Abstract
AIM: To explore gastroenterologist perceptions towards and experience with faecal microbiota transplantation (FMT).
METHODS: A questionnaire survey consisting of 17 questions was created to assess gastroenterologists’ attitude towards and experience with FMT. This was anonymously distributed in hard copy format amongst attendees at gastroenterology meetings in Australia between October 2013 and April 2014. Basic descriptive statistical analyses were performed.
RESULTS: Fifty-two clinicians participated. Twenty one percent had previously referred patients for FMT, 8% more than once. Ninety percent would refer patients with Clostridium difficile infection (CDI) for FMT if easily available, 37% for ulcerative colitis, 13% for Crohn’s disease and 6% for irritable bowel syndrome. Six percent would not refer any indication, including recurrent CDI. Eighty-six percent would enroll patients in FMT clinical trials. Thirty-seven percent considered the optimal mode of FMT administration transcolonoscopic, 17% nasoduodenal, 13% enema and 8% oral capsule. The greatest concerns regarding FMT were: 42% lack of evidence, 12% infection risk, 10% non infectious adverse effects/lack of safety data, 10% aesthetic, 10% lack of efficacy, 4% disease exacerbation, and 2% inappropriate use; 6% had no concerns. Seventy seven percent believed there is a lack of accessibility while 52% had an interest in learning how to provide FMT. Only 6% offered FMT at their institution.
CONCLUSION: Despite general enthusiasm, most gastroenterologists have limited experience with, or access to, FMT. The greatest concerns were lack of supportive evidence and safety issues. However a significant proportion would refer indications other than CDI for FMT despite insufficient evidence. These data provide guidance on where education and training are required.
Keywords: Perceptions, Gastroenterologist, Clostridium difficile, Inflammatory bowel disease, Faecal microbiota transplantation
Core tip: This is the first study assessing the experiences, attitudes and practice of gastroenterologists towards faecal microbiota transplantation (FMT) across a range of indications other than just Clostridium difficile infection. Despite general enthusiasm, most gastroenterologists have limited experience with, or access to, FMT. Views differ widely regarding the potential therapeutic role of FMT in various gastrointestinal diseases. Major concerns include lack of evidence and safety data, infection risk, aesthetic factors and possible lack of efficacy. There is limited familiarity with the current evidence base and appropriate indications for FMT highlighting the need for education on where FMT fits in to current clinical practice.
INTRODUCTION
The last few years has seen a surge in interest in faecal microbiota transplantation (FMT)[1]. While not a new treatment, until recently it was regarded as an “alternative” therapy with little scientific basis, outside the realm of mainstream medical practice and offered by only a handful of centres worldwide. The dramatic change is largely attributable to the remarkable efficacy of FMT in recurrent Clostridium difficile infection (rCDI) at a time of a global CDI epidemic[2,3]. The cure rate of approximately 90% for FMT in rCDI[4-6] is much superior to the 20%-30% success rates associated with prolonged anti-microbial therapy.
The rapid advancements in gastrointestinal microbiota research including the work of large national and multinational collaborative projects such as the Human Microbiome Project[7,8] have further fueled interest in the role of the gastrointestinal microbiota in health and disease, and the therapeutic potential of FMT. Such research has linked gastrointestinal dysbiosis to enteric[9] conditions as varied as inflammatory bowel disease (IBD)[10,11], irritable bowel syndrome (IBS), and colorectal cancer[12], and to systemic conditions including obesity and metabolic syndrome[13,14], cardiovascular disease[15], and liver disease[16]. Clinical trials are currently underway in several of these conditions.
The role of the gastrointestinal microbiota in health and disease and the “promise” of FMT has captured the attention of patients, the general community and mainstream media. Patients are attracted to FMT as they perceive it as a “natural” and “holistic” therapy which seems safer than long term medications and their associated side effects[17]. This is despite a lack of long term safety data and initial reports of potential far reaching complications[18]. Studies have demonstrated that the aesthetics of using faecal material is not as significant a deterrent for patients as previously expected[19,20]. There appears to be patient enthusiasm to make this therapy available for a range of conditions, despite the paucity of evidence outside the setting of CDI. This is reflected in the number of patient FMT self-help and do it yourself websites and forums.
