Abstract
The musculoskeletal system is significantly more complex than portrayed by traditional reductionist approaches that have focused on and studied the components of this system separately. While bone and skeletal muscle are the two largest tissues within this system, this system also includes tendons, ligaments, cartilage, joints and other connective tissue along with vascular and nervous tissue. Because the main function of this system is locomotion, the mechanical interaction among the major players of this system is essential for the many shapes and forms observed in vertebrates and even in invertebrates. Thus, it is logical that the mechanical coupling theories of musculoskeletal development exert a dominant influence on our understanding of the biology of the musculoskeletal system, because these relationships are relatively easy to observe, measure, and perturb. Certainly much less recognized is the molecular and biochemical interaction among the individual players of the musculoskeletal system.
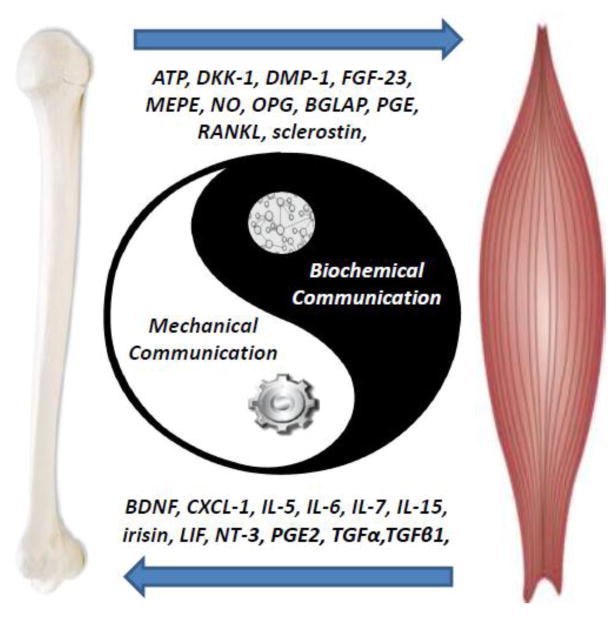
In this brief review article, we first introduce some of the key reasons why the mechanical coupling theory has dominated our view of bone-muscle interactions followed by summarizing evidence for the secretory nature of bones and muscles. Finally, a number of highly physiological questions that cannot be answered by the mechanical theories alone will be raised along with different lines of evidence that support both a genetic and a biochemical communication between bones and muscles. It is hoped that these discussions will stimulate new insights into this fertile and promising new way of defining the relationships between these closely related tissues. Understanding the cellular and molecular mechanisms responsible for biochemical communication between bone and muscle is important not only from a basic research perspective but also as a means to identify potential new therapies for bone and muscle diseases, especially for when they co-exist.
Keywords: Muscle, Bone, Secreted Factors, Gene Pleiotropy, Bone-Muscle Crosstalk
I. Introduction
The topic of bone-muscle interactions has become of interest for basic, clinical, and translational scientists because of the realization of the implications of this emerging field of research(1). The concept that bone and muscle cells communicate at the biochemical and molecular levels in ways beyond and complementary to those ways dictated by mechanical interactions is leading to new insight into how bone and muscles work together in health and disease. With life expectancy projected to surpass the centenary mark, the twin diseases osteoporosis-sarcopenia will exert additional, not yet fully understood, consequences on public health and the economy. In fact, humankind has never experienced a larger and broader demographic shift in its history with a projected 20% of the world population being 60 and older by 2050 and over 1/3 by 2150 (2, 3)
2. The mechanical coupling of bone and muscle is easier to appreciate and comprehend than biochemical cross-talk
The coupling of bones and skeletal muscles has been simplified and mainly viewed as mechanical in nature, where muscles are the load suppliers and bone simply provide the attachment sites. The musculoskeletal system is unquestionably much more complex than just bone and muscles. A broader comprehension of bone-muscle biochemical signaling could lead to unprecedented and previously unimagined therapies not only for these diseases, but likely for many other musculoskeletal diseases.
