Summary
Single-agent post-autologous transplant maintenance therapy with lenalidomide is standard of care for patients with multiple myeloma. The tolerability and effectiveness of combination post-transplant maintenance therapy is unknown, so we investigated lenalidomide and vorinostat (suberoylanilide hydroxamic acid) in this setting, hypothesizing that the regimen would be well tolerated and associated with an improved post-transplant response. This trial followed a standard 3 × 3 dose escalation phase 1 design. Vorinostat was administered beginning day +90 post-haematopoietic stem cell transplantation for days 1–7 and 15–21, and lenalidomide was started at 10 mg days 1–21, both on a 28-d cycle. The primary endpoint was maximum tolerated dose and dose limiting toxicities were assessed during the first cycle. Treatment was well tolerated in 16 enrolled patients. During Cycle 1, the most common toxicities included cytopenias, gastrointestinal complaints and fatigue. Seven patients improved their transplant response after starting combination therapy. The median follow-up was 38·4 months, and the median progression-free survival and overall survival have yet to be reached. This oral post-transplant maintenance regimen was well tolerated. This is the first trial to publish results on the use of a histone deacetylase inhibitor in the maintenance setting, and it provides rationale for the ongoing randomized trial in maintenance (ISRCTN 49407852).
Trial Registration: NCT00729118
Keywords: myeloma, autologous transplant, deacetylase inhibition, immunomodulatory agent
In transplant-eligible multiple myeloma (MM) patients, maintenance therapy with lenalidomide following autologous haematopoietic stem cell transplantation (ASCT) is associated with increased time to disease progression and improved overall survival (OS), and is currently standard of care (McCarthy et al, 2012). Effective and tolerable maintenance drug combinations may further prolong the clinical response achieved from ASCT. Numerous studies are currently underway investigating the role of proteasome inhibitors and other novel agents in this therapeutic setting. Histone deacetylase inhibitors (HDAC-I) are associated with anti-myeloma activity, and represent a promising combination approach for maintenance therapy.
In recent years, the importance of epigenetic modification in the initiation, proliferation, survival and progression of tumour cells has risen to the forefront (Herman & Baylin, 2003). A novel class of agents, the HDAC-I’s, inhibit histone deacetylase (HDAC) enzymes, effectively targeting epigenetic silencing mechanism(s) that can reverse crucial steps involved in carcinogenesis. This drug class also renders tumour cells more susceptible to immune-mediated killing (Skov et al, 2005; Gialitakis et al, 2006), and induces an anti-inflammatory effect via inhibition of cytokine release that may disrupt the tumour microenvironment (Leoni et al, 2002).
Vorinostat (suberoylanilide hydroxamic acid, SAHA), a nonspecific oral HDAC-I, was approved by the US Food and Drug Administration (FDA) in 2006 for the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma (Duvic et al, 2006, 2007). Vorinostat induces numerous anti-proliferative and pro-apoptotic effects in MM cells (Mitsiades et al, 2004), and has been investigated in myeloma patients in numerous clinical trials (Table SI). Phase 1 investigation of single agent vorinostat in relapsed/ refractory myeloma patients confirmed single agent tolerability (Richardson et al, 2008). Numerous other early phase trials have investigated vorinostat with bortezomib and have confirmed the tolerability of this combination, albeit at the cost of increased toxicity (Badros et al, 2009; Siegel et al, 2011; Weber et al, 2012; Dimopoulos et al, 2013; Wider et al, 2013). The randomized, placebo-controlled phase 3 VANTAGE (Vorinostat Clinical Trials in Haematological and Solid Malignancies) 088 trial demonstrated a significant increase in median progression-free survival (PFS) with this combination (Dimopoulos et al, 2013). When used in combination with lenalidomide and dexamethasone in relapsed patients, the addition of vorinostat was well tolerated and associated with stable disease (SD) in approximately 90% of patients (Siegel et al, 2014). Further, the three-drug regimen including bortezomib, lenalidomide and vorinostat in newly diagnosed patients was well tolerated and associated with complete response (CR) in three, very good partial response (VGPR) in one and partial response (PR) in four of the eight evaluable patients (Kaufman et al, 2010). In the relapsed/refractory setting, an overall response rate of 43% was noted and of the nine enrolled patients, 89% had a MR or better (Siegel et al, 2010). Collectively, these data indicate that vorinostat is relatively well tolerated and more efficacious when used in combination with other agents, including both proteasome inhibitors and immunomodulatory agents.
Given two randomized studies investigating lenalidomide monotherapy following ASCT (Attal et al, 2012; McCarthy et al, 2012), we hypothesized that combining vorinostat with lenalidomide in the maintenance setting would be safe and augment clinical response. We are the first to describe the use of vorinostat with lenalidomide in the post-autologous transplant maintenance setting, and our data sets the stage for further evaluation of this combination in a randomized trial.