The view of gastroenterologists towards FMT is less clear. While there is increasing research in the field of FMT, this appears to be tempered by concerns about lack of efficacy and safety data, and ongoing skepticism regarding the mechanism of action of FMT therapy[20]. There are only a few reports assessing the sentiments of gastroenterologists and other physicians with regards to FMT in CDI[21,22]. To our knowledge, the perceptions of gastroenterologists towards FMT for indications other than CDI has not been assessed. This survey of Australian gastroenterologists aimed to determine the wider gastroenterology community attitudes towards, and experience with, FMT.
MATERIALS AND METHODS
A questionnaire survey was created to assess gastroenterologists’ attitude towards and experience with FMT (Table 1). It consisted of 17 questions. This was anonymously distributed in hard copy format amongst attendees at gastroenterology meetings in Australia between October 2013 and April 2014. Basic descriptive statistical analyses were performed using SPSS Statistics Version 22.0.
Table 1.
Gastroenterologist “faecal microbiota transplantation” perceptions survey
| Gastroenterologist “faecal microbiota transplantation” (fmt) perceptions survey | |||
| 1: How would you best describe yourself? (may select more than one option) | |||
| a: General Gastroenterologist | |||
| b: Hepatology subspecialist | |||
| c: Inflammatory Bowel Disease subspecialist | |||
| d: Advanced/Therapeutic endoscopy subspecialist | |||
| e: Gastroenterology trainee | |||
| f: Other; please describe in space below | |||
| 2: What is the nature of your practice/work? (may select more than one option) | |||
| a: Staff Specialist | |||
| b: Public Hospital Visiting Medical Officer | |||
| c: Private Practice | |||
| d: > 40% Medical Research | |||
| e: Other; please describe in space below | |||
| 3: Have you been consulted by a patient who has had FMT before? If yes please circle the indication for the FMT (may select more than one option) | |||
| a. No | |||
| b: Clostridium difficile | |||
| c: Ulcerative Colitis | |||
| d: Crohn’s disease | |||
| e: Irritable bowel syndrome | |||
| f: Other; please describe in space below | |||
| 4: Have you ever referred a patient for FMT before? | |||
| a: Yes – please elaborate in space below (indication, number of referrals, outcome) | |||
| b: No | |||
| 5: Please select which of the following indications, if any, you would consider referring for FMT if easily available (may select more than one option) | |||
| a: Clostridium difficile | |||
| b: Ulcerative Colitis | |||
| c: Crohn’s disease | |||
| d: Irritable bowel syndrome | |||
| e: Other; please list in space below | |||
| f: I would not consider referring for FMT for any indication | |||
| 6: If a patient saw you and expressed interest in undergoing FMT would you (you may select more than one option) | |||
| a: Advise against it | |||
| b: Remain ambivalent | |||
| c: Acknowledge their interest and refer them for FMT | |||
| d: Only refer them for FMT for the indication of recurrent Clostridium difficile | |||
| e: Suggest they only participate in clinical trials involving FMT | |||
| f: Other; please describe in space below | |||
| 7: Please select your response in answer to each of the following potential concerns with FMT | |||
| a: I don’t believe in FMT and I don’t think it is an effective therapy | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| b: While FMT may work at present there is inadequate evidence for efficacy | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| c: There is a significant infection risk from donor stool despite screening | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| d: I have other safety concerns regarding non-infectious adverse reactions with FMT | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| e: There is a risk of disease exacerbation with FMT | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| f: I don’t think my patients would contemplate or consent to FMT | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| g: “Yuck” factor (Aesthetics) | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| h: Lack of availability/accessibility to FMT | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| i: Other; please describe in space below | |||
| 8: What is your greatest concern, if any, regarding FMT? Please select only one | |||