The close relationship and tight coupling between of the different tissues composing the musculoskeletal system is most fully appreciated during embryonic development when these tissues are formed from somites originating from the paraxial mesoderm(4). During intrauterine development, bone and muscle cells not only share a common mesenchymal precursor, but also experience organogenesis through a tightly orchestrated network of genes. It is possible that some of our own bias to more readily accept the study of mechanical coupling between these two tissues derives from embryology. As the skeleton develops, muscle contraction in the developing embryo contributes to the process of development itself and clearly to skeletal growth, and skeletal adaptations in early postnatal life are largely due to changing mechanical forces(5, 6). In addition, the immediate results of muscle load can be discerned on bones, since they adjust their shape and mass to changes in load, and such load can be easily interpreted as coming from muscle contractions. This mechanical relationship has been investigated in detail during embryonic patterning, postnatal allometric growth, and the homeostatic relationship of adult life and aging. It is therefore understandable that these many similarities and the availability of models to investigate the mechanical coupling of these two tissues has favored this perspective.
The major goal of this review article is to inform regarding muscle-bone crosstalk independent of loading. When a sound relationship between tissues is recognized and a large body of evidence supports that relationship, theories become dogmas, which can unfortunately hinder or block alternative views. The second goal is to summarize data that demonstrates the endocrine nature of skeletal muscle and bone. The third goal is to raise specific questions related to the bone-muscle relationship that are apparently unanswered by the mechanical coupling theory of bone-muscle crosstalk.
3. Muscle as a Secretory/Endocrine Organ
It was only during the last decade of research that skeletal muscles were recognized for their significant secretory capacity(7). Myostatin, discovered in Se-Jin’s laboratory in 1997 is arguably the most potent inhibitor of skeletal muscle cell proliferation and growth and is also considered one of the earliest muscle secreted factors (8, 9).
Pedersen and colleagues were the first research group to invent the term, ‘myokines,’ and since then, other muscle secreted factors have been documented by several research labs, including IL-8 that seems to stimulate angiogenesis(10), brain-derived neutrophic factor (BDNF)(11), irisin, a potent regulator of the conversion of white fat into brown fat(12), and IL-15, a muscle factor that reduces adiposity. Mice with elevated circulating levels of IL-15 show increased bone mineral content(13).
Our labs recently discovered that secreted factors from C2C12 myotubes but not from C2C12 myoblasts increased the viability of MLO-Y4 osteocytes treated with dexamethasone(14). Furthermore, ex-vivo, intact skeletal muscles when electrically stimulated also protected osteocytes against cell death, but only when muscles were electrically stimulated to contract, strongly suggesting that these effects are mediated by contraction induced muscle factors(14).
4. Bone as a Secretory/Endocrine Organ
Bone is generally not thought of as an endocrine gland such as the pituitary or adrenals. However, the definition of an endocrine gland is that it must be highly vascularized and form a system that directly secretes hormones into the bloodstream to affect distant targets. Based on this definition, bone is an endocrine organ (15). Osteoblasts have the capacity to release factors such as osteocalcin as do osteocytes. In addition, osteocytes produce circulating factors such as FGF23 and sclerostin. The osteocyte lacunocanalicular system contains bone fluid that contains factors released by osteocytes and these factors can be found in the circulation.
For decades it has been known that bone is a source of growth factors (16). Clearly bone is a source of osteogenic growth factors such as the bone morphogenetic proteins, transforming growth factor betas and insulin-like growth factors. It is thought that these factors are deposited in matrix by osteoblasts and released by osteoclasts during resorption. Potentially osteoblasts have the capacity to release factors into the circulation, but one must keep in mind that in the adult skeleton, osteoblasts may compose approximately 5% of bone cells compared to 1% of osteoclasts, whereas 90–95% are osteocytes. Considering the total mass of osteocytes and their dendritic processes in bone, these cells are likely the source of the majority of circulating factors from the adult skeleton.
One factor produced uniquely by bone is osteocalcin which has been shown to target numerous tissues and is therefore considered a hormone (17). Osteocalcin is produced by the late osteoblast before embedding into osteoid and by embedded osteocytes. Like growth factors, it was assumed that osteocalcin is mainly deposited into the bone matrix during bone formation and hypothesized to be released by osteoclasts during resorption (18). However, bone cells may also have the capacity to secrete osteocalcin into the circulation. Osteocalcin has high affinity for hydroxyapatite, but with modulation of carboxylation could be a circulating factor. Osteocalcin has effects on glucose metabolism, energy metabolism, fertility, and ectopic calcification (19) and as discussed below potentially on muscle activity. Most of these studies have been performed in rodents with some observational data in humans.