Methods
Clinical trial
This trial was a 3 + 3 phase 1 trial that followed the standard rules for dose escalation. The study protocol was approved by the Ohio State University Cancer Institutional Review Board. Patients were treated per institutional protocol with melphalan 200 mg/m2 followed by ASCT on day 0. Study treatment began day +90 ±6 days post-transplant and included vorinostat given orally for days 1–7 and 15–21 of a 28-d cycle, starting at a dose of 200 mg and escalating to a maximum of 400 mg, and lenalidomide on days 1–21 of a 28-d cycle, starting at a dose of 10 mg with escalation after Cycle 1 in 5 mg increments to a maximum of 25 mg. Within 7 d of administration of the first dose of vorinostat, patients were required to have adequate organ function, including an absolute neutrophil count >1·0 × 109/l, platelet count >75 × 109/l, total bilirubin, aspartate transaminase and ala-nine transaminase <2 times the institutional upper limit of normal, and serum creatinine ≤1·5 times the institutional upper limit of normal or a measured creatinine clearance ≤50 ml/min. Patients continued study treatment at the physician’s discretion until death, disease progression, unacceptable toxicity, patient refusal or treatment delay greater than 8 weeks. Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_Quick-Reference_8.5x11.pdf).
The primary endpoint of this trial was toxicity and any patient who received study treatment was included. Response was assessed as the proportion of patients who responded divided by the total number of patients who received any treatment on study. PFS and OS were assessed for patients across all dose levels. PFS was defined as the time from study entry to the time of progression and/or death, and OS was defined as the time from study entry to death due to any cause, censoring those who were still alive at the end of the study. Both of these time-to-event outcomes were characterized using Kaplan–Meier methods.
Patient reported outcomes
Quality of life (QoL) correlative studies were obtained on Cycle 1 Day 1 (C1D1), C1D15, C2D1, C3D1, C4D1 and off-study, and included patient responses to The Center for Epidemiologic Studies Depression Scale (CES-D-10; Irwin et al, 1999), nine-item Brief Fatigue Inventory (BFI; Mendoza et al, 1999), and the Functional Assessment of Cancer Therapy-General (FACT-G, v4.0; Cella et al, 1993). Both descriptive statistics and graphical analyses were performed to track the changes over time in these QoL scores. The changes were also quantitatively modelled using linear mixed models with repeated measures while controlling for dose level. All P-values are reported based on two-sided tests, where P < 0·05 was used to determine statistical significance.
Flow cytometry
Detailed immunophenotypic evaluation of subsets and activation status of B, T and natural killer (NK) cells and enumeration of T regulatory cells were performed in the Clinical Flow Cytometry Laboratory using a whole blood staining method with panels of directly conjugated antibodies. These analyses were conducted from samples taken on C1D1, C1D15, Day 1 of Cycles 2, 3, 4 and following trial discontinuation. Comparisons of peripheral blood immune markers by flow cytometry were done using both the nonparametric Wilcoxon signed rank sum test and linear mixed models with repeated measures.
Results
Patients
Sixteen patients with newly diagnosed MM were enrolled and demographics are summarized in Table I. Patients had a median age of 58 years (range 41–67) and 10 of the patients were male. All patients were non-Hispanic and 12 self-identified as Caucasian, two as Black/African American, and one patient each identified themselves as Caucasian and Native American, and Caucasian and African American. Three patients had disease characterized by a heavy chain monoclonal protein, seven had light chain only disease and six had both heavy and light chain disease. Of note, the dominant measurable myeloma protein was followed for assessment of response and progression. Thirteen patients had bony lytic lesions at the time of diagnosis, one patient had renal involvement only and one had anaemia only. CD138+ selected fluorescent in-situ hybridization (FISH) was not completed in four patients due to insufficient samples. In those with adequate samples for cytogenetic analyses, three patients each had normal, complex karyotype or hyperdiploid FISH, one had t(11;14) and another had t(4;14) with deletion of chromosome 17p.
Table I.
Patient characteristics.
