochromatin proteins, or changes in divalent salt concentration, can directly affect the mechanical properties of the nucleus (72, 76, 77). In contrast to the elastic nuclear lamina, interphase chromatin is more viscoelastic in nature, meaning that it undergoes plastic deformation and flow in response to applied mechanics stress, such as during micropipette aspiration (77, 78). Micropipette aspiration experiments also reveal that the sponge-like chromatin is initially quite compressible but resists further deformations once it has been compacted beyond a certain degree (15, 72). Not surprisingly, heterochromatin and euchromatin display distinct differences in their physical behavior (79). In addition to chromatin, nuclear bodies and nucleoskeletal structures could further contribute to the effective stiffness of the nucleus. For example, AFM measurements suggest that nucleoli are stiffer than the surrounding nucleoplasm, and during micropipette aspiration, nucleoli deform as cohesive, viscous structures that display permanent (i.e., plastic) deformations under high stress (77, 80). Nuclear lamins, in particular lamins A and C, form stable structures in the nuclear interior that could further increase the stability of the nucleus or aid in chromatin organization (38, 81). The relative contribution between lamins at the nuclear interior and those located at the nuclear envelope remains unclear, but the lower mobility of lamins within the nuclear lamina (38) and the-judged by electron micrographs-thicker lamin network at the nuclear envelope suggest that the lamins within the nuclear lamina predominantly provide the structural stability to the nucleus.
NUCLEAR MECHANICS IN THE CONTEXT OF CELLULAR MECHANICS
Nucleocytoskeletal Coupling
It is now well established that externally applied force can be transmitted from the cell surface through the cytoskeleton to the nucleus (82). However, the molecular players responsible for coupling the nucleus to the cytoplasm only recently have begun to emerge. Studies on Caenorhabditis elegans, Drosophila melanogaster, and mammalian cells have identified two new families of nuclear envelope proteins that are ideally suited to carry out the task of force transmission across the nuclear envelope, connecting the cytoskeleton to the nuclear interior (reviewed in 83). These studies have resulted in the current model of nucleocytoskeletal coupling, in which large nesprin isoforms located in the outer nuclear membrane bind to cytoskeletal F-actin, intermediate filaments (via plectin), or microtubules and the centrosome (via dynein and kinesin) (84–89). At the nuclear envelope, nesprins interact across the perinuclear space with inner nuclear membrane proteins, called SUN proteins, which in turn bind to lamins, nuclear pore complexes, chromatin, and possibly other, unidentified proteins, thereby completing the physical link between the cytoskeletal networks and the nucleus (90–94). This physical interaction at the nuclear envelope is now commonly referred to as the linker of nucleoskeleton and cytoskeleton (LINC) complex (91).
Nesprins
Nesprins, which stand for nuclear envelope spectrin repeat (also known as Syne, Myne, Enaptin, or Nuance) form the prototype of outer nuclear envelope components that link the nuclear envelope to the cytoskeleton. Nonetheless, smaller nesprin isoforms can also be located on the inner nuclear membrane, and some nesprin isoforms are found entirely outside the nucleus (95). Mammals have four nesprin genes, which give rise to multiple nesprin isoforms through alternative splicing and transcription initiation (96). Common to all nesprins genes (but not all isoforms) is the highly conserved C-terminal KASH (Klarsicht/ANC-1/Syne homology) domain. The KASH domain, consisting of a transmembrane domain and a short luminal domain, mediates localization of nesprins to the nuclear envelope and is required for their interaction with SUN proteins (91, 94). The genes encoding nesprin-1 and −2 express numerous isoforms; the largest of each can bind actin via their actin-binding domains at the –N terminus (97, 98). Nesprin-3 connects to cytoplasmic intermediate filaments via plectin (87), whereas nesprin-4 binds kinesin, a plus-end-directed microtubule-dependent motor protein (84). Similarly, UNC-83, a C. elegans KASH-domain protein, interacts with kinesin-1 and dynein to move nuclei during nuclear migration in the forward and backward directions, respectively (86, 99).
SUN proteins
SUN-domain proteins are conserved from yeast to humans. Mammals have four genes for SUN proteins: SUN1 and SUN2, and testis-specific SUN3 and Spag4, with only SUN1 and SUN2 found in the nucleus (91, 100, 101). SUN proteins are defined by a conserved 120-residue SUN motif located in the nuclear envelope luminal space. SUN1 and SUN2 contain three putative transmembrane domains and are localized in the inner nuclear membrane, with their C-terminal SUN domain spanning the perinuclear space between the inner and outer nuclear membrane (91, 98, 102). Their N-terminal domain is required for direct interaction with A-type lamins, whereas the coiled-coil domains are predicted to mediate homo- and heterodimerization (91, 103). Recent experiments suggest that SUN1 and SUN2 have distinct, albeit overlapping, roles (104, 105). For example, SUN1 is required for spacing of nuclear pore complexes and preferentially binds the precursor of lamin A (i.e., prelamin A), implying a role in lamin A maturation or assembly (106, 107). In contrast, SUN2 is not associated with nuclear pore complexes and binds equally well to both precursor and mature forms of lamin A (106). In addition, SUN1 and SUN2 also bind to emerin, implicating a role for emerin as a LINC complex component (90).
Linker of nucleoskeleton and cytoskeleton complex function
LINC complexes play crucial roles in many aspects of cellular structure. They determine the spacing between the inner and outer nuclear membrane (91), prevent clustering of nuclear pore complexes (106), are critical for nuclear positioning and anchorage, control nuclear size and architecture (109), and even affect cytoskeletal organization (12, 110). LINC complex proteins are also important for many cellular functions such as migration (20), polarization (12, 94), and differentiation (111). For example, nesprin-2 giant, but not nesprin-1, is required for the rearward nuclear movement in fibroblasts that initiates polarization and migration in a scratch-wound assay (20). This suggests that different combinations of nesprins and SUN proteins may mediate different functions; for example, in muscle fibers, synaptic nuclei that are normally anchored to neuromuscular junctions specifically express nesprin-1. Loss of nesprin-1, SUN1, or lamins A/C results in (partial) mislocalization of synaptic nuclei away from the neuromuscular junctions, causing dystrophic muscles (112–114). However, it remains unclear how the specific functions and interactions of SUN and KASH proteins are regulated, as biochemical assays suggest that nesprins and SUN proteins interact promiscuously through their luminal domains (94). Thus, it is likely that differential expression of specific isoforms are responsible for the particular nuclear envelope composition; another possibility is that interactions with other nuclear envelope proteins such as lamins, nuclear pore complex components, the ATPase torsin A, or emerin are required to target specific SUN or nesprin isoforms to the nuclear envelope. For example, torsin A detaches LINC complexes formed by SUN2 and nesprins-2 and −3 but does not affect the localization of SUN1 (115), suggesting a mechanism by which LINC complexes undergo active and selective assembly and retention at the nuclear envelope.
Nuclear Mechanics in Migration
When discussing nuclear mechanics, it is important to keep in mind that the nucleus exists in the context of the surrounding cytoplasm. Furthermore, the cell is anchored to neighboring cells and the extracellular matrix through integrins and other transmembrane receptors that connect to the actin and intermediate filament networks. The cytoskeleton in turn is physically linked to the nucleus, as discussed in the preceding sections. Consequently, intra- and extracellular forces and externally applied tissue strain can result in significant nuclear deformations. These nuclear deformations are relevant to many cellular processes, for example, migration or perfusion through narrow spaces. In normal cells, the nucleus is 2 to 10 times stiffer than the surrounding cytoskeleton (68, 69, 116). On the basis of its size and stiffness, the mechanical properties of the nucleus restrict the overall ability of cells to deform. Therefore, in the absence of proteolytic activity, the ability of cells to pass through narrow openings is limited by the size and deformability of their nucleus. Intact nucleocytoskeletal coupling is another requirement for effective cell migration, as nuclear movement must be precisely coordinated with the motion of the cell body. In migrating epithelial, neuronal, and mesenchymal cells, the nucleus is typically located toward the rear of the cell, with the centrosome, endoplasmic reticulum, and Golgi apparatus facing the leading edge (117). In other cell types, such as white blood cells, the nucleus can be located toward the leading edge during migration, with the centrosome positioned behind the nucleus (118). Importantly, disruption of nucleocytoskeletal coupling can interfere with this polarization and result in impaired cell mobility (11, 12, 20).
Nuclear Mechanics in Mechanosensing
Nucleocytoskeletal connection also may be relevant to cellular mechanosensing. Cells constantly rely on physical feedback from their microenvironment, including neighboring cells and the extracellular matrix. These interactions are crucial throughout development, differentiation, aging, and everyday function (119). In many of these processes, actin-associated molecular motors such as myosin adjust the cytoskeletal tension to the stiffness of the surrounding extracellular matrix, which in turn can reinforce or weaken focal adhesions, resulting in a dynamic feedback loop that modulates cytoskeletal tension, cell shape, cell adhesion, motility, and even differentiation (120–123). Because disruption of the LINC complex results in impaired cytoskeletal organization and reduced cytoskeletal stiffness (12, 75, 94, 110), it could also disturb cytoskeleton-mediated mechanosensing. Furthermore, researchers speculate that the nucleus could act as a cellular mechanosensor, detecting applied forces by induced intranuclear deformations, which may modulate transcriptional activity, for example by modulating the accessibility of transcription factor binding sites or transcriptional complexes. Supporting a role of the nucleus in mechanosensing, nuclear deformations in chondrocytes subjected to compressive loads closely correlate with resulting changes in cartilage composition and density (69), and disruption of the LINC complex perturbs mechanotransduction in C2C12 myoblasts (111). Nonetheless, more studies are necessary to elucidate further the function of the nucleus in cellular mechanotransduction.
