Abstract
We have functionalized the surface of gold nanocages with SV119, a synthetic small molecule specific to sigma-2 receptors, and then demonstrated the capability of this new class of conjugates for targeting cancer cells.
One major goal in chemotherapy is to maximize and maintain a relatively high concentration of therapeutic agents at the tumor site while minimizing their presence in normal tissues.1 This goal can be achieved by developing an efficient targeting platform that only binds to the cancer cells. A wide variety of targeting ligands have been identified and assessed for this purpose with varying degrees of success, including antibodies, antibody fragments, nucleic acids, peptides, and small molecules.2 Among these various candidates, synthetic small molecules have emerged as an important class of targeting ligands due to their many favorable attributes: small size, high purity, ease of large-scale production, and lack of immunogenicity.
Recently, sigma-2 receptors (transmembrane proteins found in mitochondria, endoplasmic reticulum, and plasma membranes)3 have received renewed interest in cancer biology research.4 And most recently, the progesterone receptor membrane component 1 (PGRMC1) protein complex was identified as a putative sigma-2 receptor binding site.5 A high-level expression of sigma-2 receptors has been observed in a diverse set of human and rodent tumor cells.6 It has also been found that proliferating tumor cells could have a density of sigma-2 receptors 10-fold greater than quiescent tumor cells.7 In addition, sigma-2 receptors can serve as a specific biomarker for differentiating solid tumors from surrounding normal tissues, suggesting that the sigma-2 receptors could be employed for targeted therapy.8 Several synthetic ligands specific to sigma-2 receptors have been successfully developed (as radioimaging or therapeutic agents for cancers) and tested in vivo.9 However, there is no report on the use of small molecule ligands specific to sigma-2 receptors for targeted delivery of nanoparticles into cancer cells. As a proof of concept, we have conjugated SV119 (Fig. 1A, a small, synthetic molecule specific to sigma-2 receptors) to Au nanocages (AuNCs, a class of hollow and porous nanostructures with great potential for theranostic applications10) and then evaluated their efficacy for targeting cancer cells.
Fig. 1.
A) Chemical structures of SV119 and (+)-pentazocine with specific binding to sigma-2 and sigma-1 receptors, respectively; B) flow cytometry analyses of MDA-MB-435 cells that were pretreated with SV119 or (+)-pentazocine for 1 h and then incubated with SW120 (50 nM) for 0.5 h (as a control, the cells were also directly incubated with SW120 for 0.5 h); C) a typical TEM image of the Au nanocages; and D) flow cytometry data showing internalization of SW120 by MDA-MB-435 cells in the absence and presence of SV119-PEG-AuNCs (1.2 nM).
We utilized MDA-MB-435 cells (a melanoma cell line) that express sigma-2 receptors as an in vitro model to evaluate the binding selectivity. The expression of sigma-2 receptors by MDA-MB-435 cells was confirmed by a competitive inhibition (blocking) assay based on SW120, a fluorescently-labeled ligand specific to sigma-2 receptors. The ligand was prepared by reacting dansyl chloride with the primary amine group of SV119. (+)-Pentazocine (Fig. 1A), a ligand known to selectively bind to sigma-1 receptors rather than sigma-2 receptors, was selected as the control. MDA-MB-435 cells were incubated with 10 μM solutions containing SV119 and (+)-pentazocine, respectively, for 1 h at 37 °C, treated with 50 nM of SW120 for 0.5 h, and their fluorescence intensities were then analyzed using flow cytometry. As shown in Figure 1B, the cells pre-treated with SV119 showed a weaker fluorescence relative to those pre-treated with (+)-pentazocine since SV119 displaced the binding of SW120 to the sigma-2 receptors on MDA-MB-435 cells, while (+)-pentazocine, the sigma-1 receptor specific ligand, failed to block the binding of SW120 to the sigma-2 receptors.
We then examined if SV119 could maintain its binding affinity for the sigma-2 receptors after conjugation to the surface of AuNCs. The nanocages with an outer edge length of 46 nm, a wall thickness of 7 nm (Fig. 1C), and a localized surface plasmon resonance (LSPR) peak at 780 nm were prepared using a protocol based on the galvanic replacement reaction between Ag nanocubes and chloroauric acid in an aqueous solution.11 Heterofunctional poly(ethylene glycol) was reacted with SV119 and then conjugated to the surface of AuNCs through a Au-thiolate linkage to produce SV119-PEG-AuNCs. Similarly PEGylated AuNCs (PEG-AuNCs) without SV119 terminal group were used as a control. The density of PEG chains on the surface of the AuNCs was found to be approximately 3.7 × 104 per AuNC (see Fig. S1 in the Supporting Information). Upon surface modification, the LSPR peak of the AuNCs slightly red-shifted due to a minor change in the refractive index on the surface (Fig. S2). As confirmed by a significant reduction in fluorescence intensity (Fig. 1D), the SV119 ligand could still inhibit the uptake of SW120 by MDA-MB-435 cells after conjugation with AuNCs. This result demonstrates that SV119 retained its binding affinity for sigma-2 receptors expressed by the MDA-MB-435 cells after it had been conjugated to the surface of AuNCs.