| ID | Gender | Age (years) | Race | ISS stage | Myeloma | CRAB | Karyotype/cytogenetics at diagnosis | Prior therapies | Dose level | Best response |
|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 61 | AA | 1 | IgG/KLC | Calcium Bone | Trisomy 3, 7, 9, 10 | Rd, ASCT | 1 | sCR |
| B | F | 67 | W | 1 | KLC | Bone | NL | Rd, Vd, ASCT | 1 | sCR |
| C | M | 44 | AA, W | 1 | LLC | Bone | Trisomy 5, 11, 19 | Vd, Rd, ASCT | 1 | PR |
| D | M | 61 | W | 1 | IgG/KLC | Bone | Trisomy 5, 11, 19, t(11;14) | Vd, VRd, ASCT | 2 | VGPR |
| E | M | 65 | W | 2 | IgA/LLC | Anaemia Bone | Complex | Rd, VRd, ASCT | 2 | VGPR |
| F | M | 56 | N, W | 2 | IgG/LLC | Anaemia Bone | NL | VRd, ASCT | 2 | sCR |
| G | M | 52 | W | 3 | IgG-K | Anaemia Bone | NA | Vd, ASCT | 3 | VGPR |
| H | M | 53 | W | 1 | IgG/LLC | Anaemia | Monosomy 4, 13, 14 | Vd, ASCT | 3 | CR |
| I | F | 59 | W | 2 | IgA/LLC | Bone | Complex | Rd, ASCT | 3 | CR |
| J | F | 48 | W | 3 | LLC | Renal Anaemia | NA | Vd, Rd, Cy/d, Cy mob, ASCT | 3 | PR |
| K | M | 66 | W | 1 | IgA-K | Bone | NA | Vd, ASCT | 3 | CR |
| L | M | 48 | AA | 1 | KLC | Bone | NA | ASCT | 3 | VGPR |
| M | F | 41 | W | NA | KLC | Bone | NL | High Dose Steroids, ASCT | 3 | sCR |
| N | F | 58 | W | 3 | IgA-K | Anaemia Bone | del 17p, t(4;14) | Vd, MPV, Anti-Kir, Rd, Pd, Cy mob, ASCT | 3 | VGPR |
| O | M | 65 | W | 1 | KLC | Renal | Complex | Vd, ASCT | 3 | VGPR |
| P | F | 58 | W | 1 | LLC | Bone | t(11;14) | Rd, VRd, ASCT | 3 | VGPR |
A total of 16 multiple myeloma patients were evaluable for analysis (patients A–P). Ten of the patients were male (M) and six were female (F). The treated population was predominantly Caucasian (W), but two patients were African American (AA), and one patient each identified themselves as W and Native American (N), and W and AA. The median International Staging System (ISS) stage was 1 (range 1–3). Seven patients had light chain disease only, four patients had IgA disease and two of these patients had an additional light chain clone, six total patients had disease characterized by two primary clones and three patients had heavy chain disease only (LLC, lambda light chain; KLC, Kappa light chain). CRAB [calcium (elevated), renal failure, anaemia, bone lesions]: Thirteen patients had bony involvement at the time of diagnosis and four of these patients had concomitant anaemia. Two patients had renal involvement and one of these patients had anaemia. Of those patients with available samples from diagnostic bone marrow aspirate, one patient had deletion of chromosome 17p and three others had complex features (NA, not available). Four of the patients received three-drug induction therapy with Velcade (V), Revlimid (R) and dexamethasone (d), one patient received no induction therapy, one was treated with high-dose steroid only, and two patients received alkylator based regimens [cyclophosphamide (Cy) and melphalan (M)]. Three patients were treated with dose level 1, three at dose level 2, and 10 at dose level 3 (mob, mobilization; MPV, melphalan, prednisone, velcade; Pd, pomalidomide; ASCT, autologous stem cell transplantation). Of the best responses following transplant, one patient had stable disease (SD), two had partial response (PR), six with very good partial response (VGPR), three with complete response and four with stringent complete response (sCR). Normal (NL) is defined as normal karyotype and no positive fluorescent in situ hybridization (FISH) probes.
Patients were accrued across three dose levels (Table II). Three patients were treated at dose level 1 (vorinostat 200 mg and lenalidomide 10 mg), three at dose level 2 (vorinostat 300 mg and lenalidomide 10 mg), and 10 at dose level 3 (vorinostat 400 mg and lenalidomide 10 mg). Fifteen patients received more than one cycle of therapy. Five patients remain on study, five were removed from the study due to treatment-related toxicities, and six were removed from the study due to progressive disease, two of whom have since expired. Of the patients that discontinued the trial due to toxicities, four remain off treatment and without evidence of disease progression, while the fifth continues 5 mg lenalidomide on days 1–21 of a 28-d cycle.
Table II.
28-d cycle oral dosing regimen.
| Dose level | Vorinostat Days 1–7, 15–21 | Lenalidomide Days 1–21 |
|---|---|---|
| 1 | 200 mg/d | 10 mg/d |
| 2 | 300 mg/d | 10 mg/d |
| 3 | 400 mg/d | 10 mg/d |
All three doses levels included starting doses of lenalidomide at 10 mg by mouth daily. Dose level 1 included a starting dose of vorinostat 200 mg PO days 1–7 and 15–21. Dose levels 2 and 3 included vorinostat on the same schedule but with 300 and 400 mg daily, respectively.
Dose modifications
Twelve months after starting study treatment, the median dose of lenalidomide was 5 mg and vorinostat was 200 mg (Table III). Approximately 6 months after starting the study treatment, eight patients were treated with lenalidomide doses higher than the 10 mg starting dose (range 15–25). At 12 months, five of these patients remained on lenalidomide doses >10 mg. In total, five patients had lenalidomide dose escalations to 25 mg, and only one patient remained on this dose at 12 months. No patient enrolled at dose level 1 required a dose reduction of vorinostat. One patient enrolled at dose level 2 had evidence of disease progression following Cycle 3. Of the remaining two patients, both required initial dose reductions to 200 mg of vorinostat in Cycle 4 and 5 respectively and, during Cycle 11, both were dose-reduced to 100 mg. Two patients treated at dose level 3 progressed before 12 months (Cycles 5 and 9, respectively). Of the remaining eight patients, half required no vorinostat dose reductions at 12 months. Of those that had a dose reduction, two patients were reduced to 300 mg, 1–200 mg and 1–100 mg. Of those four patients not requiring vorinostat dose reductions, all reached the maximum 25 mg lenalidomide dose by Cycle 5, three of which remained at doses >10 mg at 12 months. Half of the patients in this cohort were treated with 5 mg lenalidomide at 12 months.