NUCLEAR MECHANICS IN DEVELOPMENT, DIFFERENTIATION, AND AGING
Stem cells display functional and developmental plasticity in that they are fully able to differentiate into multiple lineages and activate diverse gene expression profiles (124). This property of stem cells has been linked to changes in chromatin structure and regulation by epigenetic mechanisms, which could make chromatin in stem cells more accessible to transcription factor binding (76, 125). Recent biophysical measurements have demonstrated that nuclei from stem cells also have greater mechanical plasticity (i.e., deformability) than those from differentiated cells (77, 126). For example, nuclear stiffness increases significantly (~six fold) during induced terminal differentiation of human adult stem cells and hematopoietic stem cells (77). Similarly, mouse embryonic stem cells show significantly more dynamic nuclear deformations in time-lapse imaging studies than MEFs (77). What mechanisms could contribute to these changes in nuclear deformability? One factor appears to be chromatin structure and organization. In differentiated cells, core histones form stable complexes with chromatin; in contrast, the mobility of these nucleosomal proteins is significantly increased in embryonic stem cells (76, 126). Furthermore, expression of A-type lamins is confined mostly to differentiated cells and is absent in embryonic stem cells (22). Given that lamins A and C are the main determinants of nuclear stiffness (37), this disparity in expression of A-type lamins between stem cells and differentiated cells may account for the observed softer nuclei in stem cells. In support of this idea, knockdown of A-type lamins in human epithelial cells results in nuclear deformability that is comparable to that measured in hematopoietic stem cells (77). Further evidence for the importance of lamins in (stem cell) differentiation comes from a recent report that siRNA-mediated knockdown of lamins A and C in mesenchymal stem cells inhibits cells from entering the osteoblast lineage and instead promotes adipogenic differentiation (247).
Changes in Nuclear Shape During Granulopoiesis
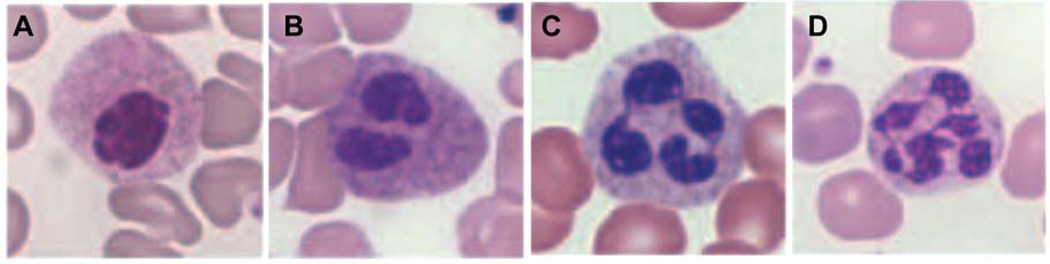
Neutrophils represent a striking example of the effect of changes in nuclear envelope composition on nuclear shape and mechanics. During granulopoiesis, i.e., the differentiation process that results in mature neutrophils, promyelocytes develop increasingly lobulated nuclei (Figure 2) (127, 128). This change in nuclear morphology is correlated with a downregulation of A-type lamins, increased expression of LBR, and additional alterations in nuclear envelope composition such as downregulation of many other stabilizing nuclear envelope proteins (i.e., emerin, B-type lamins, LAP2β) and LINC complex components, which cooperatively lead to decreased nuclear stiffness resulting in more malleable nuclei (105, 127–130). Under these circumstances, microtubules can exert the force necessary to produce nuclear distortions, but the detailed molecular mechanism underlying nuclear lobulation remains incompletely understood. Studies on human and mouse hematopoietic cell lines indicate that expression of LBR is one of the most important factors for nuclear lobulation (129, 131–135). The N-terminal domain of LBR faces the nucleoplasm and interacts with both B-type lamins and chromatin (136–139), as well as with chromatin-associated proteins such as heterochromatin protein 1 (HP1), HA95, and the methyl-CpG-binding protein MeCP2, suggesting that LBR plays a pivotal role in heterochromatin assembly and maintenance (140–142). The C-terminal domain of LBR has a primary sequence highly homologous to a family of sterol reductases (143), enzymes involved in the cholesterol biosynthesis pathway; recent experiments have confirmed that LBR is a functional sterol reductase (144, 145). The molecular mechanism by which LBR influences nuclear morphology is still unclear, and LBR’s bifunctional structure gives rise to different speculations on whether the N-terminal B-type lamin and chromatin-binding domain or the C-terminal sterol reductase domain is responsible for nuclear shape changes (146, 147). Our own unpublished observations indicate that overexpression of human LBR in any tested cell type induces nuclear hyperlobulation (Figure 3).
Figure 2. Lamin B receptor (LBR) influences nuclear morphology in mature neutrophils.
Wright-Giemsa stained blood smears showing the dosage effect of 0 to 3 functional (i.e., wild-type) LBR copies on nuclear segmentation in neutrophils. Samples were derived from (A) an individual homozygous for the splice mutation IVS12-5-10del, (B) an individual heterozygous for the splice mutation IVS12-5-10del, (C) a healthy control, and (D) an individual with a duplication of a segment of chromosome 1 (dup(1)(q32q44)) expressing three functional LBR copies. Nuclear lobulation highly correlates with LBR expression levels. Images were reprinted with kind permission from Sophia Gravemann (246).
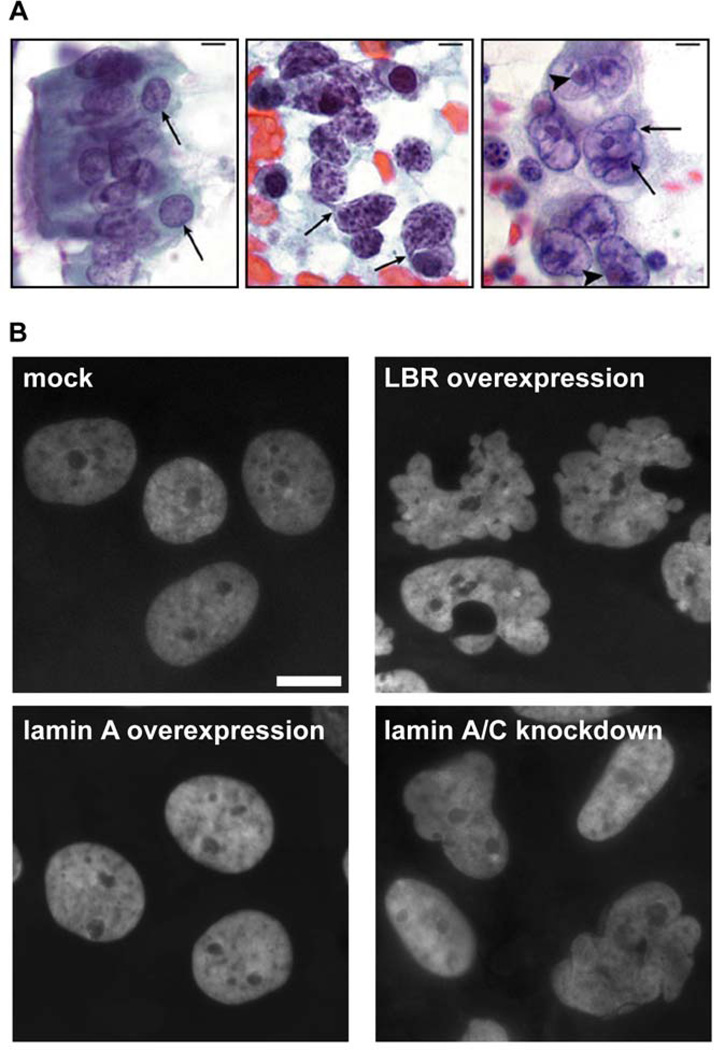
Figure 3. Abnormal nuclear shape in cancer cells.
(A) Histology sections from normal bronchial cells (left), small-cell lung carcinoma (center), and large-cell lung carcinoma (right). Cancer cells show distinct abnormalities in chromatin distribution (center panel; heterochromatin aggregates are visible as dark-violet patches) or nuclear shape (right panel, arrows). (B) Stable overexpression of lamin B receptor (LBR) or knockdown of A-type lamins in human breast epithelial cells (MCF10) results in lobulated and irregularly shaped nuclei that resemble the nuclear abnormalities often found in cancer cells. Nuclei were stained with DAPI. Scale bar: 10µm. Images in panel A reprinted from Zink et al. (222) with permission from Nature Reviews Cancer
What is the purpose of the highly unusual nuclear morphology in neutrophil granulocytes? Most likely, it supports the function of neutrophils as key components in the fast innate immune response against bacterial and fungal infections. Mature peripheral neutrophils make up approximately 60% of all white blood cells. The average life span of the mature, peripheral neutrophil is only 6–12 h. Upon activation by inflammatory stimuli, the circulating neutrophils leave the capillary beds to reach sites of infection (148). This process requires neutrophils to squeeze through tight tissue with spaces only a few micrometers wide, i.e., smaller than the nuclear diameter (127, 149). The lobulated nuclear shape and the downregulation of A-type lamins and other nuclear envelope proteins is thought to facilitate this migration process and allow the cells to rapidly reach infected sites. Evidently, the lobulated nuclear morphology of neutrophil granulocytes represents an evolutionary adaptation of nuclear mechanics to cellular function to optimize (temporally) the immune response against invading pathogens, highlighting the role of nuclear mechanics in physiology and disease.
Dynamic Changes in Nuclear Shape in Neurons
Changes in nuclear shape not only occur during development and cellular differentiation but can also represent adaptation to alterations in intrinsic or extrinsic signaling. As an intriguing example, Wittmann and coworkers reported dramatic and very rapid changes of nuclear morphology in response to neuronal activity (150). In particular, they observed that action potential bursting in hippocampal neurons dramatically increased the number of nuclei with large invaginations and inward-directed folding of the nuclear envelope. These nuclei usually displayed distinct nuclear subcompartments because of numerous nuclear envelope infoldings, a larger surface area, an increased number of nuclear pore complexes, and changes in chromatin structure, as compared with nonactivated, near-spherical nuclei. These findings match previous reports on neurons in the frontal cortex of hamsters that develop increasingly deeper and more frequent nuclear invaginations during development and adulthood (151). The precise role of the substantial and rapid nuclear shape changes in neuronal function remains unclear, but notably these cells represent an additional example for the adaptation of nuclear morphology to the specific functions of a cell.
Altered Nuclear Mechanics in Aging
Several recent studies suggest that abnormal nuclear shape and mechanics may also contribute to the normal aging process. In C. elegans, most nonneuronal cells display progressive age-dependent changes in nuclear structure, including abnormal nuclear shape and loss of peripheral heterochromatin (152). Many of these changes are reminiscent of the nuclear defects observed in HGPS, a segmental premature aging disease caused by mutations in the LMNA gene (discussed in more detail below). Accumulation of progerin, i.e., the mutant lamin A, which results from abnormal splicing of prelamin A and is responsible for HGPS, has recently been detected in blood vessels (153), skin (154, 155), and skin fibroblasts (35) from normally aged individuals, implicating disturbed lamin A processing in normal aging. This idea is further supported by a recent study that found accumulation of farnesylated prelamin A in vascular smooth muscle cells (VSMCs) from normally aged individuals, whereas prelamin A was undetectable in cells from young donors (156). It remains unclear whether the low levels of progerin detected in elderly individuals are sufficient to cause cellular dysfunction and contribute to the aging process. Studies on HGPS patients with atypical mutations and on a mouse model of HGPS suggest that progerin exerts dose-dependent effects (157–159); thus, future experiments should be aimed at determining whether expression of progerin above a critical threshold is necessary to cause functional defects and whether accumulation of incompletely processed prelamin A may cause similar defects as progerin.