| a: Lack of efficacy | |||
| b: Lack of evidence | |||
| c: Infection risk from donor stool despite screening | |||
| d: Non infectious adverse reaction and lack of safety data | |||
| e: Possible disease exacerbation | |||
| f: “Yuck” factor of donor stool | |||
| g: None; I have no concerns regarding FMT | |||
| h: Other; please list in space below | |||
| 9: How do you feel the potential risks of FMT compare with blood transfusion or other biologic product administration? | |||
| a: More risk with blood transfusion than FMT | |||
| b: More risk with FMT than blood transfusion | |||
| c: Not sure | |||
| d: Other; please describe in space below | |||
| 10: What do you think is the optimal modality through which to deliver FMT? | |||
| a: Transcolonoscopic | |||
| b: Enema based | |||
| c: Nasoduodenal/jejunal | |||
| d: Other; please list in space below | |||
| e: I don’t have an opinion | |||
| 11: If your patient had exhausted all other medical options and was facing surgery for refractory disease in which FMT has been suggested as a potential therapeutic option, would you consider FMT as a last resort therapy? | |||
| a: Yes | |||
| b: Yes but only for Clostridium difficile | |||
| c: Yes but only in a clinical trial | |||
| d: Not sure | |||
| e: No | |||
| f: Other; please describe in space below | |||
| 12: Do you think FMT holds promise as a potential future therapy for certain gastrointestinal diseases? | |||
| a: Yes | |||
| b: No | |||
| c: Not Sure | |||
| d: Other; please describe in space below | |||
| 13: Would you be willing to enroll your patients in clinical trials assessing FMT? | |||
| a: Yes | |||
| b: No | |||
| c: Not Sure | |||
| d: Other; please describe in space below | |||
| 14: In the next 3 yr, do you foresee a situation where you would consider referring a patient for FMT outside a clinical trial if a trusted service was available? Please select your answer for each of the following indications | |||
| a. No, I would not consider referring for FMT for any indication | |||
| b: Recurrent Clostridium difficile infection | |||
| Highly Likely | Somewhat Likely | Somewhat Unlikely | Highly unlikely |
| c: Ulcerative Colitis | |||
| Highly Likely | Somewhat Likely | Somewhat Unlikely | Highly unlikely |
| d: Crohn’s disease | |||
| Highly Likely | Somewhat Likely | Somewhat Unlikely | Highly unlikely |
| e: Irritable bowel syndrome or other functional gut disorder | |||
| Highly Likely | Somewhat Likely | Somewhat Unlikely | Highly unlikely |
| 15: With regards to FMT, please select your response to the following statements | |||
| a: I already offer FMT as a therapeutic option in my practice | |||
| b: I have an interest in learning how to process and administer FMT so that I or my institution can arrange such therapy for our patients independently | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| c: I believe a few select centres that satisfy appropriate regulatory requirements should be available in my city to offer FMT | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| d: I don’t believe the therapy should be available for routine clinical use | |||
| Strongly Disagree | Somewhat Disagree | Somewhat Agree | Strongly Agree |
| 16: After reviewing the attached FOCUS study letter of invitation, protocol summary and selection criteria | |||
| a: Are you likely to refer patients who meet selection criteria to this study? | |||
| Highly Likely | Somewhat Likely | Somewhat Unlikely | Highly unlikely |
| b: Do you have any actual patients in mind that you would consider referring to this study? | |||
| Highly Likely | Somewhat Likely | Somewhat Unlikely | Highly unlikely |
| 17: Any other comments regarding FMT that you wish to make? | |||
RESULTS
Respondents
52 clinicians participated in the survey. Subspecialty breakdown of respondents is shown in Figure 1. The general physicians included in the data set are those with dual training or a specialty interest in gastroenterology. With regards to nature of practice, 14 (27%) were public hospital staff specialists, 13 (25%) visiting medical officers, 11 (21%) solely in private practice, 10 (19%) trainee gastroenterologists in the public hospital system, 3 (6%) public hospital staff specialists with associated private practice, and 1 (2%) a predominantly research-based gastroenterologist.
Figure 1.