Table III.
Vorinostat and lenalidomide dosing in evaluable patients.
| Vorinostat dose (mg)
|
Lenalidomide dose (mg)
|
Time to first dose reduction (cycle)
|
|||||
|---|---|---|---|---|---|---|---|
| ID | Starting | Ending | Starting | Max | Ending | Vorinostat | Lenalidomide |
| A | 200 | 200 | 10 | 25 | 5 | NA | 6 |
| B | 200 | 200 | 10 | 10 | 5 | NA | 7 |
| C | 200 | 200 | 10 | 20 | 10 | NA | 4 |
| D | 300 | 300 | 10 | 10 | 10 | NA | NA |
| E | 300 | 100 | 10 | 20 | 5 | 3 | 3 |
| F | 300 | 100 | 10 | 10 | 5 | 6 | 1 |
| G | 400 | 300 | 10 | 15 | 5 | 9 | 5 |
| H | 400 | 400 | 10 | 25 | 20 | NA | 7 |
| I | 400 | 300 | 10 | 25 | 5 | 17 | 5 |
| J | 400 | 300 | 10 | 10 | 5 | 1 | 1 |
| K | 400 | 400 | 10 | 25 | 25 | NA | NA |
| L | 400 | 400 | 10 | 25 | 15 | NA | 5 |
| M | 400 | 100 | 10 | 10 | 5 | 1 | 1 |
| N | 400 | 400 | 10 | 20 | 5 | NA | 3 |
| O | 400 | 300 | 10 | 20 | 15 | 2 | 12 |
| P | 400 | 200 | 10 | 15 | 5 | 3 | 2 |
Vorinostat and lenalidomide dose escalations and reductions are listed by patient. ID, patient identification; Max, maximum; NA, not applicable.
Toxicities
Of the adverse events that were possibly, probably or definitely related to therapy that occurred during the first year of trial participation, the most common toxicities were neutropenia (14·4% of total patients), fatigue (13·5%), leucopenia (12·7%), thrombocytopenia (11·9%), lymphopenia (11·0%) diarrhoea (9·3%), anaemia (8·5%), hypokalaemia (7·6%), rash (5·9%) and nausea (5·1%). In total, only two patients (12·5%) had grade 2 or lower adverse events, while all others had one or more grade 3 toxicity throughout their treatment course. Grade 4 toxicities include one patient each with neutropenia and thrombocytopenia. Grade 3 toxicities included neutropenia (n = 7), thrombocytopenia (n = 4), leucopenia (n = 2), fatigue (n = 1), diarrhoea (n = 1) and hypokalaemia (n = 1). As shown in, the median time to the first grade 3 or higher event was 172 d (Fig S1).
During Cycle 1, cytopenias (n = 20), gastrointestinal complaints (n = 9) and fatigue (n = 4) were the most common toxicities. Of note, there were no grade 4 events during Cycle 1. Grade 3 adverse events included one patient each with neutropenia, fatigue and hypokalaemia, grade 2 events included nausea (n = 2), emesis (n = 2), lymphopenia (n = 2) and one patient each with neutropenia, diarrhoea and fatigue. All other Cycle 1 events were grade 1 (Fig 1).
Fig 1.
Ten worst grade adverse events occurring during Cycle 1. Treatment was generally well tolerated and no grade 4 events were experienced by any patient. Grade 3 events included neutropenia, fatigue and hypokalaemia. Grade 2 events included neutropenia, nausea, diarrhoea, fatigue, lymphopenia and emesis. The most frequently experienced grade 1 events included cytopenias (neutropenia, leucopenia and thrombocytopenia), nausea, diarrhoea and hypokalaemia.
Patient report outcomes
Analysis of the QoL from the start of therapy through the first three cycles found no significant change in the BFI, FACT-G and CES-D (P = 0·425, P = 0·140, P = 0·897, respectively) (Table IV). CES-D indicated that patients did not have significantly worse depressive scores between the start of therapy (day +90) and the end of the trial. Likewise, the FACT-G functional well-being tool revealed relatively stable functional status, and patients did not have significant changes in fatigue over the course of treatment. Additionally, descriptive statistics showed that fatigue, functional well-being, and the CES-D depression scale did not appear to differ significantly between dose levels, however, we recognize the limitations of strict comparisons between dose levels given the limited numbers of patients involved.
Table IV.