CHANGES OF NUCLEAR STRUCTURE AND MECHANICS IN PATHOLOGICAL CONDITIONS AND IMPLICATIONS FOR DISEASE MECHANISMS
Nuclear Mechanics in Laminopathies
Ever since the discovery that mutations in the inner nuclear membrane protein emerin cause EDMD, numerous other nuclear envelope proteins have been linked to a broad spectrum of human diseases (14, 160). A large subset of these nuclear envelopathies comprises the laminopathies, which are caused by mutations in the genes encoding lamins. To date, at least 344 different LMNA mutations have been identified, resulting in 266 protein variants (see online database at http://www.umd.be/LMNA/). In contrast, mutations in the human B-type lamins are rare, and only a complete duplication of the LMNB1 gene and two amino acid substitutions in human lamin B2 have been described: Duplication of LMNB1 is associated with autosomal dominant leukodystrophy (24) and adult-onset leukoencephalopathy (25), whereas LMNB2 mutations result in acquired partial lipodystrophy (161). As discussed above, these findings strongly suggest that B-type lamins are essential for cell viability (see online database at http://www.interfil.org).
LMNA mutations can cause at least 11 different diseases, although it has been suggested that the laminopathies often represent a spectrum of overlapping diseases rather than distinct diseases (14). Laminopathies include (a) premature aging syndromes such as HGPS ; (b) striated muscle diseases such as autosomal dominant EDMD, limb-girdle muscular dystrophy, and dilated cardiomyopathy 1A; (c) lipodystrophy syndromes such as familiar partial lipodystrophy (FPLD) type 2 (also known as Dunnigan type); and (d) a peripheral neuropathy (reviewed in 14). One of the most puzzling questions in the nuclear envelope field is how mutations in a single, nearly ubiquitously expressed gene, LMNA, can cause such a variety of disease phenotypes, with many mutations specifically affecting striated muscle tissue, whereas others have little or no effect on muscles. The broad range of phenotypes suggests that LMNA mutations may interfere with basic cellular mechanisms as diverse as replication, gene expression, proliferation, or stem cell differentiation, and the molecular mechanism(s) underlying the various laminopathies remains a topic of debate and intense research (162, 163). Two hypotheses explain some of the tissue-specific phenotypes. The structural hypothesis proposes that mutations in A-type lamins cause a loss of structural function, mechanically weakening the nucleus and rendering it more fragile. These defects result in cell death in mechanically stressed tissues such as muscle and lead to the progressive disease phenotypes observed in many laminopathies. The gene-regulation hypothesis proposes that mutations in lamins result in perturbed gene regulation, which might be the underlying cause for the development of different disease phenotypes (164). Additional variations of these basic concepts relate to alterations in DNA repair mechanisms, disturbed stem cell differentiation, and disruption of nucleocytoskeletal coupling (92, 165–167). Importantly, these hypotheses are not mutually exclusive. Most likely, a combination of molecular disturbances determines the disease outcome of specific LMNA mutations, which may be further modulated by the genetic background and/or epigenetic factors (168). In the following sections, we focus on the role of disturbed physical properties of the nucleus in laminopathies and discuss how alterations in nuclear stiffness may contribute to the disease phenotypes. For a detailed overview of laminopathies and the diverse cellular defects associated with lamin mutations (e.g., impaired signaling pathways, DNA repair, and differentiation), the reader is referred to some excellent recent reviews (14, 169–171).
Decreased nuclear stiffness and impaired nucleocytoskeletal coupling in muscular laminopathies
EDMD is a rare skeletal and cardiac muscle condition, which is inherited in either an X-linked (i.e., through mutations in the EMD gene encoding emerin located on the X-chromosome) or autosomal manner (i.e., mutations in the LMNA gene on chromosome 1) (172, 173). Symptoms include contractions of tendons, typically starting in the first decade of life, and slow progressive skeletal muscle weakness and wasting at the onset of disease that is later accompanied by a life-threatening cardiac disease with conduction defects and congestive heart failure (174).
Skeletal muscle fibers from EDMD patients often contain fragmented nuclei, lending support to the structural hypothesis (65, 175, 176). Similarly, mouse models expressing human mutations that cause dilated cardiomyopathy or EDMD display disorganized and fragile nuclei, and mechanical load results in increased physical damage (177–179). Further support for the structural hypothesis comes from studies on mice and cells that completely lack A-type lamins. Lmna−/− mice develop severe muscular dystrophy and dilated cardiomyopathy and die at 4–8 weeks of age (23). Importantly, biomechanical studies on cultured MEFs show that A-type lamins, and in particular lamin A, are the primary contributors to nuclear mechanics (37). Accordingly, MEFs from Lmna−/− mice have significantly decreased nuclear and cytoskeletal mechanical stiffness, more fragile nuclei, impaired activation of mechanosensitive genes, and reduced viability under mechanical strain compared with MEFs from wild-type littermates (2, 180). These studies indicate a direct influence of lamins A/C on the physical properties of nuclei and the structural support of cells; furthermore, the finding that mechanotransduction is impaired in Lmna−/− MEFs illustrates how tightly the structural and the gene regulation hypothesis are interrelated. The collective results of cellular and animal models as well as those obtained from laminopathy patients indicate that specific LMNA mutations can increase the cells’ susceptibility to mechanical stress. This structural defect is most likely accompanied by further defects in nucleocytoskeletal coupling (see below), mechanotransduction signaling, tissue regeneration, cell proliferation, and cell differentiation (2, 110, 179, 181–183), resulting in a dual disease mechanism where physically more fragile cells are lost in mechanically stressed tissues and defects in mechanotransduction signaling, gene regulation, and stem cell differentiation, cause insufficient repair and maintenance of the tissue.
The importance of nucleocytoskeletal coupling has been emphasized by observations in human patients and animal disease models. Recently, mutations in the genes encoding nesprins-1 and −2 have been linked to EDMD (184), and disruption of the KASH domain in the nuclear envelope proteins nesprin-1 and −2 cause muscular dystrophy in mice (112). In Lmna−/− mice, synaptic muscle fiber nuclei are mislocalized from the neuromuscular junction (114), and cardiac myocytes have disturbed organization of desmin filaments at the nuclear surface (179). MEFs derived from Lmna−/− animals have impaired organization of the perinuclear actin and vimentin networks (180, 185) and significantly reduced cytoskeletal stiffness (2, 110). A possible explanation may be that loss of lamins A/C results in increased mobility of SUN1/2 and nesprin-2 (19). Interestingly, the microtubule organizing center is often detached from the nucleus in Lmna−/− and emerin-deficient cells (12, 110, 186). These latter findings have led to speculation that emerin, which is responsible for the X-linked form of EDMD, may constitute an active component of the LINC complex, where it may mediate interaction with microtubules and the centrosome by directly interacting with β-tubulin (186). As a whole, these studies indicate substantial and intricate physical interactions between the nucleoskeleton and the cytoskeleton components and the effect mutations in nuclear envelope proteins can have on nucleocytoskeletal coupling.
Nuclear stiffening in Hutchinson-Gilford progeria syndrome
Although very rare (affecting 1 in 4 to 8 million newborns), HGPS, is one of the most dramatic laminopathies. Children with HGPS appear normal at birth but fail to thrive shortly thereafter and die in their early teens from myocardial infarctions or stroke (187). Clinical symptoms include alopecia (i.e., hair loss), beaked nose, sclerodermatous skin, dwarfism, lipodystrophy, osteoporosis, and progressive arteriosclerotic disease that is ultimately responsible for more than 90% of all deaths (187–189). HGPS is typically caused by a de novo mutation, c.1824C>T (p.G608G), in the LMNA gene (190, 191). The mutation activates a cryptic splice site, resulting in a truncated form of prelamin A, referred to as progerin. Progerin lacks 50 amino acids near its C terminus and remains-unlike mature lamin A-permanently farnesylated, and therefore stays tightly associated with the inner nuclear membrane. Importantly, the G608G mutation is located in an exon specific to lamin A, so that lamin C is not affected by this mutation. Several recent studies on skin fibroblasts from HGPS patients have revealed important insights into the morphologic, structural, and mechanical alterations of the nucleus that result from progerin expression and how these alterations may contribute to the disease mechanism.
Fibroblasts from HGPS patients are characterized by specific changes in nuclear shape and organization, including lobulation of the nuclear envelope, thickening of the nuclear lamina, and loss of peripheral heterochromatin (192). These structural defects accumulate with increasing passage number as HGPS cells age in cell culture (192); concomitantly, HGPS fibroblasts develop progressively stiffer nuclei with increasing passage number, possibly owing to accumulation of progerin at the nuclear envelope or through secondary changes in nuclear organization (193). Interestingly, a recent study found that cells from a mouse model of HGPS have impaired nucleocytoskeletal coupling, similar to the defects reported in Lmna−/− mice (12), providing an additional aspect of impaired nuclear mechanics in HGPS.
Because progerin remains permanently farnesylated, farnesyl-transferase-inhibitors (FTIs) have been proposed as therapeutic agents for HGPS, as they might block accumulation of progerin at the nuclear envelope. Treatment of cells from HGPS patients and mouse models of the disease reduces the localization of progerin to the inner nuclear membrane and improves the nuclear abnormalities induced by the progerin expression (158, 194–197), indicating that part of the disease mechanism results from abnormal membrane association of the mutant protein. However, completely eliminating progerin farnesylation in a mouse model of HGPS does not completely eliminate the disease phenotype, although it significantly extends the life of the animals and ameliorates several disease aspects (159).