Osteocytes send signals to not only other osteocytes, but both osteoblasts and osteoclasts and their precursors on the bone surface acting as ‘orchestrators’ of bone remodeling. Clearly bone responds to both anabolic loading and to immobilization or unloading and mechanical loading regulates osteocyte production of factors such as sclerostin (20). The Wnt/β-catenin pathway in osteocytes is an important regulator of bone mass and important in osteocyte transmission of mechanical loading signals to cells on the bone surface(21). The pathway is triggered by crosstalk with the prostaglandin pathway in response to loading resulting in a decrease in negative regulators of bone formation such as Sost/sclerostin and Dkk1(22). It has recently been shown that two products, prostaglandin E2 and Wnt3a, both produced by osteocytes in response to shear stress will support myogenesis and muscle function (23–26). While the WNTs are thought to mainly act locally, Wnt3a can be quantitated in serum. It is not known if this circulating form is active or not. Whether osteocytes secrete sufficient levels into the circulation to have effects on muscle is not known at this time.
Since its identification in 2000, FGF23 has become one of the most important osteocyte-secreted endocrine factors(27–30). Not only do osteocytes secrete small signaling molecules but also factors that regulate mineral homeostasis. Osteocytes appear to regulate phosphate through molecules such as Phosphate Regulating Neutral Endopeptidase on Chromosome X, (Phex), Dentin Matrix Protein 1, (Dmp1), and Fibroblast Growth Factor 23, (FGF23)(31). Both Dmp1 and Phex down regulate FGF23 in osteocytes allowing reabsorption of phosphate by the kidney to maintain sufficient circulating phosphate to maintain normal bone mineral content. In the absence of either Dmp1 or Phex, FGF23 is elevated in the osteocyte and systemically, leading to phosphate excretion by the kidney resulting in osteomalacia and rickets. It was previously proposed that the osteocyte lacuno-canalicular network can function as an endocrine system, targeting distant organs such as kidney(28). Interestingly, FGF23 is also able to act on the parathyroid gland to decrease PTH secretion, identifying the parathyroid gland as another endocrine target of osteocyte signaling(32, 33).
FGF23 is known to have effects on cardiac muscle. Recent studies have linked raised levels of circulating FGF23 to an increased risk of heart disease. Elevated levels of FGF23 were found to be independently associated with left ventricular hypertrophy in human population studies (34, 35). Increased serum FGF23 has also been linked with impaired vascular function (34), vascular calcification (36) and increased fat mass (37), indicating the importance of osteocyte-regulated proteins in health and disease. These findings of altered cardiac and vascular function identify these tissues as target organs of osteocyte-secreted FGF23. The effects of elevated FGF23 on skeletal muscle function is not known.
4. Unanswered Questions
A major goal of our review article is to stimulate questions that could help advance the research of both bone and muscle and this emerging field of bone-muscle crosstalk. Some examples of intriguing questions that have not been answered in the context of the mechanical coupling theory alone are:
Why do fractures heal better with muscle flaps?
Why does the deletion/inhibition of a muscle specific genes lead to bone gains?
What are the roles of bone-muscle pleiotropic genes? Why would similar molecular signaling pathways govern bone and muscle if the relationship was only one of load imposed by one tissue on the other tissue?
In a known model of bone disease, Osteogenesis Imperfecta, why is there muscle weakness even in the absence of myopathy?
Why should the muscle-bone relationship be unidirectional with only muscles influencing bones and not vice-versa? If bones secrete a myriad of factors, such factors could influence or even optimize muscle function.
Is there a connection between sarcopenia and osteoporosis?
4.1 Fracture Healing
In the broader context of bone-muscle interactions, it seems largely ignored that with open bone fractures where skeletal muscle is simultaneously injured, the resulting fracture healing is significantly compromised(38–41). Likely, a quick answer to this question would be that since muscles are injured, contractions are reduced or weaker leading to the reduced healing. Nevertheless, if this assumption was correct, why would healing improve with specific conditions where substantial muscle force is not occurring? Harry et al reported studies in mice using the open tibial fracture model showing that when the fracture area was surrounded with muscle flaps, bone repair was significantly enhanced; both faster and the quality of the repair was superior(42). These findings have gained even stronger clinical significance in light of the fact that the same holds true in humans with open tibial fractures(43). Certainly, a plausible answer to these intriguing effects of muscle flaps is that muscles are producing and releasing factors that can travel either through specific tissue-tissue transport mechanisms or through the general circulation to exert direct, specific effects on bone healing and repair. It is fascinating to consider that muscles might function as a second periosteum layer as recently proposed by Little and colleagues in their study showing that muscle stem cells contribute to bone repair (44, 45).