Quality of life indices.
| BFI
|
FACT-G FWB
|
CES-D
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Quality of life (Timepoint) | X̄ (SE) | P | X̄ (SE) | P | X̄ (SE) | P | |||
| C1D1 (+90) | 2·7 (0·54) |
|
0·425 | 2·3 (0·20) |
|
0·140 | 7·6 (1·22) |
|
0· (897) |
| C1D15 (+105) | 3·2 (0·62) | 2·5 (0·20) | 7·5 (1·64) | ||||||
| C2D1 (+120) | 2·4 (0·58) | 2·6 (0·20) | 7·4 (1·84) | ||||||
| C3D1 (+150) | 3·0 (0·66) | 2·5 (0·21) | 7·9 (1·55) | ||||||
| C4D1 (+180) | 2·5 (0·52) | 2·6 (0·18) | 6·8 (1·39) | ||||||
| EOT | 3·2 (1·70) | 2·7 (0·52) | 9·5 (4·17) | ||||||
Quality of life was measured by the Brief Fatigue Inventory (BFI), Functional Assessment of Cancer Therapy-General (FACT-G), and the Center for Epidemiologic Studies Depression Scale (CES-D) at the initiation of study treatment (Cycle 1, Day 1; C1D1), Day 15 of Cycle 1 (C1D15), Day 1 of each successive cycle from Cycles 2 to 4, and at the end of the trial (EOT). Though there was variation in all three quality of life indices over the course of treatment, there were no significant changes in the quality of life for patients over the course of treatment.
Flow cytometry
At days +56–60 post-transplant (n = 16), prior to starting combination vorinostat and lenalidomide maintenance therapy, the median percentage of leucocyte subsets was quantified. These patients were generally CD19+ B cell depleted (17·9%). The majority of leucocytes were CD4+ and CD8+ T cells (67·9%) of which most were CD8+ T cells (43·4%). The other predominant cell subset was NK cells (CD3−/CD16+/ CD56+), which made up 15·1% of the total cell population.
Flow cytometric analyses were then obtained on post-transplant days +120 and +150, corresponding to the beginning of Cycles 2 and 3 following treatment with vorinostat and lenalidomide. There was a significant increase in NK-and CD4+ T-cells, and significant decrease in CD19+ B-cells between days +54–66 and +150 (P = 0·03, 0·0001 and 0·003, respectively) (Table SII; Fig S2). There was no significant change between these same time points for CD8+ T-cells.
Response
Following transplant (day +90) and before starting the study maintenance regimen, three patients had SD, two had PR, seven had VGPR, two had CR and two had stringent CR (sCR) (Fig 2). Seven patients had improved response following the initiation of study treatment, and this improvement was noted in ≤5 cycles in four of these patients. The best improvement in response was SD to sCR, but two patients were noted to improve from PR to CR. The changes in the dominant monoclonal protein for each patient are displayed in Fig 3. Of note, in those patients with IgA disease, the total IgA was followed for response and progression.
Fig 2.
Changes in response from transplant to trial discontinuation. Seven of the evaluable 16 patients had an improvement of response following treatment. Two patients improved from stable disease (SD) to partial response (PR), one patient improved from SD to stringent complete response (sCR), two patients improved from PR to very good partial response (VGPR), one patient improved from VGPR to complete response and one patient improved from VGPR to sCR. The remaining nine patients did not have an improvement in response, but all had VGPR or better following autologous stem cell transplantation.
*Deceased patients.
Fig 3.

Changes in monoclonal protein from before transplant to the end of treatment. The predominant clone for each patient is depicted over time from prior to transplant to screening and through the duration of treatment. 100% represents the pre-transplant baseline and each subsequent data point is the monoclonal protein at a specific time point divided by this baseline value. For example, if a patient had a 50% decrease of the monoclonal protein from the pre-transplant baseline, this would be represented as a monoclonal protein of 50%, and if a patient had evidence of no monoclonal spike, then this would be depicted as 0%. Six patients continue therapy as of 1 September 2014 (dark solid lines). Four patients discontinued study treatment due to toxicities (light solid line) and six patients were found to have disease progression (dotted line). Although patient M’s monoclonal protein more than doubled by Cycle 14, the patient did not meet criteria for progressive disease. C, cycle; D, day (e.g., C1D1 = Cycle 1, Day 1)
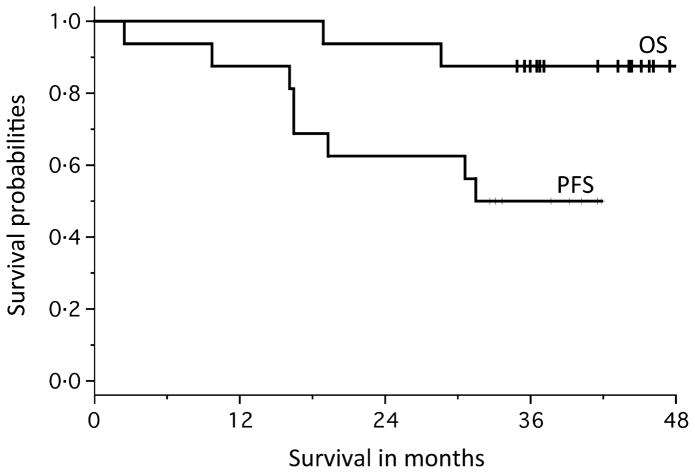
The median follow-up was 38·4 months (range 31·6–43·1 months). The median PFS and OS have not yet been met (Fig 4). Six patients remain on trial and have been treated for an average of 36 cycles. Two patients have died due to disease progression, and both patients (G and J) had evidence in improvement in response following the initiation of combination maintenance therapy (SD to PR and PR to VGPR). Three patients discontinued the trial due to progressive disease and remain alive (Patients A, N, P). One patient was taken off treatment due to toxic megacolon that was not attributable the study treatment, but rather to transplant. Four other patients were taken off treatment for gastrointestinal intolerance (n = 2) and cytopenias (n = 2); all but one of these remain off-treatment without disease progression.