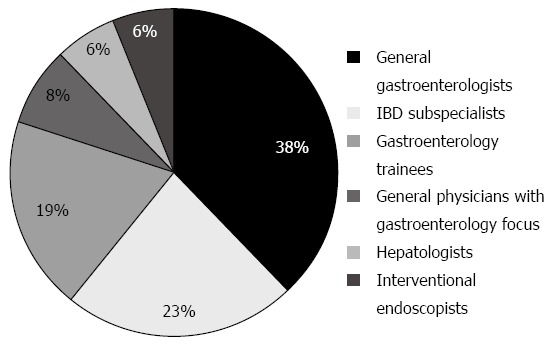
Subspecialty characteristics of respondents.
Experience with FMT
Twenty-seven respondents (52%) had never been consulted by a patient who had received FMT before. Eleven (21%) reported having referred a patient for FMT: 7 respondents (13%) had referred a patient for FMT once, 1 respondent (2%) three times, 1 respondent (2%) four times, 1 respondent (2%) six times and 1 respondent (2%) over one hundred times. Three respondents (6%) were offering FMT as a therapeutic option at their practice or institution.
Current stance on FMT indications
The current stance of respondents towards various FMT indications is shown in Figure 2. Forty-seven respondents (90%) would refer patients with CDI for FMT if it were easily available. Regarding other indications, 19 (37%) would refer patients with ulcerative colitis, 7 (13%) for Crohn’s disease and 3 (6%) for IBS. Three (6%) would not consider referring for FMT for any indication. No respondent reported that they would advise against FMT if approached by a patient interested in undergoing such treatment; 3 (6%) reported they were ambivalent, 15 (29%) stated they would acknowledge the patient’s interest and refer for FMT, 26 (50%) would only refer for FMT for the indication of rCDI while 21 (40%) would suggest patients only participate in clinical trials of FMT. Forty five respondents (86%) would be willing to enroll their patients in clinical trials assessing FMT, three (6%) were unsure and 1 (2%) was not willing [3 (6%) non respondents]. Twenty-six (50%) would consider FMT as a last resort therapy for a medical condition where FMT was speculated to have benefit if their patient had refractory disease and was facing surgery while 12 (23%) said they would only do so in the context of a clinical trial.
Figure 2.
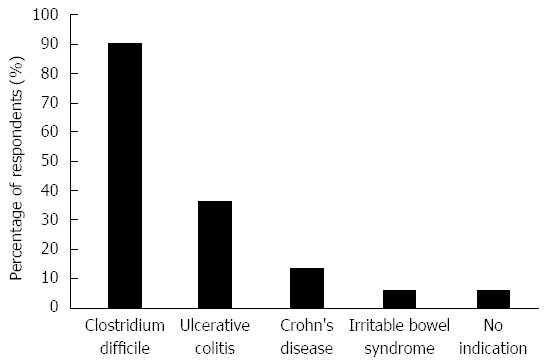
Perceived faecal microbiota transplant indications.
Perceived efficacy of FMT
Regarding the statement “I don’t believe in FMT and I don’t think it is an effective therapy”: 1 (2%) strongly agreed, 7 (14%) somewhat agreed, 20 (38%) somewhat disagreed and 22 (42%) strongly disagreed [2 (4%) non respondents]. Regarding the statement “While FMT may work at present there is inadequate evidence for efficacy”: 6 (12%) strongly agreed, 25 (48%) somewhat agreed, 13 (25%) somewhat disagreed and 6 (12%) strongly disagreed [2 (4%) non respondents].
Perceived safety of FMT
Thirteen respondents (25%) somewhat agreed that there was a significant infection risk from donor stool despite screening, while 27 (52%) somewhat disagreed and 10 (19%) strongly disagreed [2 (4%) non respondents]. Regarding safety concerns pertaining to non infectious adverse reactions with FMT, 1 (2%) strongly agreed, 18 (34%) somewhat agreed, 26 (50%) somewhat disagreed and 6 (12%) strongly disagreed [1 (2%) non respondents]. 21 respondents (40%) somewhat agreed there was a risk of disease exacerbation with FMT, 26 (50%) somewhat disagreed and 3 (6%) strongly disagreed [2 (4%) non respondents]. Twenty four respondents (46%) felt the potential risks of FMT were less than for a blood transfusion or other biologic product administration, 24 (46%) were unsure and 2 (4%) felt FMT was more risky than a blood transfusion or other biologic product administration [2 (4%) non respondents].