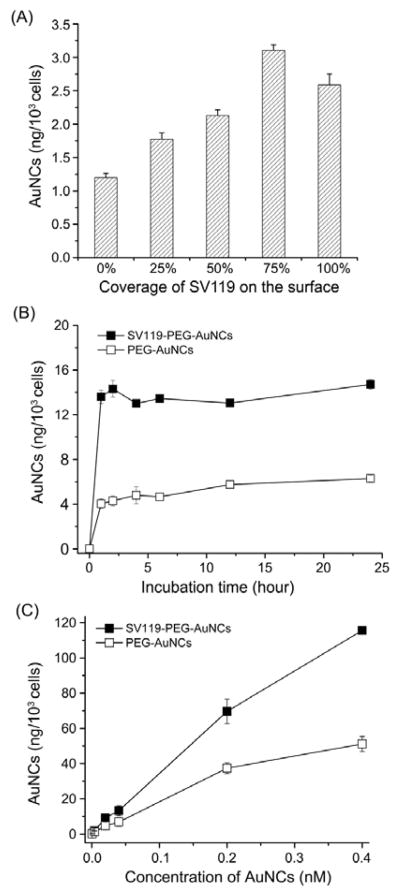
The sigma-2 receptors on the membrane of MDA-MB-435 cells belong to a family of recycling endocytotic receptors. After conjugation with SV119, the AuNCs could bind to the sigma-2 receptors on the cell membrane and then enter the cell via SV119-mediated endocytosis. To quantify the effect of SV119 on cellular uptake, AuNCs with different coverage densities of SV119 (0, 25, 50, 75 and 100%) were prepared by varying the ratio of SV119-PEG to PEG during conjugation (see experimental section in the Supporting Information). After incubation for 2 h, the medium was discarded and the amount of AuNCs taken up by the cells was determined using inductively coupled plasma mass spectroscopy (ICP-MS). As shown in Figure 2A, for MDA-MB-435 cells incubated with the same amount of AuNCs, cellular uptakes of AuNCs increased with increasing surface coverage of SV119. This result is consistent with that cellular uptake of the AuNCs was greatly enhanced by SV119 functionalization due to a receptor-mediated endocytosis mechanism.
Fig. 2.
A) Uptakes of AuNCs by MDA-MB-435 cells after incubation for 2 h, where the AuNCs had different coverage densities of SV119 on the surface; B) uptakes of SV119-PEG-AuNCs and PEG-AuNCs, respectively, by MDA-MB-435 cells after incubation for different periods of time; and C) uptakes of SV119-PEG-AuNCs and PEG-AuNCs, respectively, by MDA-MB-435 cells after incubation with the AuNCs at different concentrations for 2 h.
We then quantified the internalization of the different AuNCs by MDA-MB-435 cells as a function of incubation time (Fig. 2B). At any time point, the quantity of SV119-PEG-AuNCs up-taken by the cells was larger than that of PEG-AuNCs. This result confirms that SV119 could specifically interact with the sigma-2 receptors and facilitate cellular uptake. We further incubated MDA-MB-435 cells with SV119-PEG-AuNCs and PEG-AuNCs supplied at different concentrations. It was found that at each concentration, SV119-PEG-AuNCs always showed a much higher cellular uptake than PEG-AuNCs. The discrepancy was more pronounced at higher concentrations (Fig. 2C).
The photoluminescence from AuNCs provides a convenient means to evaluate their in vitro targeting capability using two-photon microscopy.12 Here MDA-MBD-435 cells were incubated with SV119-PEG-AuNCs and PEG-AuNCs, respectively, at 37 °C for 4 h in the presence of FM4-64 dye, a marker for both the cell membrane and endosome. As shown in Figure 3, the photoluminescence from AuNCs is green in color while the photoluminence from FM4-64 dye is in red. For the cells incubated with SV119-PEG-AuNCs, two-photon images revealed a strong intensity for the green color. The red color was also co-localized with the green color, implying that SV119-PEG-AuNCs bind to the sigma-2 receptors and then entered the cells. In contrast, cancer cells incubated with the PEG-AuNCs under the same condition showed limited green color, implying that very few PEG-AuNCs entered the cancer cells. This observation is consistent with the data obtained from ICP-MS analysis.
Fig. 3.
Two-photon fluorescence confocal images of MDA-MBD-435 cells after incubation with SV119-PEG-AuNCs and PEG-AuNCs, respectively, for 3 h. The green color represents the photoluminescence from the AuNCs and the red color came from the FM4-64 dye added for cell membrane and plasma staining.