Fig 4.
Progression-free and overall survival. At the time of analysis (1 September 2014), median progression-free survival (PFS) and overall survival (OS) had not been reached (median days on treatment = 965).
Discussion
In this phase 1 dose escalation trial of maintenance oral lenalidomide and vorinostat, the maximum administered doses were 400 mg vorinostat, days 1–7 and 15–21 and 25 mg le-nalidomide, days 1–21, each of a 28-d cycle. No dose limiting toxicities occurred, however, 60% of the patients in the final cohort required dose reduction of vorinostat and lenalidomide due to onset of adverse events. Of those patients that required dose reductions, the median time to regimen augmentation was 3 (range 1–17) and 4·5 (range 1–12) cycles for vorinostat and lenalidomide, respectively. While most patients (73%) tolerated dose escalation of lenalidomide over their course of treatment, 36% reached the 25 mg dose, but 91% required dose reduction primarily due to neutropenia. Considering that the majority of patients required dose reductions and that the median dose of vorinostat and lenalidomide at 1 year were 200 mg and 5 mg, respectively, it is likely that the most well tolerated dose of vorinostat includes vorinostat 200 mg days 1–7 and 15–21 of a 28-d cycle. In fact, in those patients treated at the 200 mg dose (Cohort 1), all three patients had an improvement in their post-transplant response, two of whom achieved sCR.
The most common observed adverse events over the course of the trial included cytopenias, fatigue, diarrhoea and hypokalaemia. During Cycle 1, no grade 4 events were experienced. Grade 2 and 3 events were minimal and included neutropenia, nausea, diarrhoea, fatigue, hypokalaemia, emesis and lymphopenia (Fig 1). In parallel with prior experience describing common vorinostat side effects when used as single agent (Richardson et al, 2008) and in combination regimens (Badros et al, 2009; Siegel et al, 2014), diarrhoea and hypokalaemia occurred, but the majority of these events were grade 1 or 2 in severity. Interestingly, though fatigue was the third most common adverse event recorded (12·4% of patients), the Global Fatigue Score did not significantly increase over the course of the trial (P = 0·425) (Table IV). Additionally, patient functionality and depression indices did not significantly change (P = 0·140 and P = 0·897, respectively). Collectively, our data confirm tolerability.
Of the 16 patients enrolled, seven improved their response after starting lenalidomide/vorinostat. These patients achieved SD (n = 3), PR (n = 2) and VGPR (n = 2) following transplant, and all but two improved their response to VGPR or better. Of those patients that did not improve on maintenance, the best responses included five VGPR, two CR, and two sCR (Fig 2). Interestingly, this data suggests that those patients that benefited least from transplant derived the most benefit from this maintenance regimen. In total, 14/16 (87·5%) of the enrolled patients remain alive with a median follow-up of 38·4 months. The median PFS and OS have not yet been reached (Fig 4), limiting our ability to make conclusions regarding the clinical benefit gained by the addition of vorinostat to lenalidomide monotherapy.
The biological mechanism underlying the lenalidomide and vorinostat combination has not been elucidated, but it has been previously shown that various HDAC-Is show synergism when combined with immunomodulatory agents and/ or proteasome inhibitors (Mitsiades et al, 2004; Pei et al, 2004; Campbell et al, 2010; Kikuchi et al, 2010). HDAC-Is can modulate the immune response via increased expression of major histocompatibility complex class I-related chain A and B (MICA/B) (Skov et al, 2005) to enhance NK-cell mediated killing (Carbone et al, 2005). Additionally, it is known that lenalidomide stimulates T-cells, improves dendritic cell function and inhibits regulatory T-cell expansion (Quach et al, 2010). The resulting increase in production of interferon-γ and interleukin-2 can reverse the impaired innate immune response by indirectly enhancing NK cell proliferation, cytolytic activity and antibody-dependent cell-mediated cytotoxicity (Davies et al, 2001; Hayashi et al, 2005).
In the early post-transplant setting, it has been shown that patients with early NK cell recovery after autologous transplant have prolonged OS and PFS (Porrata et al, 2008). Though conclusions are limited by the sample size, our correlative flow cytometric analyses indicate that the percentage of NK cells (CD3−/CD16+/CD56+) increased following combination therapy, potentially explaining at least in part the improvement in response of nearly half of our patients. Ultimately, data regarding immune constitution following autologous transplantation and the effects of maintenance therapy on NK-, T- and B-cellular subsets in myeloma patients remains limited, and further investigation is warranted to define the significance of these changes over the course of treatment.