Despite recent advances, the relevance of altered nuclear structure and increased nuclear stiffness to the disease mechanism of HGPS remains unclear. Cells from HGPS patients have an increased sensitivity to mechanical strain, resulting in increased cell death when subjected to repetitive mechanical strain (193). We recently observed similar results in VSMCs from a mouse model of HGPS and in VSMCs expressing human progerin (J. Lammerding, unpublished data). Because large blood vessels are continuously subjected to repetitive vessel strain, the increased mechanical sensitivity of HGPS cells could provide a potential mechanism for the progressive loss of VSMCs in the medial layer of major arteries and their replacement by fibrous material observed in HGPS patients (198, 199) and recapitulated in two mouse models of HGPS (200, 201). So far, the molecular mechanism responsible for the increased sensitivity to mechanical strain remains elusive. In experiments on human HGPS fibroblasts, cells underwent increased cell death in response to mechanical strain even at early passages when nuclear stiffness was still normal, and treatment of later passage cells with FTIs restored nuclear stiffness but did not improve the mechanical sensitivity (193). These findings suggest that changes in nuclear stiffness are not directly responsible for the increased mechanical sensitivity; instead, disturbed extracellular matrix synthesis could render HGPS cells more sensitive (153, 202). This idea is supported further by the fact that in contrast to patients with EDMD, patients with HGPS have normal muscle function.
Correlation between abnormal nuclear mechanics and various laminopathies
Despite extensive research, the question remains why some LMNA mutations result in the progressive loss of muscle cells and an impaired ability to repair muscular damage, whereas other, often similar, mutations have no effect at all on muscle tissue and involve, for example, only adipose tissue. One striking example for the diverging phenotypes caused by similar mutations is amino acid T528 of the human lamin A/C proteins: Mutating this position to lysine (T528L) causes EDMD, whereas a mutation to methionine (T528M) results in minor symptoms of FPLD without skeletal muscle involvement (203, 204). In addition, a compound heterozygous missense mutation involving T528M and M540T causes atypical HGPS (205).
To gain better insight into genotype-phenotype correlations of laminopathies, several studies have compared the effect of different lamin A mutations on nuclear structure and mechanics. One report demonstrated a link between protein mobility as analyzed by fluorescence recovery after photobleaching experiments in human cells expressing GFP-tagged lamin A variants: Mutations causing EDMD displayed increased protein movement, whereas an FPLD-associated lamin A mutant was indistinguishable from wild-type lamin A (206). Similarly, in another study, a subset of lamin A mutations causing EDMD displayed dramatically abnormal intranuclear localization when expressed in C2C12 cells, whereas mutations causing FPLD did not (207). Despite these reports, consistent evidence for a correlation between altered nuclear mechanics and striated muscle diseases is still lacking; for example, a study of nuclear shape and organization in skin fibroblasts from a panel of human laminopathy patients found no correlation between nuclear shape abnormalities and disease (208).
The analysis of mutation-specific effects is further complicated because mutations that cause striated muscle diseases are distributed throughout the entire LMNA gene, although the majority affects the central rod domain (173, 209, 210). In contrast, mutations found in FPLD patients are all found in the C-terminal domain of the protein that displays a conformation similar to that of an immunoglobulin fold (211–213), and the mutations localize to more external regions of the lamin molecule. These findings have led to speculations that mutations associated with FPLD do not significantly perturb the overall structure or assembly of lamins A/C, but instead result in disturbed interaction of the lamin Ig-fold with specific proteins, such as SREBP-1. In contrast, mutations that involve muscular phenotypes are thought to result in major structural perturbations of lamins or improper higher-order lamin assembly that could destabilize the nucleus (210, 214). Although this appears to be the case for at least some of the mutations involving striated muscle, it does not fit for all the reported mutations, suggesting additional molecular mechanisms (210), such as impaired nucleocytoskeletal coupling or impaired muscle regeneration. In HGPS, the majority of cases is caused by the G608G mutations near the lamin A/C-terminus, but atypical HGPS can also result from lamin A/C rod domain mutations (S143F, E145K), homozygous recessive mutations (K542N), or compound mutations (T528M and M540T), and in some cases without affecting the farnesylation of the mutant protein (205, 215–217). As mentioned above, there are many examples where genetic background and/or epigenetic factors modulate disease outcome, and intrafamilial and interfamilial clinical heterogeneity are clearly a hallmark of laminopathies (218).
In summary, although there is ample evidence that increased sensitivity to mechanical strain contributes to at least some laminopathies, either by direct damage to more fragile nuclei or by modulating mechanosensitive signaling pathways, more work is needed to fully elucidate the complex interplay between altered nuclear mechanics and their contribution to laminopathies.
Altered Nuclear Structure in Pelger-Huët Anomaly
Another nuclear envelope protein that can dramatically affect nuclear shape is the LBR, a multifunctional inner nuclear membrane protein (135). As discussed in the sections above, induction of LBR expression is important for the development of hyperlobulated nuclei during granulopoiesis. This role of LBRs in nuclear morphology first became evident when Hoffmann and coworkers linked mutations in the LBR gene to a benign autosomal dominant hematologic trait termed the Pelger-Huët anomaly (134). In heterozygous carriers of the associated LBR mutations, peripheral blood smears reveal neutrophils with bilobulated nuclei and coarse chromatin, instead of the highly lobulated nuclear morphology seen in normal neutrophils (Figure 2) (128, 219). Besides hypolobulated granulocytes, no other clinical symptoms occur, and individuals are phenotypically healthy. Rare homozygous carriers, however, have neutrophils with completely ovid nuclei, and these individuals display varying degrees of developmental delay, epilepsy, and skeletal abnormalities (134).
As discussed above, the normally hyperlobulated nuclear shape is thought to facilitate this migration process of neutrophils and allow the cells to rapidly reach infected sites. In accordance with this view, neutrophils from individuals with the Pelger-Huët anomaly show impaired ability to traverse filters with small pore size (5 µm) during chemotaxis (149). In addition, in vitro differentiated neutrophils from LBR knockout mice revealed that, in addition to lack of nuclear lobulation, loss of LBR results in significant retardation of transmembrane migration (131). Thus, the incompletely segmented neutrophil nuclei may be less malleable and less deformable than are those of healthy individuals, which results in mechanical hindrance during migration through tight tissue. Nonetheless, individuals with the Pelger-Huët anomaly do not display significantly compromised immune responses (220, 221). In addition, other granulocytic cells, the eosinophils and basophils, have to traverse tissue and display only bi- or trilobed nuclei. However, these cells fulfill different functions in immune defense, and the pressure for rapid defense against the invading pathogens is not as demanding as on neutrophil granulocytes.
Nuclear Structure and Mechanics in Cancer Cells
Abnormal nuclear shape and structure remains one of the gold standards for histological diagnosis and characterization of cancer, for example, in the Pap test for detection of potentially precancerous or cancerous cells in the cervix (222). Aberrations in nuclear structure and morphology associated with cancer include irregular nuclear shape, nucleolar alterations, loss of nuclear domains, and changes in chromatin organization. In histological preparations, nuclei from cancer cells often appear lobulated, folded, more malleable, and more fragile compared with nuclei from noncancer cells (222, 223). The molecular changes underlying these characteristic changes in nuclear morphology, such as altered nuclear envelope composition or changes in chromatin structure, are incompletely understood, and the relationship between morphological features of cancer cells and their role in cancer progression remains mostly descriptive.
Over the past few years, increasing evidence has accumulated to suggest that changes in nuclear structure and protein composition are directly linked to the malignant transformation and metastatic potential of cells. For example, nuclear matrix proteins, such as NMP22 (NUMA) or PC1 (B23) have the potential to serve as cancer biomarkers in prostate, bladder, and breast cancer (224–226), and lamins are differentially expressed in many cancers. In prostate cancer, upregulation of B-type lamins strongly correlates with tumor differentiation (227), and in hepatocellular carcinoma, the expression level of lamin B1 correlates positively with tumor stages, tumor sizes, and number of nodules (228, 229).Conversely, downregulation of lamin B is observed in gastrointestinal and lung cancer (230, 231). A-type lamins are upregulated in skin or ovarian cancers (232, 233) but are reduced in leukemias and lymphomas (234, 235). Notably, A-type lamin expression can vary within some cancer subtypes; for example, colorectal and basal cell carcinomas can be positive, reduced, or negative for lamin A/C expression (236). In gastric carcinoma, the presence of cells with reduced expression of A-type lamins correlates with a poorer prognosis (237), whereas patients with colorectal cancer tumors have significantly poorer prognosis when lamins A/C are expressed (238). These studies illustrate that lamins and other nuclear proteins may serve as valuable prognostic biomarkers; however, the particular modulation, i.e., whether expression of specific nuclear proteins increase or decrease in cancer cells, is strongly dependent on the given cancer type, tumor subtype, and stage, which complicates the interpretation of the above findings. Furthermore, it remains unclear whether the disturbed nuclear structure is merely a consequence of other molecular events occurring during tumor initiation and progression or whether it directly contributes to the disease mechanism. How could disturbed nuclear structure contribute to cancer progression? That so many cancers display distinct nuclear morphologic characteristics indicates that changes in nuclear structure and organization-including possible changes in the physical properties of the nucleus-may confer a selective advantage to these cells during cancer progression. From a biophysical point of view, changes in nuclear stiffness and plasticity could enhance the ability of metastatic cancer cells to invade neighboring tissues, enter and exit the blood stream or lymphatic system, and spread to other parts of the body. For example, during invasion, breast cancer cells degrade the basement membrane and form invasive protrusion (invadopodia), resulting in microscopic openings in the basement membrane only a few micrometers in diameter through which the cells squeeze into the underlying tissue, and the deformation of the nucleus is a rate-limiting factor (239). Similarly, within the tissue and during transendothelial migration (intravasation), migrating cells have to pass though narrow spaces in the extracellular matrix and blood or lymphatic vessels, where deformations of the normally stiff nucleus can be one of the rate-limiting steps during amoeboid migration (240, 241). During these processes, more deformable nuclei might facilitate passage through narrow capillaries and promote wider distribution. In this setting, downregulation of A-type lamins in cancer cells would parallel the physiological modulation in nuclear envelope composition during granulopoiesis, whereas downregulation of lamins and nesprins and upregulation of LBR is thought to enhance rapid transendothelial migration and passage through narrow tissue spaces (127).