Perceived patient acceptance of FMT
One respondent (2%) strongly believed that their patients would not contemplate or consent to FMT, 13 (25%) somewhat agreed, 28 (54%) somewhat disagreed and 9 (17%) strongly disagreed [1 (2%) non respondent]. Nine respondents (17%) strongly believed their patients would be put off by the aesthetics of FMT, 24 (46%) somewhat agreed, 12 (23%) somewhat disagreed while 6 (12%) strongly disagreed [1 (2%) non respondent].
Greatest concerns regarding FMT
The greatest concerns regarding FMT are shown in Figure 3. Lack of evidence was the most commonly cited concern (42%) with safety/adverse events (infectious and non infectious), lack of efficacy and aesthetic factors also reported frequently.
Figure 3.
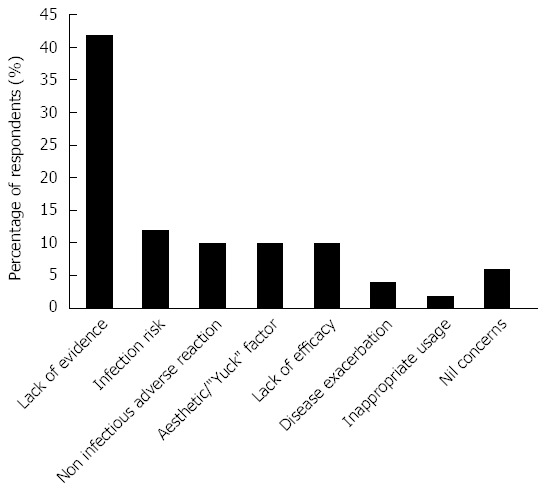
Greatest concerns about faecal microbiota transplant.
Availability and accessibility of FMT
Seventy-seven percent of respondents agreed that there is a lack of availability or accessibility to FMT. Fifty two percent had an interest in learning how to process and administer FMT so their institution could offer the therapy. Seventy-nine percent agreed (35% strongly agreed, 44% somewhat agreed) with the statement that a few centres that satisfy appropriate regulatory requirements should be available in any area or region to offer FMT. Regarding the statement that FMT should not be available for routine clinical use, 3 (6%) strongly agreed, 14 (27%) somewhat agreed, 22 (42%) somewhat disagreed and 8 (15%) strongly disagreed [5 (10%) non respondents].
Route of administration and future of FMT
Figure 4 shows the perceived optimal modality of FMT administration with the transcolonoscopic route most popular (37%) followed by nasoduodenal (17%), while a significant proportion had no opinion. When asked if they thought FMT held promise as a future therapy for certain gastrointestinal diseases, 77% said yes, 15% were unsure and 4% said no. (4% non respondent). When asked whether in the next 3 years they could foresee referring for FMT outside a clinical trial if a trusted service was available, none stated no for all indications, 60% said highly likely and 29% somewhat likely for rCDI, 13% said highly likely and 50% somewhat likely for UC, 4% said highly likely and 44% somewhat likely for Crohn’s disease, 31% said somewhat likely, 33% somewhat unlikely and 31% said highly unlikely for IBS.
Figure 4.
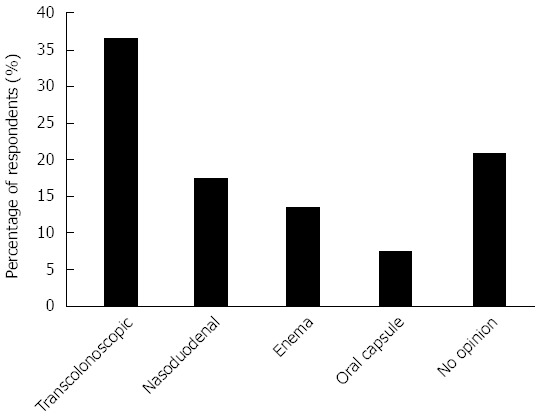
Optimal modality of administration.