Combination with novel therapeutics, such as lenalidomide and bortezomib, in patients with MM is associated with improved clinical response; findings that continue to drive the pursuit to define optimal combination strategies. Ongoing trials have suggested in the phase 2 setting that multi-drug maintenance may be more effective in patients with higher risk disease (Nooka et al, 2014). Our trial is the first to investigate HDAC-Is in the maintenance setting, but this phase 1 study only sets the stage to compare multi-drug regimens in the randomized setting against lenalidomide monotherapy. Additionally, pan HDAC-Is may not be the most effective therapy, and more selective HDAC-Is are being actively investigated. For example, ACY-1215 (HDAC-6-I) has been well-tolerated in the phase 1 setting in combination with both IMiDs (Vorhees et al, 2013) and proteasome inhibitors (Vogl et al, 2013), and preclinical investigation highlights that BG45 (HDAC-3-I) effectively induces MM cell apoptosis and increases the cytotoxic effects of bortezomib (Minami et al, 2014); promising findings that could lead to the eventual incorporation of these agents into effective and well tolerated MM treatment regimens.
Supplementary Material
Acknowledgments
This research is supported by the National Cancer Institute of the National Institutes of Health under Award Number U01CA076576 (PI Michael Grever). DWS is supported under Award Number T32CA165998 (PI Miguel Villalona and Steven Devine). The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Authorship
CCH designed the study, analysed the data and wrote the manuscript. YH, DWS analysed data, wrote the manuscript. CCH, YE, DMB and SD enrolled patients. All authors revised the manuscript for intellectual content and approved the final version.
Disclosures
CCH reports personal fees and non-financial support from Celgene, personal fees from Millenium, personal fees from Onyx, outside the submitted work. SD reports personal fees from Amgen, Celgene, and GlaxoSmithKline. DWS, DMB, NW, YH, MAB, KH and YE have no disclosures to report.
Additional Supporting Information may be found in the online version of this article:
Fig S1. Number of days to first grade >3 adverse event.
Fig S2. Box plot representation of cellular subset changes over the course of two cycles.
Table SI. Clinical trials investigating vorinostat in patients with multiple myeloma.
Table SII. Cell population subsets over time.
References
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L, Garderet L, Decaux O, Leyvraz S, Vekemans MC, Voillat L, Michallet M, Pegourie B, Dumontet C, Roussel M, Leleu X, Mathiot C, Payen C, Avet-Loiseau H, Harousseau JL. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. New England Journal of Medicine. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, Harris C, Zwiebel J, Wright JJ, Espinoza-Delgado I, Baer MR, Holleran JL, Egorin MJ, Grant S. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clinical Cancer Research. 2009;15:5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RA, Sanchez E, Steinberg J, Shalitin D, Li ZW, Chen H, Berenson JR. Vorinostat enhances the antimyeloma effects of melphalan and bortezomib. European Journal of Haematology. 2010;84:201–211. doi: 10.1111/j.1600-0609.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, Catalano L, Tassone P, Rotoli B, Venuta S. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, Eckberg K, Lloyd S, Puri S, Blendowski C, Goodman M, Bornicle M, Stewart I, McHale M, Bonomi P, Kaplan E, Taylor S, Thomas CR, Harris J. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, Treon SP, Richardson PG, Schlossman RL, Morgan GJ, Muller GW, Stirling DI, Anderson KC. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M, Siegel DS, Lonial S, Qi J, Hajek R, Facon T, Rosinol L, Williams C, Blacklock H, Goldschmidt H, Hungria V, Spencer A, Palumbo A, Graef T, Eid JE, Houp J, Sun L, Vuocolo S, Anderson KC. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): a multicentre, randomised, double-blind study. The Lancet Oncology. 2013;14 :1129–1140. doi: 10.1016/S1470-2045(13)70398-X. [DOI] [PubMed] [Google Scholar]
- Duvic M, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Randolph S, Richon VM, Olsen EA. Vorinostat (suberoylanilide hydroxamic acid, SAHA) provides prolonged clinical benefit to advanced cutaneous T-cell lymphoma patients: updated results of the phase IIb multicenter clinical trial. Blood (ASH Annual Meeting Abstracts) 2006;108:399. [Google Scholar]
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialitakis M, Kretsovali A, Spilianakis C, Kravariti L, Mages J, Hoffmann R, Hatzopoulos AK, Papamatheakis J. Coordinated changes of histone modifications and HDAC mobilization regulate the induction of MHC class II genes by Trichostatin A. Nucleic Acids Research. 2006;34:765–772. doi: 10.1093/nar/gkj462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, Kumar S, Chauhan D, Treon SP, Richardson P, Anderson KC. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. British Journal of Haematology. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hyper-methylation. New England Journal of Medicine. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Archives of Internal Medicine. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Kaufman JL, Shah JJ, Laubach JP, Heffner L, Francis D, Harvey RD, Lewis C, Tighiouart M, Richardson P, Orlowski RZ, Lonial S. Lenalidomide, bortezomib, and dexamethasone (RVD) in combination with vorinostat as front-line therapy for patients with multiple myeloma (MM): initial results of a phase 1 study. Blood (ASH Annual Meeting Abstracts) 2010;116:3034. [Google Scholar]