Although this biophysical approach provides a strong argument for a selective advantage of reduced expression of A-type lamins and other modulations in nuclear envelope composition that could enhance nuclear deformability, it is insufficient to explain fully the variable changes in lamin expression reported across the different cancers. In this context, it is important to consider the various functions of A-type lamins and how they could contribute to tumorigenesis and cancer progression. As illustrated above, lamins are important structural components supporting nuclear stiffness and maintaining the LINC complex; nonetheless, they are also implicated in various nuclear functions such as DNA replication, repair and genome stability, transcriptional regulation, and gene expression (171). Moreover, they play a dynamic role in several cell processes, including cell division, differentiation, migration, aging, and apoptosis (242, 243). Lamins A and C are expressed in differentiated cells (181), and may play a role in controlling stem cell self-renewal and differentiation (167). In general, the poorer the differentiation state of a given tumor type, the worse the prognosis (236). Thus, downregulation of A-type lamins and the consequences on chromatin distribution and gene expression may contribute to adjusting a more stem cell-like character to tumor cells and to regaining the characteristic functional plasticity. Noteworthy are also the multiple interactions of A-type lamins with major cancer gene pathways such as tumor suppressor pathways that control cell proliferation, including Rb, p53, and SMADs, and with pathways that trigger apoptosis or senescence (7). In addition, A-type lamins interact (directly or indirectly) with nuclear components involved in epigenetic modifications, such as histone deacetylases , Rb, and HP1. Altered expression of A-type lamins may thus have a variety of effects on cells, including procancerous and anticancerous, and depending on the primary tumor cell type, malignant transformation, as well as differentiation stage, overexpression, or silencing of A-type lamins, may promote tumor growth, invasion, and metastasis (244, 245).
CONCLUSIONS
Recent experiments identify the nucleus as a critical cellular element that is fully integrated with the surrounding cytoskeleton. Consequently, changes in nuclear structure and composition can have dramatic consequences on cellular polarization, cytoskeletal organization, and function. In return, forces from the extracellular matrix and the cytoskeleton are transmitted across the nuclear envelope to the nuclear interior, where they can result in significant nuclear deformations. These nuclear deformations factor into several important cellular processes. During cell migration in three-dimensional environments, the mechanical properties of the large and, compared with the surrounding cytoskeleton, stiff nucleus can present a rate-limiting step in the ability of cells to squeeze through narrow pores. At the same time, the nucleus must be able to withstand significant forces in mechanically active tissues such as muscle, and mutations that impair the structural stability of the nucleus can result in muscular dystrophies and cardiomyopathies. Despite the impressive recent advances, several open questions remain. The functions of many nuclear envelope proteins are incompletely defined, and the structural organization within the nucleus, or the nuclear lamin network, continue to puzzle researchers. One challenge is the multifaceted function of many nuclear proteins; for example, lamins act as intranuclear structural elements but also interact with transcriptional regulators. Targeted engineering of select domains or analysis of specific mutations will be necessary to separate these functions. Ultimately, a better understanding of the molecular components governing nuclear shape, deformability, and linkage to the cytoskeleton, as well as their interplay with diverse cellular signaling pathways, may provide further insight into the role of nuclear mechanics under physiological conditions and its contribution to human disease and stimulate new therapeutic approaches.
SUMMARY POINTS.
The nucleus is physically coupled to the surrounding cytoskeleton, and, via cell-cell junctions and transmembrane receptors, to the cellular microenvironment. Thus, mechanical forces are transmitted throughout the cytoskeletal networks and to the nucleus via LINC complexes found at the nuclear envelope, consisting of nesprins, SUN proteins, and other nuclear envelope proteins.
The elastic nuclear lamina, composed of a fibrous lamin network, and viscoelastic components of the nuclear interior, including chromatin and other intranuclear structures, predominantly determine the mechanical properties of the nucleus.
Nuclear shape alterations, typically associated with modulations in nuclear envelope composition, frequently occur as part of the normal development and differentiation process and may contribute to aging; likewise, dynamic changes in nuclear shape can also result from intrinsic or extrinsic cell signaling.
The nuclear envelopathies and, more specifically, laminopathies often involve dramatic changes in nuclear shape and mechanics, including altered nuclear stiffness and impaired nucleocytoskeletal coupling, which may further impact mechanotransduction and gene regulation processes.
The size and stiffness of the nucleus can impose a rate-limiting step in cells migrating through narrow tissue spaces. Changes in nuclear envelope composition that result in more deformable nuclei can be found in physiological processes (granulopoiesis), but may also contribute to cancer progression, as cancer cells often have altered nuclear shape and lamin expression.
FUTURE ISSUES.
What is the function of the many nuclear membrane proteins that remain incompletely characterized? To what extent do they contribute to nuclear organization, nuclear stiffness, or nucleocytoskeletal coupling.
What is the structural organization of nuclear lamins, both at the nuclear envelope and the nuclear lamina? And what are the functional effects of specific lamin mutations that result in different diseases?
How can nuclear structure and mechanics modulate cellular signaling and function? Can lamins and other nuclear proteins act as signaling scaffolds or sequester transcriptional regulators? And can physical deformations of nuclear elements, for example, opening of chromatin under mechanical stress, contribute to mechanotransduction signaling?
What is the role of abnormal nuclear shape and structure in cancer? Cancer cells often display characteristic nuclear abnormalities, but it remains unclear whether such changes can functionally contribute to cancer initiation and progression.
ACKNOWLEDGMENTS
We apologize in advance to the investigators whose research could not be appropriately cited owing to space limitations. The authors thank Maria Lucia Lombardi for critical reading and discussion. This work was supported by the National Institutes of Health grants HL082792 and NS059348 and the Cardiovascular Leadership Group Award to J.L.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Monika Zwerger, Email: monika.zwerger@gmail.com.
Chin Yee Ho, Email: cyho@partners.org.
Jan Lammerding, Email: jlammerding@rics.bwh.harvard.edu.
LITERATURE CITED
- 1.Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004;117:4779–4786. doi: 10.1242/jcs.01357. [DOI] [PubMed] [Google Scholar]
- 2.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Investig. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naetar N, Korbei B, Kozlov S, Kerenyi MA, Dorner D, et al. Loss of nucleoplasmic LAP2α-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat. Cell Biol. 2008;10:1341–1348. doi: 10.1038/ncb1793. [DOI] [PubMed] [Google Scholar]
- 4.Zastrow MS, Vlcek S, Wilson KL. Proteins that bind A-type lamins: integrating isolated clues. J. Cell Sci. 2004;117:979–987. doi: 10.1242/jcs.01102. [DOI] [PubMed] [Google Scholar]
- 5.González JM, Navarro-Puche A, Casar B, Crespo P, Andrés V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J. Cell Biol. 2008;183:653–666. doi: 10.1083/jcb.200805049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, et al. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc. Natl. Acad. Sci. USA. 2004;101:9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson KL, Foisner R. Lamin-binding proteins. Cold Spring Harb. Perspect. Biol. 2010;2:a000554. doi: 10.1101/cshperspect.a000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mekhail K, Moazed D. The nuclear envelope in genome organization, expression and stability. Nat. Rev. Mol. Cell Biol. 2010;11:317–328. doi: 10.1038/nrm2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature. 2007;445:379–781. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- 10.Towbin BD, Meister P, Gasser SM. The nuclear envelope-a scaffold for silencing? Curr. Opin. Genet. Dev. 2009;19:180–186. doi: 10.1016/j.gde.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 2010;99:115–123. doi: 10.1016/j.bpj.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, et al. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 2008;95:5462–5475. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 14.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J. Clin. Investig. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowat AC, Lammerding J, Herrmann H, Aebi U. Towards an integrated understanding of the structure and mechanics of the cell nucleus. BioEssays. 2008;30:226–236. doi: 10.1002/bies.20720. [DOI] [PubMed] [Google Scholar]
- 16.Moroianu J. Nuclear import and export pathways. J. Cell. Biochem. 1999;32–33(Suppl.):76–83. doi: 10.1002/(sici)1097-4644(1999)75:32+<76::aid-jcb10>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Schirmer EC, Florens L, Guan T, Yates JR3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 18.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev. Cell. 2009;17:626–638. doi: 10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J. Cell Sci. 2009;122:4099–4108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, et al. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J. Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- 22.Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat. Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 25.Brussino A, Vaula G, Cagnoli C, Mauro A, Pradotto L, et al. A novel family with lamin B1 duplication associated with adult-onset leucoencephalopathy. J. Neurol. Neurosurg. Psychiatry. 2009;80:237–240. doi: 10.1136/jnnp.2008.147330. [DOI] [PubMed] [Google Scholar]
- 26.Vergnes LPM, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl. Acad. Sci. USA. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, et al. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. USA. 2010;107:5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl. Acad. Sci. USA. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 30.Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broers JL, Bronnenberg NM, Kuijpers HJ, Schutte B, Hutchison CJ, Ramaekers FC. Partial cleavage of A-type lamins concurs with their total disintegration from the nuclear lamina during apoptosis. Eur. J. Cell Biol. 2002;81:677–691. doi: 10.1078/0171-9335-00282. [DOI] [PubMed] [Google Scholar]
- 32.Moir RD, Yoon M, Khuon S, Goldman RD. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 2000;151:1155–1168. doi: 10.1083/jcb.151.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J. Cell Sci. 2008;121:215–225. doi: 10.1242/jcs.022020. [DOI] [PubMed] [Google Scholar]