DISCUSSION
To our knowledge this is the first report assessing the perception and practice of gastroenterologists towards FMT across a range of indications other than rCDI. This study suggests that views vary widely amongst gastroenterologists regarding the role of FMT. Despite general enthusiasm, experience with FMT remains limited and lack of accessibility appears to be a contributing factor.
The most commonly reported concern by gastroenterologists regarding FMT was the lack of evidence about efficacy. Almost 60% felt that while FMT may be effective, at present there is inadequate supportive evidence and this was the major concern cited by almost half of respondents. However at the same time, despite a limited evidence base many gastroenterologists advocated FMT for indications other than rCDI. Over a third reported they would refer their UC patients for FMT if easily available, 10% would refer Crohn’s disease, and 6% for IBS. Almost a third were happy to refer patients with non CDI indications for FMT outside a clinical trial setting. At the other extreme, around 15% did not believe FMT was an effective therapy and a small proportion would not refer any patient for FMT, even in the setting of CDI, despite the growing body of evidence demonstrating efficacy and short term safety of this therapy in a condition with significant morbidity and mortality.
The majority of surveyed gastroenterologists did not express reservations regarding the safety of FMT from infection transmission, other non infectious adverse events or disease exacerbation, despite relatively limited short term data and negligible long term data.
Almost three quarters of gastroenterologists surveyed believed their patients would contemplate or consent to FMT, though almost two thirds believed they would be concerned by the aesthetic factor. Published studies on patient perception towards FMT have found a majority would consider such therapy and that the aesthetic factor is not a major issue, suggesting the perception of gastroenterologists are only partly consistent with those of their patients[17,19]. However there appears to be improvement in gastroenterologist awareness of patient attitudes towards FMT compared to the earliest report assessing gastroenterologist perceptions of FMT in which 71% cited lack of patient acceptance and tolerability as the main barrier to FMT for CDI[21].
Half the respondents felt a lower gastrointestinal route was the optimal mode of FMT administration, with only a quarter advocating an upper gastrointestinal route and the remainder not having an opinion. These findings may be influenced to some degree by the ease of endoscopic access and administration available to gastroenterologists. A small number volunteered that oral capsule would be the optimal method despite this not being listed as a pre-specified choice on the questionnaire and minimal publications at the time of survey distribution reporting its use; the evidence for such a mode of delivery in rCDI is only just appearing in clinical trials.
Over three quarters of respondent gastroenterologists believe FMT holds promise as a potential therapy for certain gastrointestinal diseases, and would be willing to enroll their patients in FMT clinical trials. In the next 3 years, the majority expected they would be referring patients for FMT outside a clinical trial setting for both rCDI and UC if a trusted service was available, almost 50% for Crohn’s disease and one third for IBS. This represents a significant shift in the last few years from when less than half of respondent gastroenterologists would consider FMT in the setting of CDI[21], despite arguably more convincing evidence at that stage for FMT in CDI than currently exists for FMT in non CDI settings.
A limitation of this study is the relatively small total respondent number. Furthermore, it was not possible to determine the response rate as the method of survey distribution involved circulating hard copies of the questionnaire at gastroenterology meetings rather than formal mailbox or email distribution. Finally, all respondents were Australian gastroenterologists, the majority from Sydney, potentially limiting the generalisability of the responses.
This study is the first report of gastroenterologist practice and perceptions regarding the use of FMT to include indications beyond rCDI. It highlights that while there is a large degree of interest in FMT amongst the profession, experience remains limited and opinions conflicting regarding its therapeutic potential and safety, sometimes inconsistent with the current medical evidence base. It indicates areas of educational need, and the need to address patients’ expectations.
COMMENTS
Background
Faecal microbiota transplantation (FMT) has attracted substantial interest over recent years from researchers, clinicians, patients and mainstream media due to its extraordinary efficacy in the treatment of recurrent Clostridium difficile infection (rCDI), a condition with significant morbidity and mortality. As a result, there is growing interest in exploring the potential for FMT in the treatment of other disease states where pathogenesis is presumed to be secondary to dysbiosis. However concerns have been raised about the lack of efficacy and safety data along with limited accessibility and experience outside specialized centres.