- Kikuchi J, Wada T, Shimizu R, Izumi T, Akutsu M, Mitsunaga K, Noborio-Hatano K, Nobuyoshi M, Ozawa K, Kano Y, Furukawa Y. Histone deacetylases are critical targets of bortezomib-induced cytotoxicity in multiple myeloma. Blood. 2010;116:406–417. doi: 10.1182/blood-2009-07-235663. [DOI] [PubMed] [Google Scholar]
- Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Dona G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2002;99 :2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, Giralt S, Stadtmauer EA, Weisdorf DJ, Vij R, Moreb JS, Callander NS, Van Besien K, Gentile T, Isola L, Maziarz RT, Gabriel DA, Bashey A, Landau H, Martin T, Qazilbash MH, Levitan D, McClune B, Schlossman R, Hars V, Postiglione J, Jiang C, Bennett E, Barry S, Bressler L, Kelly M, Seiler M, Rosenbaum C, Hari P, Pasquini MC, Horowitz MM, Shea TC, Devine SM, Anderson KC, Linker C. Lenalidomide after stem-cell transplantation for multiple myeloma. New England Journal of Medicine. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Minami J, Suzuki R, Mazitschek R, Gorgun G, Ghosh B, Cirstea D, Hu Y, Mimura N, Ohguchi H, Cottini F, Jakubikova J, Munshi NC, Haggarty SJ, Richardson PG, Hideshima T, Anderson KC. Histone deacetylase 3 as a novel therapeutic target in multiple myeloma. Leukemia. 2014;28:680–689. doi: 10.1038/leu.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann TA, Richon VM, Marks PA, Anderson KC. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, Casbourne D, Saxe D, Boise LH, Lonial S. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28 :690–693. doi: 10.1038/leu.2013.335. [DOI] [PubMed] [Google Scholar]
- Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clinical Cancer Research. 2004;10:3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Litzow MR, Winters JL, Markovic SN. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biology of Blood and Marrow Transplantation. 2008;14 :807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24 :22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Mitsiades C, Colson K, Reilly E, McBride L, Chiao J, Sun L, Ricker J, Rizvi S, Oerth C, Atkins B, Fearen I, Anderson K, Siegel D. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leukaemia & Lymphoma. 2008;49:502–507. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- Siegel DS, McBride L, Bilotti E, Schmidt L, Gao Z, Tufail M, Lendvai N, McNeill A, Donadio K, Olivo K, Bednarz U, Graef T, Vesole DH. Vorinostat overcomes lenalidomide-dexamethasone and lenalidomide-bortezomib-dexamethasone resistance in relapsed/ refractory multiple myeloma. Blood (ASH Annual Meeting Abstracts) 2010;116:3065. [Google Scholar]
- Siegel DS, Dimopoulos MA, Yoon SS, Laubach JP, Kaufman JL, Goldschmidt H, Reece DE, Leleu X, Durrant S, Offner FC, Cavo M, Nagler A, Jagannath S, Graef T, Houp J, Sun L, Howe J, Wear SM, Anderson KC. Vantage 095: vorinostat in combination with bortezomib in salvage multiple myeloma patients: final study results of a global phase 2b trial. Blood (ASH Annual Meeting Abstracts) 2011;118:480. [Google Scholar]
- Siegel DS, Richardson P, Dimopoulos M, Moreau P, Mitsiades C, Weber D, Houp J, Gause C, Vuocolo S, Eid J, Graef T, Anderson KC. Vorinostat in combination with lenalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood Cancer Journal. 2014;4:e182. doi: 10.1038/bcj.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Research. 2005;65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- Vogl DT, Hari PN, Jagannath S, Jones SS, Supko JG, Leone G, Wheeler C, Orlowski RZ, Richardson PG, Lonial S. ACY-1215, a selective histone deacetylase (HDAC) 6 inhibitor: interim results of combination therapy with bortezomib in patients with multiple myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2013;122:759. [Google Scholar]
- Vorhees P, Bensinger WI, Berdeja J, Supko JG, Richardson PG, Jones SS, Patrick G, Wheeler C, Raje N. ACY-1215, a selective histone deacetylase (HDAC) 6 inhibitor, in combination with lenalidomide and dexamethasone (dex), is well tolerated without dose limiting toxicity (DLT) in patients (Pts) with multiple myeloma (MM) at doses demonstrating biologic activity: interim results of a phase 1b trial. Blood (ASH Annual Meeting Abstracts) 2013;122:3190. [Google Scholar]
- Weber DM, Graef T, Hussein M, Sobecks RM, Schiller GJ, Lupinacci L, Hardwick JS, Jagannath S. Phase I trial of vorinostat combined with bortezomib for the treatment of relapsing and/or refractory multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia. 2012;12:319–324. doi: 10.1016/j.clml.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Wider D, Keller K, Groβ B, Reinhardt H, Jakobs D, Moeller MD, Burbeck M, Pantic M, May A, Jung M, Waesch R, Engelhardt M. Vorinostat (V) in combination with bortezomib (B), doxorubicin (D) and dexamethasone (D) (VBDD) in patients with refractory or relapsed multiple myeloma: an interim phase I/II analysis. Onkologie. 2013;36:152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.