- 35.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 38.Broers JL, Kuijpers HJ, Ostlund C, Worman HJ, Endert J, Ramaekers FC. Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization. Exp. Cell Res. 2005;304:582–592. doi: 10.1016/j.yexcr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum. Mol. Genet. 2002;11:769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- 40.Dreuillet C, Tillit J, Kress M, Ernoult-Lange M. In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res. 2002;30:4634–4642. doi: 10.1093/nar/gkf587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozaki T, Saijo M, Murakami K, Enomoto H, Taya Y, Sakiyama S. Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene. 1994;9:2649–2653. [PubMed] [Google Scholar]
- 42.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raska I, Shaw PJ, Cmarko D. Structure and function of the nucleolus in the spotlight. Curr. Opin. Cell Biol. 2006;18:325–334. doi: 10.1016/j.ceb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011;3:a000646. doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deryusheva S, Gall JG. Dynamics of coilin in Cajal bodies of the Xenopus germinal vesicle. Proc. Natl. Acad. Sci. USA. 2004;101:4810–4814. doi: 10.1073/pnas.0401106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimber A, Nguyen QD, Gespach C. Nuclear bodies and compartments: functional roles and cellular signalling in health and disease. Cell. Signal. 2004;16:1085–1104. doi: 10.1016/j.cellsig.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Platani M, Goldberg I, Swedlow JR, Lamond AI. In vivo analysis of Cajal body movement, separation, and joining in live human cells. J. Cell Biol. 2000;151:1561–1574. doi: 10.1083/jcb.151.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Shiels C, Sasieni P, Wu PJ, Islam SA, et al. Promyelocytic leukemia nuclear bodies associate with transcriptionally active genomic regions. J. Cell Biol. 2004;164:515–526. doi: 10.1083/jcb.200305142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bourdeau V, Baudry D, Ferbeyre G. PML links aberrant cytokine signaling and oncogenic stress to cellular senescence. Front. Biosci. 2009;14:475–485. doi: 10.2741/3256. [DOI] [PubMed] [Google Scholar]
- 50.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 51.Gieni RS, Hendzel MJ. Actin dynamics and functions in the interphase nucleus: moving toward an understanding of nuclear polymeric actin. Biochem. Cell Biol. 2009;87:283–306. doi: 10.1139/O08-133. [DOI] [PubMed] [Google Scholar]
- 52.Hofmann WA, Johnson T, Klapczynski M, Fan JL, de Lanerolle P. From transcription to transport: emerging roles for nuclear myosin I. Biochem. Cell Biol. 2006;84:418–426. doi: 10.1139/o06-069. [DOI] [PubMed] [Google Scholar]
- 53.Young KG, Kothary R. Spectrin repeat proteins in the nucleus. BioEssays. 2005;27:144–152. doi: 10.1002/bies.20177. [DOI] [PubMed] [Google Scholar]
- 54.Pederson T, Aebi U. Actin in the nucleus: What form and what for? J. Struct. Biol. 2002;140:3–9. doi: 10.1016/s1047-8477(02)00528-2. [DOI] [PubMed] [Google Scholar]
- 55.Zastrow MS, Flaherty DB, Benian GM, Wilson KL. Nuclear titin interacts with A- and B-type lamins in vitro and in vivo. J. Cell Sci. 2006;119:239–249. doi: 10.1242/jcs.02728. [DOI] [PubMed] [Google Scholar]
- 56.Bettinger BT, Gilbert DM, Amberg DC. Actin up in the nucleus. Nat. Rev. Mol. Cell Biol. 2004;5:410–415. doi: 10.1038/nrm1370. [DOI] [PubMed] [Google Scholar]
- 57.Jockusch BM, Schoenenberger CA, Stetefeld J, Aebi U. Tracking down the different forms of nuclear actin. Trends Cell Biol. 2006;16:391–396. doi: 10.1016/j.tcb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Pederson T, Aebi U. Nuclear actin extends, with no contraction in sight. Mol. Biol. Cell. 2005;16:5055–5060. doi: 10.1091/mbc.E05-07-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoenenberger CA, Buchmeier S, Boerries M, Sütterlin R, Aebi U, Jockusch BM. Conformation-specific antibodies reveal distinct actin structures in the nucleus and the cytoplasm. J. Struct. Biol. 2005;152:157–168. doi: 10.1016/j.jsb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Hofmann WA, Johnson T, Klapczynski M, Fan JL, Lanerolle P. From transcription to transport: emerging roles for nuclear myosin I. Biochem. Cell Biol. 2006;84:418–426. doi: 10.1139/o06-069. [DOI] [PubMed] [Google Scholar]
- 61.Percipalle P, Visa N. Molecular functions of nuclear actin in transcription. J. Cell Biol. 2006;172:967–971. doi: 10.1083/jcb.200512083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moir RD, Spann TP, Herrmann H, Goldman RD. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J. Cell Biol. 2000;149:1179–1192. doi: 10.1083/jcb.149.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manju K, Muralikrishna B, Parnaik VK. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J. Cell Sci. 2006;119:2704–2714. doi: 10.1242/jcs.03009. [DOI] [PubMed] [Google Scholar]
- 64.Gotic I, Leschnik M, Kolm U, Markovic M, Haubner BJ, et al. Lamina-associated polypeptide 2α loss impairs heart function and stress response in mice. Circ. Res. 2010;106:346–353. doi: 10.1161/CIRCRESAHA.109.205724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fidzianska A, Hausmanowa-Petrusewicz I. Architectural abnormalities in muscle nuclei. Ultrastructural differences between X-linked and autosomal dominant forms of EDMD. J. Neurol. Sci. 2003;210:47–51. doi: 10.1016/s0022-510x(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 66.Kumaran RI, Muralikrishna B, Parnaik VK. Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription. J. Cell Biol. 2002;159:783–793. doi: 10.1083/jcb.200204149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spann TP, Goldman AE, Wang C, Huang S, Goldman RD. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J. Cell Biol. 2002;156:603–608. doi: 10.1083/jcb.200112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J. Biomech. 2002;35:177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 69.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem. Biophys. Res. Commun. 2000;269:781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 70.Kha HN, Chen BK, Clark GM, Jones R. Stiffness properties for nucleus standard straight and contour electrode arrays. Med. Eng. Phys. 2004;26:677–685. doi: 10.1016/j.medengphy.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Vaziri A, Mofrad MR. Mechanics and deformation of the nucleus in micropipette aspiration experiment. J. Biomech. 2007;40:2053–2062. doi: 10.1016/j.jbiomech.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 72.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys. J. 2005;89:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schape J, Prausse S, Radmacher M, Stick R. Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy. Biophys. J. 2009;96:4319–4325. doi: 10.1016/j.bpj.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newport JW, Wilson KL, Dunphy WG. A lamin-independent pathway for nuclear envelope assembly. J. Cell Biol. 1990;111:2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann. N. Y. Acad. Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
- 76.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rowat AC, Lammerding J, Ipsen JH. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys. J. 2006;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazumder A, Shivashankar GV. Gold-nanoparticle-assisted laser perturbation of chromatin assembly reveals unusual aspects of nuclear architecture within living cells. Biophys. J. 2007;93:2209–2216. doi: 10.1529/biophysj.106.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melling M, Hochmeister S, Blumer R, Schilcher K, Mostler S, et al. Atomic force microscopy imaging of the human trigeminal ganglion. NeuroImage. 2001;14:1348–1352. doi: 10.1006/nimg.2001.0924. [DOI] [PubMed] [Google Scholar]
- 81.Goldman AE, Moir RD, Montag-Lowy M, Stewart M, Goldman RD. Pathway of incorporation of microinjected lamin A into the nuclear envelope. J. Cell Biol. 1992;119:725–735. doi: 10.1083/jcb.119.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, et al. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc. Natl. Acad. Sci. USA. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, et al. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fridolfsson HN, Starr DA. Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. J. Cell Biol. 2010;191:115–128. doi: 10.1083/jcb.201004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, et al. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp. Cell Res. 2004;295:330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J. Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- 90.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, et al. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Méjat A, Misteli T. LINC complexes in health and disease. Nucleus. 2010;1:40–52. doi: 10.4161/nucl.1.1.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Razafsky D, Hodzic D. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J. Cell Biol. 2009;186:461–472. doi: 10.1083/jcb.200906068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 2008;314:1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 96.Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev. Cell. 2009;17:587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, et al. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J. Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- 98.Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, et al. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 99.Fridolfsson HN, Ly N, Meyerzon M, Starr DA. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev. Biol. 2010;338:237–250. doi: 10.1016/j.ydbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shao XTH, Lee JP, Oko R, Van Der Hoorn FA. Spag4, a novel sperm protein, binds outer dense fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev. Biol. 1999;211:109–123. doi: 10.1006/dbio.1999.9297. [DOI] [PubMed] [Google Scholar]
- 101.Starr DA, Fischer JA. KASH ‘n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. BioEssays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- 102.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J. Biol. Chem. 2004;279:25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q, Du X, Cai Z, Greene MI. Characterization of the structures involved in localization of the SUN proteins to the nuclear envelope and the centrosome. DNA Cell Biol. 2006;25:554–562. doi: 10.1089/dna.2006.25.554. [DOI] [PubMed] [Google Scholar]
- 104.Lei K, Zhang X, Ding X, Guo X, Chen M, et al. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl. Acad. Sci. USA. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olins AL, Hoang TV, Zwerger M, Herrmann H, Zentgraf H, et al. The LINC-less granulocyte nucleus. Eur. J. Cell Biol. 2009;88:203–214. doi: 10.1016/j.ejcb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, et al. Functional association of Sun1 with nuclear pore complexes. J. Cell Biol. 2007;178:785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, et al. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J. Biol. Chem. 2010;285:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Deleted in proof. [Google Scholar]
- 109.Kandert S, Luke Y, Kleinhenz T, Neumann S, Lu W, et al. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum. Mol. Genet. 2007;16:2944–2959. doi: 10.1093/hmg/ddm255. [DOI] [PubMed] [Google Scholar]