4.2 Myostatin Paradox
Inactivation of myostatin, which results in muscle hypertrophy or “double muscling” in animals(46–51) and humans(52), is a prime example of how a mutation presumably restricted to one tissue can lead to altered properties in the other. Hamrick and colleagues investigated the potential effects of increased muscle mass on bone mineral content and density utilizing the myostatin deficient mouse model. Myostatin or growth and differentiation factor 8, GDF8, is a member of the TGF-β superfamily and is a secreted myokine that circulates in the blood, making it an attractive candidate to be involved in muscle-bone endocrine signaling(53). The GDF8 null mouse is a fascinating model, since the significantly larger muscles of these mice are actually weaker when force is normalized per unit of cross-sectional area. Therefore, a clear argument is that loading per unit of muscle is actually lessened in these mice, but their muscle volume is certainly larger. Even though the normalized force is less, there is still greater total force compared to controls due to the dramatic increase in muscle mass. Hamrick found that there was an increased periosteal circumference along the humerus and increased cortical bone mineral density in the distal femur exactly two bones of the extremities and two bones exposed to the largest muscle volume in the body (54, 55). In agreement with the explanation provided above for the effects of muscle flaps on bone healing, the significantly larger volume of muscle could favor a larger amount of secretion of muscle factors, which in turn can influence bone mass.
The development of myostatin inhibitors was recently reviewed and has further stimulated the discussion around tissue crosstalk (56). Studies in humans have found that myostatin inhibition leads to increased lean mass, decreased fat mass, and increased bone formation. Myostatin neutralizing antibody (Mstn-mAb), and a soluble myostatin decoy receptor (ActRIIB-Fc) have both been used to inhibit myostatin. They are both considered myostatin inhibitors, but in 2014, Bialek and colleagues, showed that while both increased muscle mass, only ActRIIB-Fc increased both muscle mass and bone mass. Since they obtained the same bone mass increase with ActRIIB-Fc in myostatin deficient mice, they suggested that this soluble decoy receptor might be binding a ligand other than myostatin that plays a role in regulating bone mass, but in muscle AcTRIIB does work as a myostatin inhibitor(57).
4.3 Bone-Muscle Pleiotropic Genes
More recently innovative bivariate GWAS has been used to identify pleiotropic candidate genes/SNPs/regions associated with traits in both bone and muscle(58–63). These GWAS based studies produced a list of potential bone-muscle pleiotropic genes that now require validation studies. One of the genes identified in these studies was MEF2C, a gene that encodes a transcription factor (myocyte enhancer factor 2C) which was demonstrated to be involved in cardiac and skeletal muscle development(64). A mouse model where Mef2C was specifically deleted in osteocytes challenged these initial observations since these mice have an increased bone density through complex mechanisms that involve reduced Sost expression, increased OPG expression resulting in a reduced RANKL/OPG ratio, and reduced osteoclastogenesis(65).
Our group has recently attempted to begin the validation of another potentially pleiotropic gene, METTL21C(66). This gene is part of the METTL21family of the methyltransferase superfamily and has protein-lysine N-methyltransferase activity(67), and methylates valosin containing protein (VCP) chaperones, which harbor specific mutations that are causal for Inclusion Body Myositis (muscle) with early onset of Paget’s Disease (bone) (68). We found that molecular-genetic down-regulation of this gene in C2C12 muscle cells led to reduced myogenic differentiation, a dramatic decrease in myotube cell area (i.e., cellular atrophy), and reduced calcium release from the sarcoplasmic reticulum, while MLO-Y4 osteocyte cells became significantly more prone to dexamethasone induced cell death. Surprisingly, the effects mediated by Mettl21C in bone and muscle cells was linked to the alteration of common signaling pathway, the NF-kB pathway. Together, these findings suggest important roles for MEF2C and METTL21C in both bone and muscle and support the concept that shared genetic determinants and commonly shared signaling pathways are operational in both muscle and bone growth and development. What remains to be answered is the nature of the potential biochemical factors mediating the modulation of bone and muscle cells in pleiotropy.