We then investigated the targeting ability of SV119-functionalized AuNCs for other types of cancer cells. In this study, we found that MDA-MB-231 and PC-3 cell lines had similar levels of sigma-2 receptor expression, which were greater than that of Hela cells (a cervical cancer cell line), as demonstrated by flow cytometry using SW120 as the specific fluorescence dye (Fig. 4A). It was found that the uptake of SV119-PEG-AuNCs by cancer cells (e.g., MDA-MB-231 and PC-3 cells) expressing sigma-2 receptors was about 3 times higher than that by Hela cells (Fig. 4B). This observation suggests that the SV119-PEG-AuNCs conjugates have the capability to target different types of cancer cells.
Fig. 4.

A) Flow cytometry analyses of Hela, MDA-MB-231, and PC-3 cells after incubation with SW 120 (50 nM) for 0.5 h; (B) uptakes of SV119-PEG-AuNCs by Hela, MDA-MB-231, and PC-3 cells after incubation for 2 h.
In summary, we have developed a new class of conjugates comprised of Au nanocages and a small molecule ligand specific to sigma-2 receptors. Since sigma-2 receptors are highly expressed by various cancer cells, the conjugates provide a new platform for cancer targeting. The conjugates are suitable for use as tracers for in vitro imaging tools such as two-photon or three-photon confocal fluorescence microscopy,10 and as contrast agents for in vivo imaging modalities like photoacoustic tomography (PAT) and optical coherence tomography (OCT).10 Combined with the ability of AuNCs for loading chemotherapeutic drugs, as well as their photothermal effect, this multifunctional conjugate is potentially suitable for a range of theranostic applications.10 Although further in vivo studies are needed to investigate their targeting efficiency after systemic administration, this novel class of conjugates implies an improvement over the traditional conjugation paradigms utilizing antibodies or peptides.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the NCI (R01 CA13852701), an NIH Director’s Pioneer Award (DP1 OD000798), and startup funds from Washington University in St. Louis.
References
- 1.Petros RA, DeSimone JM. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Proc Natl Acad Sci USA. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li Z, Zhang H, Xia Y, Li X. Nano Lett. 2007;7:1318–1322. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Samori C, Ali-Boucetta H, Sainz R, Guo C, Toma FM, Fabbro C, da Ros T, Prato M, Kostarelos K, Bianco A. Chem Commun. 2009;46:1494–1496. doi: 10.1039/b923560d. [DOI] [PubMed] [Google Scholar]; (d) Huang X, Peng X, Wang Y, Wang Y, Shin DM, El-Sayed MA, Nie S. ACS Nano. 2010;4:5887–5896. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Mach RH, Wheeler KT. Cent Nerv Syst Agents Med Chem. 2009;9:230–245. doi: 10.2174/1871524910909030230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hashimoto K, Ishiwata K. Curr Pharm Des. 2006;12:3857–3876. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- 4.Zeng C, Vangveravong S, Xu J, Chang KC, Hotchkiss RS, Wheeler KT, Shen D, Zhuang ZP, Kung HF, Mach RH. Cancer Res. 2007;67:6708–6716. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- 5.Xu JB, Zeng CB, Chu WH, Pan FH, Rothfuss JM, Zhang FJ, Tu ZD, Zhou D, Zeng DX, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH. Nat Commun. 2011;2:380–386. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Hornick JR, Xu J, Vangveravong S, Tu Z, Mitchem JB, Spitzer D, Goedegebuure P, Mach RH, Hawkins WG. Mol Cancer. 2010;9:298–308. doi: 10.1186/1476-4598-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kashiwagi H, McDunn JE, Simon PO, Goedegebuure PS, Vangveravong S, Chang K, Hotchkiss RS, Mach RH, Hawkins WG. J Transl Med. 2009;7:24–31. doi: 10.1186/1479-5876-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kashiwagi H, McDunn JE, Simon PO, Goedegebuure PS, Xu J, Jones L, Chang K, Johnston F, Trinkaus K, Hotchkiss RS, Mach RH, Hawkins WG. Mol Cancer. 2007;6:48–59. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) van Waarde A, Rybczynska AA, Ramakrishnan NK, Ishiwata K, Elsinga PH, Dierckx RAJO. Curr Pharm Des. 2010;16:3519–3537. doi: 10.2174/138161210793563365. [DOI] [PubMed] [Google Scholar]; (b) Collier TL, Waterhouse RN, Kassiou M. Curr Pharm Des. 2007;13:51–72. doi: 10.2174/138161207779313740. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa O, Ming X, Huang L, Juliano RL. J Am Chem Soc. 2010;132:8848–8849. doi: 10.1021/ja102635c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashiwagi H, McDunn JE, Simon PO, Goedegebuure PS, Vangveravong S, Chang K, Hotchkiss RS, Mach RH, Hawkins WG. J Transl Med. 2009;7:24–31. doi: 10.1186/1479-5876-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK. Acc Chem Res. 2011 in press. [Google Scholar]
- 11.Skrabalak SE, Au L, Li XD, Xia Y. Nat Protoc. 2007;2:2182–2190. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]
- 12.Au L, Zheng D, Zhou F, Li Z, Li X, Xia Y. ACS Nano. 2008;2:1645–1652. doi: 10.1021/nn800370j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.