- 110.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int. J. Biochem. Cell Biol. 2010;42:1717–1728. doi: 10.1016/j.biocel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum. Mol. Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lei K, Zhang X, Ding X, Guo X, Chen M, et al. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl. Acad. Sci. USA. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Méjat A, Decostre V, Li J, Renou L, Kesari A, et al. Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J. Cell Biol. 2009;184:31–44. doi: 10.1083/jcb.200811035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vander Heyden AB, Naismith TV, Snapp EL, Hodzic D, Hanson PI. LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation. Mol. Biol. Cell. 2009;20:2661–2672. doi: 10.1091/mbc.E09-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caille N, Tardy Y, Meister JJ. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann. Biomed. Eng. 1998;26:409–416. doi: 10.1114/1.132. [DOI] [PubMed] [Google Scholar]
- 117.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 118.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat. Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 119.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp. Cell Res. 2007;313:3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 123.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, et al. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 125.Bibikova M, Laurent LC, Ren B, Loring JF, Fan JB. Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell. 2008;2:123–134. doi: 10.1016/j.stem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 126.Bhattacharya D, Talwar S, Mazumder A, Shivashankar GV. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys. J. 2009;96:3832–3839. doi: 10.1016/j.bpj.2008.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olins AL, Zwerger M, Herrmann H, Zentgraf H, Simon AJ, et al. The human granulocyte nucleus: unusual nuclear envelope and heterochromatin composition. Eur. J. Cell Biol. 2008;87:279–290. doi: 10.1016/j.ejcb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoffmann K, Sperling K, Olins AL, Olins DE. The granulocyte nucleus and lamin B receptor: avoiding the ovoid. Chromosoma. 2007;116:227–235. doi: 10.1007/s00412-007-0094-8. [DOI] [PubMed] [Google Scholar]
- 129.Olins AL, Herrmann H, Lichter P, Kratzmeier M, Doenecke D, Olins DE. Nuclear envelope and chromatin compositional differences comparing undifferentiated and retinoic acid- and phorbol ester-treated HL-60 cells. Exp. Cell Res. 2001;268:115–127. doi: 10.1006/excr.2001.5269. [DOI] [PubMed] [Google Scholar]
- 130.Yabuki M, Miyake T, Doi Y, Fujiwara T, Hamazaki K, et al. Role of nuclear lamins in nuclear segmentation of human neutrophils. Physiol. Chem. Phys. Med. NMR. 1999;31:77–84. [PubMed] [Google Scholar]
- 131.Gaines P, Tien CW, Olins AL, Olins DE, Shultz LD, et al. Mouse neutrophils lacking lamin B-receptor expression exhibit aberrant development and lack critical functional responses. Exp. Hematol. 2008;36:965–976. doi: 10.1016/j.exphem.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Olins AL, Olins DE. Cytoskeletal influences on nuclear shape in granulocytic HL-60 cells. BMC Cell Biol. 2004;5:30. doi: 10.1186/1471-2121-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zwerger M, Herrmann H, Gaines P, Olins AL, Olins DE. Granulocytic nuclear differentiation of lamin B receptor-deficient mouse EPRO cells. Exp. Hematol. 2008;36:977–987. doi: 10.1016/j.exphem.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoffmann K, Dreger CK, Olins AL, Olins DE, Shultz LD, et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huet anomaly) Nat. Genet. 2002;31:410–414. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- 135.Olins AL, Rhodes G, Welch DB, Zwerger M, Olins DE. Lamin B receptor: multi-tasking at the nuclear envelope. Nucleus. 2010;1:53–70. doi: 10.4161/nucl.1.1.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duband-Goulet I, Courvalin JC. Inner nuclear membrane protein LBR preferentially interacts with DNA secondary structures and nucleosomal linker. Biochemistry. 2000;39:6483–6488. doi: 10.1021/bi992908b. [DOI] [PubMed] [Google Scholar]
- 137.Polioudaki H, Kourmouli N, Drosou V, Bakou A, Theodoropoulos PA, et al. Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. EMBO Rep. 2001;2:920–925. doi: 10.1093/embo-reports/kve199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pyrpasopoulou A, Meier J, Maison C, Simos G, Georgatos SD. The lamin B receptor (LBR) provides essential chromatin docking sites at the nuclear envelope. EMBO J. 1996;15:7108–7119. [PMC free article] [PubMed] [Google Scholar]
- 139.Worman HJ, Yuan J, Blobel G, Georgatos SD. A lamin B receptor in the nuclear envelope. Proc. Natl. Acad. Sci. USA. 1988;85:8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guarda A, Bolognese F, Bonapace IM, Badaracco G. Interaction between the inner nuclear membrane lamin B receptor and the heterochromatic methyl binding protein, MeCP2. Exp. Cell Res. 2009;315(11):1895–1903. doi: 10.1016/j.yexcr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 141.Martins SB, Eide T, Steen RL, Jahnsen T, Skalhegg BS, Collas P. HA95 is a protein of the chromatin and nuclear matrix regulating nuclear envelope dynamics. J. Cell Sci. 2000;113(Pt. 21):3703–3713. doi: 10.1242/jcs.113.21.3703. [DOI] [PubMed] [Google Scholar]
- 142.Ye Q, Callebaut I, Pezhman A, Courvalin JC, Worman HJ. Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J. Biol. Chem. 1997;272:14983–14989. doi: 10.1074/jbc.272.23.14983. [DOI] [PubMed] [Google Scholar]
- 143.Holmer L, Pezhman A, Worman HJ. The human lamin B receptor/sterol reductase multigene family. Genomics. 1998;54:469–476. doi: 10.1006/geno.1998.5615. [DOI] [PubMed] [Google Scholar]
- 144.Prakash A, Sengupta S, Aparna K, Kasbekar DP. The erg-3 (sterol delta 14,15-reductase) gene of Neurospora crassa: generation of null mutants by repeat-induced point mutation and complementation by proteins chimeric for human lamin B receptor sequences. Microbiology. 1999;145(Pt. 6):1443–1451. doi: 10.1099/13500872-145-6-1443. [DOI] [PubMed] [Google Scholar]
- 145.Silve S, Dupuy PH, Ferrara P, Loison G. Human lamin B receptor exhibits sterol C14-reductase activity in Saccharomyces cerevisiae . Biochim. Biophys. Acta. 1998;1392:233–244. doi: 10.1016/s0005-2760(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 146.Clayton P, Fischer B, Mann A, Mansour S, Rossier E, et al. Mutations causing Greenberg dysplasia but not Pelger anomaly uncouple enzymatic from structural functions of a nuclear membrane protein. Nucleus. 2010;1:354–366. doi: 10.4161/nucl.1.4.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wassif CA, Brownson KE, Sterner AL, Forlino A, Zerfas PM, et al. HEM dysplasia and ichthyosis are likely laminopathies and not due to 3β-hydroxysterol Δ14-reductase deficiency. Hum. Mol. Genet. 2007;16:1176–1187. doi: 10.1093/hmg/ddm065. [DOI] [PubMed] [Google Scholar]
- 148.Gaines P, Berliner N. Granulocytopoiesis. In: Young NS, Gerson SL, High KA, editors. Clinical Hematology. Philadelphia: Mosby Elsevier; 2006. pp. 61–70. [Google Scholar]
- 149.Park BH, Dolen J, Snyder B. Defective chemotactic migration of polymorphonuclear leukocytes in Pelger-Huet anomaly. Proc. Soc. Exp. Biol. Med. 1977;155:51–54. doi: 10.3181/00379727-155-39743. [DOI] [PubMed] [Google Scholar]
- 150.Wittmann M, Queisser G, Eder A, Wiegert JS, Bengtson CP, et al. Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling. J. Neurosci. 2009;29:14687–14700. doi: 10.1523/JNEUROSCI.1160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Buschmann MB, LaVelle A. Morphological changes of the pyramidal cell nucleolus and nucleus in hamster frontal cortex during development and aging. Mech. Ageing Dev. 1981;15:385–397. doi: 10.1016/0047-6374(81)90043-9. [DOI] [PubMed] [Google Scholar]
- 152.Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans . Proc. Natl. Acad. Sci. USA. 2005;102:16690–16695. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Olive M, Harten I, Mitchell R, Beers J, Djabali K, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rodriguez S, Coppede F, Sagelius H, Eriksson M. Increased expression of the Hutchinson-Gilford progeria syndrome truncated lamin A transcript during cell aging. Eur. J. Hum. Genet. 2009;17:928–937. doi: 10.1038/ejhg.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 157.Yang SH, Qiao X, Farber E, Chang SY, Fong LG, Young SG. Eliminating the synthesis of mature lamin A reduces disease phenotypes in mice carrying a Hutchinson-Gilford progeria syndrome allele. J. Biol. Chem. 2008;283:7094–7099. doi: 10.1074/jbc.M708138200. [DOI] [PubMed] [Google Scholar]
- 158.Moulson CL, Fong LG, Gardner JM, Farber EA, Go G, et al. Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum. Mutat. 2007;28:882–889. doi: 10.1002/humu.20536. [DOI] [PubMed] [Google Scholar]
- 159.Yang SH, Andres DA, Spielmann HP, Young SG, Fong LG. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J. Clin. Investig. 2008;118:3291–3300. doi: 10.1172/JCI35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp. Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, et al. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am. J. Hum. Genet. 2006;79:383–389. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- 163.Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol. Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 164.Hutchison CJ, Worman HJ. A-type lamins: Guardians of the soma? Nat. Cell Biol. 2004;6:1062–1067. doi: 10.1038/ncb1104-1062. [DOI] [PubMed] [Google Scholar]
- 165.Espada J, Varela I, Flores I, Ugalde AP, Cadinanos J, et al. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J. Cell Biol. 2008;181:27–35. doi: 10.1083/jcb.200801096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lees-Miller SP. Dysfunction of lamin A triggers a DNA damage response and cellular senescence. DNA Repair. 2006;5:286–289. doi: 10.1016/j.dnarep.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 167.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat. Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tazir M, Azzedine H, Assami S, Sindou P, Nouioua S, et al. Phenotypic variability in autosomal recessive axonal Charcot-Marie-Tooth disease due to the R298C mutation in lamin A/C. Brain. 2004;127:154–163. doi: 10.1093/brain/awh021. [DOI] [PubMed] [Google Scholar]
- 169.Burtner CR, Kennedy BK. Progeria syndromes and ageing: What is the connection? Nat. Rev. Mol. Cell Biol. 2010;11:567–578. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 170.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 171.Wilson KL, Berk JM. The nuclear envelope at a glance. J. Cell Sci. 2010;123:1973–1978. doi: 10.1242/jcs.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 173.Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 174.Emery AE. Emery-Dreifuss muscular dystrophy-a 40 year retrospective. Neuromuscul. Disord. 2000;10:228–232. doi: 10.1016/s0960-8966(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 175.Fidzianska A, Toniolo D, Hausmanowa-Petrusewicz I. Ultrastructural abnormality of sarcolemmal nuclei in Emery-Dreifuss muscular dystrophy (EDMD) J. Neurol. Sci. 1998;159:88–93. doi: 10.1016/s0022-510x(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 176.Markiewicz E, Venables R, Mauricio Alvarez R, Quinlan R, Dorobek M, et al. Increased solubility of lamins and redistribution of lamin C in X-linked Emery-Dreifuss muscular dystrophy fibroblasts. J. Struct. Biol. 2002;140:241–253. doi: 10.1016/s1047-8477(02)00573-7. [DOI] [PubMed] [Google Scholar]