4.4 Osteogenesis Imperfecta-Why muscle weakness without muscle disease?
Osteogenesis imperfect (OI) is a congenital bone disorder where mutations is COL1A1 and COL1A2 lead to a deficiency in collagen, which makes bones prone to fracture (brittle bones) (69). In a series of very elegant studies, Phillips and collaborators developed OI animal models and have characterized them in the last decade. They found that even in the total absence of muscle myopathy, significant muscle weakness was present in OI mice(70). The clinical significance of their findings in this animal model is further enhanced, since essentially the same type of muscle weakness had been reported in humans in at least three independent studies (71–73). These studies not only provide a robust and clinically relevant example of bone-muscle crosstalk, but also provide strong evidence that muscle applied load is not the only dictating factor in the bone-muscle relationship. Do weaker bones release factors that negatively influence muscle function or conversely do weaker bones not release factors that support muscle function?
4.5 Why should the muscle-bone relationship be unidirectional with only muscles influencing bones and not vice-versa? If bones secrete a myriad of factors, why shouldn’t such factors influence or even optimize muscle function?
As discussed above, osteocytes can signal to tissues other than bone via secretion of factors. One of the targets of pathological elevated levels of FGF23 is cardiac muscle resulting in left ventricular hypertrophy(74). This therefore raises an interesting possibility; can osteocytes directly influence muscle mass by secreting muscle regulatory factors? Such interplay between bone and muscle seems highly plausible, especially as muscle and bone mass are tightly correlated from development through aging. In vitro support for this hypothesis was shown recently. MLO-Y4 osteocyte-like cells and primary osteocytes secrete factors that induce muscle myogenesis and activate the Wnt/β-catenin pathway in C2C12 pluripotent cells (75) (25). These findings suggest that this pathway not only plays a role in muscle development but also in muscle repair and myogenesis. Two factors produced by osteocytes in response to shear stress, PGE2 and Wnt3a, were found to enhance myogenesis and ex vivo primary muscle function. These studies suggest other potential endocrine and/or paracrine functions for osteocytes.
Recently, it has also been suggested that osteocalcin can have effects on muscle(76, 77). Muscle strength is associated with total osteocalcin levels(76), exercise increases blood levels of uncarboxylated osteocalcin (77) and osteocalcin may have a direct effect on muscle strength(78). Osteocalcin is such an incredibly multifunctional molecule that it will be important to determine the receptors and signaling pathways responsible for its actions.
4.6 Is there a connection between sarcopenia and osteoporosis?
Osteoporosis and Sarcopenia are major clinical problems in the aging population and in many patients these two conditions occur concurrently. It is unclear whether one condition precedes the other or if the conditions are linked. The mechanical perspective implies that as muscle function declines, this would result in decreased loading of the skeleton and therefore would result in a decrease in bone mass. However, muscle atrophy alone cannot fully explain the totality of osteoporosis and, reciprocally, aging associated decreases in b one mass do not fully explain sarcopenia.
Osteocytes are descendants of osteoblasts embedded within bone matrix. The osteocyte is an aging, senescent cell. As osteocytes reside in human bone for decades while osteoblasts and osteoclasts live for only days or weeks, the osteocyte is very susceptible to the effects of aging. The osteocyte cannot be replaced except by bone remodeling, therefore the osteocyte could be considered the senescent cell in bone. Osteocyte cell death can result in micropetrosis where mineral fills the lacuna resulting in a cell that is a ‘living fossil’ (79). Fewer numbers of osteocyte lacunae were found in patients suffering from fractures compared to controls(80). It has been found that an age-dependent decrease occurs in osteocyte lacunar density with an increased amount of hypermineralized calcium phosphate occlusions(81). These observations have important implications for the flow of canalicular fluid, for the osteocyte’s ability to sense mechanical loading and the cell’s capacity to sense microdamage. The aging osteocyte in a compromised lacuno-canalicular system is less likely to produce factors to support muscle myogenesis and function. We have found that muscle secretes low molecular weight factors that protect osteocytes against glucocorticoid induced apoptosis(23). Muscle, especially with contraction, is most likely producing osteocyte viability factors, and these are lost with aging. It will be important to identify muscle factors that protect osteocytes and maintain their function and vice versa to identify bone factors that maintain muscle function in order to design therapeutics for treatment/prevention of the twin syndrome of osteoporosis and sarcopenia.