Research frontiers
While interest in FMT is growing, controversy exists regarding potential indications, efficacy and safety for FMT. While patient perceptions of FMT have been reported and suggest widespread interest and enthusiasm, the overall opinions and experience of gastroenterologists related to FMT are not clear and have not been studied for conditions other than just CDI. The research hotspot this study addresses is to explore gastroenterologist attitudes towards, and experience with, FMT in general.
Innovations and breakthroughs
In recent years, uncontrolled and controlled studies have demonstrated that FMT is highly effective in the treatment of CDI. However data is still lacking regarding long term safety and non infectious adverse events. Controlled efficacy data for other potential indications are required though several clinical trials are currently underway.
Applications
This study suggests that while there is general interest in FMT, experience and accessibility are major limiting factors for most gastroenterologists that need to be addressed. Knowledge of current evidence based indications was suboptimal suggesting the need for further education and training. The greatest concerns were lack of supportive evidence and safety issues, highlighting areas for future research.
Terminology
FMT involves the transfer of faecal material (and associated microbiota) from a healthy donor to a recipient for the purpose of treating an underlying disease. The mechanism of action is generally believed to be via correction of underlying disease dysbiosis. Dysbiosis is a disturbance in the natural balance of the microbial ecology of a part of the body.
Peer-review
An informative paper, suitable for educational purposes and with potential to be of general interest because the topic is controversial and current.
Footnotes
Supported by The Clinical trial (ClinicalTrials.gov Identifier: NCT01896635) this study was performed in conjunction with has received funding from the Broad Medical Research Program at CCFA and the GESA IBD Clinical Research Grant.
Institutional review board statement: The clinical trial (ClinicalTrials.gov Identifier: NCT01896635) this study (involving a voluntary anonymous survey of gastroenterology colleagues) was performed in conjunction with was reviewed and approved by the St Vincent’s Hospital Human Research Ethical Committee.
Informed consent statement: All respondents provided written informed consent acknowledged by completion and return of the anonymous survey.
Conflict-of-interest statement: Paramsothy S, Walsh AJ, Samuel D, van den Bogaerde J, Leong RWL, Connor S, Ng W, Mitchell HM, Kaakoush NO and Kamm MA have no financial or potential competing interest or affiliation with any institution, organization, or company relating to the manuscript. Borody T has an interest in the Centre for Digestive Diseases, where faecal microbiota transplantation is a treatment option for patients and has filed patents in this field.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 31, 2015
First decision: June 19, 2015
Article in press: August 31, 2015
P- Reviewer: Zuluaga AF S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Smith MB, Kelly C, Alm EJ. Policy: How to regulate faecal transplants. Nature. 2014;506:290–291. doi: 10.1038/506290a. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 4.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 5.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 6.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 12.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 14.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn SA, Vachon A, Rodriquez D, Goeppinger SR, Surma B, Marks J, Rubin DT. Patient perceptions of fecal microbiota transplantation for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1506–1513. doi: 10.1097/MIB.0b013e318281f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55:1652–1658. doi: 10.1093/cid/cis809. [DOI] [PubMed] [Google Scholar]
- 20.Brandt LJ. Editorial commentary: fecal microbiota transplantation: patient and physician attitudes. Clin Infect Dis. 2012;55:1659–1660. doi: 10.1093/cid/cis812. [DOI] [PubMed] [Google Scholar]
- 21.Kelly C, de Leon L, Kerstetter D, Okpara N. Barriers to Greater Utilization of Fecal Bacteriotherapy for Chronic Clostridium difficile Infection (abstract). American College of Gastroenterology annual meeting; San Antonio, Texas; 15-20 October. 2010. [Google Scholar]
- 22.Sofi AA, Georgescu C, Sodeman T, Nawras A. Physician outlook toward fecal microbiota transplantation in the treatment of Clostridium difficile infection. Am J Gastroenterol. 2013;108:1661–1662. doi: 10.1038/ajg.2013.207. [DOI] [PubMed] [Google Scholar]