- 177.Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, et al. Mouse model carrying H222P–Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 178.Mounkes LC, Kozlov SV, Rottman JN, Stewart CL. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 2005;14:2167–2180. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- 179.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Investig. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 181.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 182.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, et al. Nesprin-1 and −2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 185.Houben F, Ramaekers FC, Snoeckx LH, Broers JL. Role of nuclear lamina-cytoskeleton interactions in the maintenance of cellular strength. Biochim. Biophys. Acta. 2007;1773:675–686. doi: 10.1016/j.bbamcr.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 186.Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J. Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N. Engl. J. Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- 189.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ. Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 190.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, et al. Lamin A truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 191.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Glynn MW, Glover TW. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 2005;14:2959–2969. doi: 10.1093/hmg/ddi326. [DOI] [PubMed] [Google Scholar]
- 195.Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2005;102:14416–14421. doi: 10.1073/pnas.0503712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, et al. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. USA. 2005;102:12873–12878. doi: 10.1073/pnas.0505767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang SH, Meta M, Qiao X, Frost D, Bauch J, et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Investig. 2006;116:2115–2121. doi: 10.1172/JCI28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stehbens WE, Delahunt B, Shozawa T, Gilbert-Barness E. Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc. Pathol. 2001;10:133–136. doi: 10.1016/s1054-8807(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 199.Stehbens WE, Wakefield SJ, Gilbert-Barness E, Olson RE, Ackerman J. Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc. Pathol. 1999;8:29–39. doi: 10.1016/s1054-8807(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 200.Varga R, Eriksson M, Erdos MR, Olive M, Harten I, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hernandez L, Roux KJ, Wong ES, Mounkes LC, Mutalif R, et al. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev. Cell. 2010;19:413–425. doi: 10.1016/j.devcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc. Natl. Acad. Sci. USA. 2008;105:15902–15907. doi: 10.1073/pnas.0807840105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bonne G, Mercuri E, Muchir A, Urtizberea A, Becane HM, et al. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann. Neurol. 2000;48:170–180. [PubMed] [Google Scholar]
- 204.Savage DB, Soos MA, Powlson A, O’Rahilly S, McFarlane I, et al. Familial partial lipodystrophy associated with compound heterozygosity for novel mutations in the LMNA gene. Diabetologia. 2004;47:753–756. doi: 10.1007/s00125-004-1360-4. [DOI] [PubMed] [Google Scholar]
- 205.Verstraeten VL, Broers JL, van Steensel MA, Zinn-Justin S, Ramaekers FC, et al. Compound heterozygosity for mutations in LMNA causes a progeria syndrome without prelamin A accumulation. Hum. Mol. Genet. 2006;15:2509–2522. doi: 10.1093/hmg/ddl172. [DOI] [PubMed] [Google Scholar]
- 206.Gilchrist S, Gilbert N, Perry P, Ostlund C, Worman HJ, Bickmore WA. Altered protein dynamics of disease-associated lamin A mutants. BMC Cell Biol. 2004;5:46. doi: 10.1186/1471-2121-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ostlund C, Bonne G, Schwartz K, Worman HJ. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J. Cell Sci. 2001;114:4435–4445. doi: 10.1242/jcs.114.24.4435. [DOI] [PubMed] [Google Scholar]
- 208.Muchir A, Medioni J, Laluc M, Massart C, Arimura T, et al. Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve. 2004;30:444–450. doi: 10.1002/mus.20122. [DOI] [PubMed] [Google Scholar]
- 209.Raffaele Di Barletta M, Ricci E, Galluzzi G, Tonali P, Mora M, et al. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am. J. Hum. Genet. 2000;66:1407–1412. doi: 10.1086/302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Scharner J, Gnocchi VF, Ellis JA, Zammit PS. Genotype-phenotype correlations in laminopathies: How does fate translate? Biochem. Soc. Trans. 2010;38:257–262. doi: 10.1042/BST0380257. [DOI] [PubMed] [Google Scholar]
- 211.Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat. Genet. 2000;24:153–156. doi: 10.1038/72807. [DOI] [PubMed] [Google Scholar]
- 212.Speckman RA, Garg A, Du F, Bennett L, Veile R, et al. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am. J. Hum. Genet. 2000;66:1192–1198. doi: 10.1086/302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vigouroux C, Magre J, Vantyghem MC, Bourut C, Lascols O, et al. Lamin A/C gene: sex-determined expression of mutations in Dunnigan-type familial partial lipodystrophy and absence of coding mutations in congenital and acquired generalized lipoatrophy. Diabetes. 2000;49:1958–1962. doi: 10.2337/diabetes.49.11.1958. [DOI] [PubMed] [Google Scholar]
- 214.Vantyghem MC, Pigny P, Maurage CA, Rouaix-Emery N, Stojkovic T, et al. Patients with familial partial lipodystrophy of the Dunnigan type due to a LMNA R482W mutation show muscular and cardiac abnormalities. J. Clin. Endocrinol. Metab. 2004;89:5337–5346. doi: 10.1210/jc.2003-031658. [DOI] [PubMed] [Google Scholar]
- 215.Plasilova M, Chattopadhyay C, Pal P, Schaub NA, Buechner SA, et al. Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome. J. Med. Genet. 2004;41:609–614. doi: 10.1136/jmg.2004.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kirschner J, Brune T, Wehnert M, Denecke J, Wasner C, et al. p.S143F mutation in lamin A/C: a new phenotype combining myopathy and progeria. Ann. Neurol. 2005;57:148–151. doi: 10.1002/ana.20359. [DOI] [PubMed] [Google Scholar]
- 217.Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc. Natl. Acad. Sci. USA. 2009;106:20788–20793. doi: 10.1073/pnas.0911895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Benedetti S, Merlini L. Laminopathies: from the heart of the cell to the clinics. Curr. Opin. Neurol. 2004;17:553–560. doi: 10.1097/00019052-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 219.Pelger K. Demonstratie van een paar zeldzaam voorkomende typen van bloedlichaampjesen bespreking der patiënten. Ned. Tijdschr. Geneeskd. 1928;72:1487. [Google Scholar]
- 220.Cunningham JM, Patnaik MM, Hammerschmidt DE, Vercellotti GM. Historical perspective and clinical implications of the Pelger-Huet cell. Am. J. Hematol. 2009;84:116–119. doi: 10.1002/ajh.21320. [DOI] [PubMed] [Google Scholar]
- 221.Speeckaert MM, Verhelst C, Koch A, Speeckaert R, Lacquet F. Pelger-Huet anomaly: a critical review of the literature. Acta Haematol. 2009;121:202–206. doi: 10.1159/000220333. [DOI] [PubMed] [Google Scholar]
- 222.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat. Rev. Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 223.DeMay RM. The Art and Science of Cytopathology. Chicago: Am. Soc. Clin. Pathol. Press; 1996. [Google Scholar]
- 224.Leman ES, Getzenberg RH. Nuclear matrix proteins as biomarkers in prostate cancer. J. Cell. Biochem. 2002;86:213–223. doi: 10.1002/jcb.10218. [DOI] [PubMed] [Google Scholar]
- 225.Lever E, Sheer D. The role of nuclear organization in cancer. J. Pathol. 2010;220:114–125. doi: 10.1002/path.2651. [DOI] [PubMed] [Google Scholar]
- 226.Luftner D, Possinger K. Nuclear matrix proteins as biomarkers for breast cancer. Expert Rev. Mol. Diagn. 2002;2:23–31. doi: 10.1586/14737159.2.1.23. [DOI] [PubMed] [Google Scholar]
- 227.Coradeghini R, Barboro P, Rubagotti A, Boccardo F, Parodi S, et al. Differential expression of nuclear lamins in normal and cancerous prostate tissues. Oncol. Rep. 2006;15:609–613. [PubMed] [Google Scholar]
- 228.Lim SO, Park SJ, Kim W, Park SG, Kim HJ, et al. Proteome analysis of hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2002;291:1031–1037. doi: 10.1006/bbrc.2002.6547. [DOI] [PubMed] [Google Scholar]
- 229.Sun S, Xu MZ, Poon RT, Day PJ, Luk JM. Circulating lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. J. Proteome Res. 2010;9:70–78. doi: 10.1021/pr9002118. [DOI] [PubMed] [Google Scholar]
- 230.Moss SF, Krivosheyev V, de Souza A, Chin K, Gaetz HP, et al. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut. 1999;45:723–729. doi: 10.1136/gut.45.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Broers JL, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS, Ramaekers FC. Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am. J. Pathol. 1993;143:211–220. [PMC free article] [PubMed] [Google Scholar]
- 232.Hudson ME, Pozdnyakova I, Haines K, Mor G, Snyder M. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc. Natl. Acad. Sci. USA. 2007;104:17494–17499. doi: 10.1073/pnas.0708572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tilli CM, Ramaekers FC, Broers JL, Hutchison CJ, Neumann HA. Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. Br. J. Dermatol. 2003;148:102–109. doi: 10.1046/j.1365-2133.2003.05026.x. [DOI] [PubMed] [Google Scholar]
- 234.Agrelo R, Setien F, Espada J, Artiga MJ, Rodriguez M, et al. Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J. Clin. Oncol. 2005;23:3940–3947. doi: 10.1200/JCO.2005.11.650. [DOI] [PubMed] [Google Scholar]
- 235.Venables RS, McLean S, Luny D, Moteleb E, Morley S, et al. Expression of individual lamins in basal cell carcinomas of the skin. Br. J. Cancer. 2001;84:512–519. doi: 10.1054/bjoc.2000.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Foster CR, Przyborski SA, Wilson RG, Hutchison CJ. Lamins as cancer biomarkers. Biochem. Soc. Trans. 2010;38:297–300. doi: 10.1042/BST0380297. [DOI] [PubMed] [Google Scholar]
- 237.Wu Z, Wu L, Weng D, Xu D, Geng J, Zhao F. Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. J. Exp. Clin. Cancer Res. 2009;28:8. doi: 10.1186/1756-9966-28-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Willis ND, Cox TR, Rahman-Casans SF, Smits K, Przyborski SA, et al. Lamin A/C is a risk biomarker in colorectal cancer. PLoS ONE. 2008;3:e2988. doi: 10.1371/journal.pone.0002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wolf K, Friedl P. Molecular mechanisms of cancer cell invasion and plasticity. Br. J. Dermatol. 2006;154(Suppl. 1):11–15. doi: 10.1111/j.1365-2133.2006.07231.x. [DOI] [PubMed] [Google Scholar]
- 241.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 242.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–547. doi: 10.1101/gad.960502. [DOI] [PubMed] [Google Scholar]
- 244.Prokocimer M, Margalit A, Gruenbaum Y. The nuclear lamina and its proposed roles in tumorigenesis: projection on the hematologic malignancies and future targeted therapy. J. Struct. Biol. 2006;155:351–360. doi: 10.1016/j.jsb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 245.Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar DZ, et al. Nuclear lamins: key regulators of nuclear structure and activities. J. Cell. Mol. Med. 2009;13:1059–1085. doi: 10.1111/j.1582-4934.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gravemann S, Schnipper N, Meyer H, Vaya A, Nowaczyk M, et al. Dosage effect of zero to three functional LBR-genes in vivo and in vitro. Nucleus. 2010;1:79–189. doi: 10.4161/nucl.1.2.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Akter R, Rivas D, Geneau G, Drissi H, Duque G. Effect of lamin A/C knockdown on osteoblast differentiation and function. J Bone Miner Res. 2009;24(2):283–293. doi: 10.1359/jbmr.081010. [DOI] [PubMed] [Google Scholar]