5. Future Directions
In summary, the mechanical and biochemical theories of bone-muscle crosstalk are not exclusive or one more important or relevant than the other theory. These theories are most likely complementary whereby mechanical force might prime bone and muscle for regulation and release of specific factors to exert their effects on the opposing tissue. Continued and progressive research aimed at merging these two theories will bring advances in knowledge that will translate into new therapies that could revolutionize current treatments by targeting not only one but both tissues simultaneously.
Supplementary Material
Figure 1.
Highlights.
The major goal of this review article is to inform regarding muscle-bone crosstalk independent of loading. The second goal is to summarize data that demonstrates the endocrine nature of skeletal muscle and bone. The third goal is to raise specific questions related to the bone-muscle relationship that are apparently unanswered by the mechanical coupling theory of bone-muscle crosstalk.
Acknowledgments
The research supported by the authors in muscle bone crosstalk is supported by NIAMS NIA PO1AG039355.
Footnotes
Conflict of Interest
Authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonewald LF, Kiel DP, Clemens TL, Esser K, Orwoll ES, O’Keefe RJ, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(9):1857–65. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benefield LE, Holtzclaw BJ. Aging in place: merging desire with reality. The Nursing clinics of North America. 2014;49(2):123–31. doi: 10.1016/j.cnur.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Sex and Age Distribution of the World Population, the 1998 Revision. United Nations Publication, No E99XII81998.
- 4.Pourquié O. VERTEBRATE SOMITOGENESIS. Annual Review of Cell and Developmental Biology. 2001;17(1):311–50. doi: 10.1146/annurev.cellbio.17.1.311. [DOI] [PubMed] [Google Scholar]
- 5.Rauch F, Schoenau E. The Developing Bone: Slave or Master of Its Cells and Molecules? Pediatr Res. 2001;50(3):309–14. doi: 10.1203/00006450-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Land C, Schoenau E. Fetal and postnatal bone development: reviewing the role of mechanical stimuli and nutrition. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22(1):107–18. doi: 10.1016/j.beem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Kurek JB, Bower JJ, Romanella M, Koentgen F, Murphy M, Austin L. The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve. 1997;20(7):815–22. doi: 10.1002/(sici)1097-4598(199707)20:7<815::aid-mus5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, et al. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294(5):E918–27. doi: 10.1152/ajpendo.00798.2007. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3(3):1337–62. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103(3):1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen L, Olsen CH, Pedersen BK, Hojman P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am J Physiol Endocrinol Metab. 2012;302(7):E831–40. doi: 10.1152/ajpendo.00339.2011. [DOI] [PubMed] [Google Scholar]
- 12.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. American journal of physiology Endocrinology and metabolism. 2009;296(1):E191–202. doi: 10.1152/ajpendo.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012;24:197–209. doi: 10.22203/ecm.v024a14. discussion -10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocrine reviews. 2013;34(5):658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19(1 Suppl):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein-Nulend J, Bonewald LF. The Osteocyte. In: Bilezikian JP, Raisz LG, editors. Principles of Bone Biology. Vol. 1. Academic Press; 2008. [Google Scholar]
- 21.Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30(12):3071–85. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonewald LF, Johnson ML. Osteocytes, Mechanosensing, and Wnt Signaling. Bone. 2008;42:606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012;24:197–210. doi: 10.22203/ecm.v024a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent patents on biotechnology. 2012;6(3):223–9. doi: 10.2174/1872208311206030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo CL, Romero-Suarez S, Bonewald LF, Johnson ML, Brotto M. Evidence for Biochemical and Functional Communication from the MLO-Y4 Osteocyte-like Cell to the C2C12 Muscle Cells. Cell Cycle. 2012 In Submission. [Google Scholar]
- 26.Huang J, Mo C, Bonewald L, Brotto M. Wnt3a potentiates myogenesis in C2C12 myoblasts through changes of signaling pathways including Wnt and NFkB. ASBMR 2014 Annual Meeting; 2014; p. s266. [Google Scholar]
- 27.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. The Journal of clinical investigation. 2004;113(4):561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Lu Y, Xie Y, Zhou J, Quarles LD, Bonewald L, et al. Elevated levels of FGF23 in Dentin Matrix Protein 1 (DMP1) Null Mice Potentially Explain Phenotypic Similarities to Hyp Mice. J Bone Min Res. 2006 [Google Scholar]
- 30.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonewald LF. Osteocytes as Dynamic, Multifunctional Cells. Ann N Y Acad Sci. 2007;1116:281–90. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 32.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195(1):125–31. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. The Journal of clinical investigation. 2007;117(12):4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–90. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. The Journal of clinical investigation. 2011;121(11):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 37.Mirza MA, Alsio J, Hammarstedt A, Erben RG, Michaelsson K, Tivesten A, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31(1):219–27. doi: 10.1161/ATVBAHA.110.214619. [DOI] [PubMed] [Google Scholar]
- 38.Zacks SI, Sheff MF. Periosteal and metaplastic bone formation in mouse minced muscle regeneration. Lab Invest. 1982;46(4):405–12. [PubMed] [Google Scholar]
- 39.Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. The Journal of biological chemistry. 2001;276(31):28961–8. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 40.Utvag SE, Iversen KB, Grundnes O, Reikeras O. Poor muscle coverage delays fracture healing in rats. Acta Orthop Scand. 2002;73(4):471–4. doi: 10.1080/00016470216315. [DOI] [PubMed] [Google Scholar]
- 41.Yamashiro T, Fukunaga T, Kobashi N, Kamioka H, Nakanishi T, Takigawa M, et al. Mechanical stimulation induces CTGF expression in rat osteocytes. J Dent Res. 2001;80(2):461–5. doi: 10.1177/00220345010800021201. [DOI] [PubMed] [Google Scholar]
- 42.Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26(9):1238–44. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Chen H, Feng JQ, Windle JJ, Koop BA, Harris MA, Bonewald LF, Boyce BF, Wozney JM, Mundy GR, Harris SE. Osteoblastic cell lines derived from a transgenic mouse containing the osteocalcin promoter driving SV40 T-antigen. Mol Cell Diff. 1995;3:193–212. [Google Scholar]
- 44.Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation. 2009;77(1):12–8. doi: 10.1016/j.diff.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Schindeler A, Little DG. The potential role of muscle in bone repair. Journal of musculoskeletal & neuronal interactions. 2010;10(1):71–6. [PubMed] [Google Scholar]
- 46.Grobet L, Royo Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17(1):71–4. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 47.Kambadur R, Sharma M, Smith TPL, Bass JJ. Mutations in myostatin (GDF8) in Double-Muscled Belgian Blue and Piedmontese Cattle. Genome Research. 1997;7(9):910–5. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 48.McPherron AC, Lee S-J. Double muscling in cattle due to mutations in the myostatin gene. Proceedings of the National Academy of Sciences. 1997;94(23):12457–61. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38(7):813–8. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 50.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, et al. A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLoS Genet. 2007;3(5):e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang GX, Zhao XH, Wang JY, Ding FX, Zhang L. Effect of an exon 1 mutation in the myostatin gene on the growth traits of the Bian chicken. Animal Genetics. 2012;43(4):458–9. doi: 10.1111/j.1365-2052.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 52.Williams M. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;351(10):1030–1. [PubMed] [Google Scholar]
- 53.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 54.Elkasrawy M, Fulzele S, Bowser M, Wenger K, Hamrick M. Myostatin (GDF-8) inhibits chondrogenesis and chondrocyte proliferation in vitro by suppressing Sox-9 expression. Growth factors. 2011;29(6):253–62. doi: 10.3109/08977194.2011.599324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. Journal of musculoskeletal & neuronal interactions. 2010;10(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- 56.Buehring B, Binkley N. Myostatin--the holy grail for muscle, bone, and fat? Current osteoporosis reports. 2013;11(4):407–14. doi: 10.1007/s11914-013-0160-5. [DOI] [PubMed] [Google Scholar]
- 57.Bialek P, Parkington J, Li X, Gavin D, Wallace C, Zhang J, et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone. 2014;60:162–71. doi: 10.1016/j.bone.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Karasik D, Zhou Y, Cupples LA, Hannan MT, Kiel DP, Demissie S. Bivariate Genome-Wide Linkage Analysis of Femoral Bone Traits and Leg Lean Mass: Framingham Study. Journal of Bone and Mineral Research. 2009;24(4):710–8. doi: 10.1359/JBMR.081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46(5):1226–37. doi: 10.1016/j.bone.2010.01.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta M, Cheung C-L, Hsu Y-H, Demissie S, Cupples LA, Kiel DP, et al. Identification of homogeneous genetic architecture of multiple genetically correlated traits by block clustering of genome-wide associations. Journal of Bone and Mineral Research. 2011;26(6):1261–71. doi: 10.1002/jbmr.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun L, Tan L-J, Lei S-F, Chen X-D, Li X, Pan R, et al. Bivariate Genome-Wide Association Analyses of Femoral Neck Bone Geometry and Appendicular Lean Mass. PLoS ONE. 2011;6(11):e27325. doi: 10.1371/journal.pone.0027325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karasik D, Cohen-Zinder M. Osteoporosis genetics: year 2011 in review. Bonekey Rep. 2012;1:Article Number: 114, 1–5. doi: 10.1038/bonekey.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Y-F, Zhang L-S, Liu Y-J, Hu H-G, Li J, Tian Q, et al. Suggestion of GLYAT gene underlying variation of bone size and body lean mass as revealed by a bivariate genome-wide association study. Hum Genet. 2013;132(2):189–99. doi: 10.1007/s00439-012-1236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120(5):1251–63. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 65.Kramer I, Baertschi S, Halleux C, Keller H, Kneissel M. Mef2c deletion in osteocytes results in increased bone mass. Journal of Bone and Mineral Research. 2012;27(2):360–73. doi: 10.1002/jbmr.1492. [DOI] [PubMed] [Google Scholar]
- 66.Huang J, Hsu YH, Mo C, Abreu E, Kiel DP, Bonewald LF, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014;29(7):1531–40. doi: 10.1002/jbmr.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kernstock S, Davydova E, Jakobsson M, Moen A, Pettersen S, Maelandsmo GM, et al. Lysine methylation of VCP by a member of a novel human protein methyltransferase family. Nat Commun. 2012;3:1038. doi: 10.1038/ncomms2041. [DOI] [PubMed] [Google Scholar]
- 68.Cloutier P, Lavallee-Adam M, Faubert D, Blanchette M, Coulombe B. A newly uncovered group of distantly related lysine methyltransferases preferentially interact with molecular chaperones to regulate their activity. PLoS Genet. 2013;9(1):e1003210. doi: 10.1371/journal.pgen.1003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–85. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 70.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix biology: journal of the International Society for Matrix Biology. 2010;29(7):638–44. doi: 10.1016/j.matbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donohoe M, Cintas H. Ankle strength and functional limitations in children and adolescents with type I osteogenesis imperfecta. Pediatric physical therapy: the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2010;22(3):295. doi: 10.1097/PEP.0b013e3181eb6d35. [DOI] [PubMed] [Google Scholar]
- 72.Caudill A, Flanagan A, Hassani S, Graf A, Bajorunaite R, Harris G, et al. Ankle strength and functional limitations in children and adolescents with type I osteogenesis imperfecta. Pediatric physical therapy: the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2010;22(3):288–95. doi: 10.1097/PEP.0b013e3181ea8b8d. [DOI] [PubMed] [Google Scholar]
- 73.Boot AM, de Coo RF, Pals G, de Muinck Keizer-Schrama SM. Muscle weakness as presenting symptom of osteogenesis imperfecta. European journal of pediatrics. 2006;165(6):392–4. doi: 10.1007/s00431-006-0083-6. [DOI] [PubMed] [Google Scholar]
- 74.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin e2: from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–9. doi: 10.2174/1872208311206030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, et al. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. 2014;64:8–12. doi: 10.1016/j.bone.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Levinger I, Zebaze R, Jerums G, Hare DL, Selig S, Seeman E. The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(5):1621–6. doi: 10.1007/s00198-010-1370-7. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gomez-Ambrosi J, Moreno-Navarrete JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. The Journal of clinical endocrinology and metabolism. 2009;94(1):237–45. doi: 10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- 79.Bell LS, Kayser M, Jones C. The mineralized osteocyte: a living fossil. Am J Phys Anthropol. 2008;137(4):449–56. doi: 10.1002/ajpa.20886. [DOI] [PubMed] [Google Scholar]
- 80.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Reduced iliac cancellous osteocyte density in patients with osteoporotic vertebral fracture. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2003;18(9):1657–63. doi: 10.1359/jbmr.2003.18.9.1657. [DOI] [PubMed] [Google Scholar]
- 81.Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9(6):1065–75. doi: 10.1111/j.1474-9726.2010.00633.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.