Abstract
Background:
The emergence of multidrug-resistant Acinetobacter baumannii complicates the therapy of the related infections. Hospital isolates of A. baumannii are usually multidrug-resistant. The problem is compounded by increasing resistance to broad-spectrum antibiotics including carbapenems.
Objectives:
The aim of this study was to determine antimicrobial susceptibility patterns and distribution of blaOXA-type carbapenemases genes among A. baumannii isolates from hospitalized patients in Shiraz, Southwest Iran.
Materials and Methods:
Two hundred A. baumannii isolates were recovered from different clinical specimens in four Shiraz teaching hospitals. Isolates were detected as A. baumannii by Microgen kit and PCR with specific primers of blaOXA-51-like gene. Antimicrobial susceptibility testing was determined by disk diffusion method for all the isolates. Multiplex PCR assays were performed for detection of blaOXA-23-like, blaOXA-24-like and blaOXA-58-like genes.
Results:
All the isolates were susceptible to colistin and polymyxin B. Moreover, all of them were resistant to piperacillin, piperacillin-tazobactam, ampicillin, ceftazidime, cefoxitin and aztreonam. Eighty (40%) isolates had positive results for blaOXA-23-like, 14 (7%) for blaOXA-24-like and 1 (0.5%) isolate for blaOXA-58-like. The co-existence of studied genes was detected for blaOXA-23-like plus blaOXA-24-like in nine (4.5%) isolates.
Conclusions:
The prevalence of carbapenem resistant A. baumannii isolates in Shiraz hospitals is high. The blaOXA-23-like gene was the most frequent carbapenemase identified among resistant A. baumannii isolated in Shiraz hospitals. The increasing incidence of A. baumannii is a serious concern, therefore control of this pathogen and taking preventive measures are emphasized.
Keywords: OXA-51 beta-lactamase, Oxacillinase, Acinetobacter baumannii
1. Background
Acinetobacter baumannii is a glucose non-fermentative Gram-negative bacillus classified as an opportunistic pathogen responsible for nosocomial infections, especially in Intensive Care Units (ICU) and Burn Therapy Units (BTU) (1, 2). A. baumannii is responsible for nosocomial pneumonia, wound infections, bacteremia, urinary tract infection, meningitis, endocarditis, osteomyelitis and keratitis (3). Also infections of respiratory tract, central nervous system, urinary tract and peritonitis can be caused by A. baumannii especially in immunocompromised patients (3). In the recent years, the emergence of multidrug-resistant A. baumannii has complicated the therapy of A. baumannii infections (4). Hospital strains of Acinetobacter are usually multidrug-resistant. Multidrug-Resistance (MDR) is defined as resistance to three or more representatives of the following classes of antibiotics: fluoroquinolones, third generation cephalosporins, aminoglycosides and carbapenems (5).
MDR is compounded by increasing resistance to broad-spectrum antibiotics including carbapenems as one of the most effective antibiotics against Gram-negative rods (6, 7). Although, most of A. baumannii isolates were susceptible to carbapenems previously and imipenem was the most effective treatment for A. baumannii infections, widespread use of carbapenem has caused emergence of resistant strains (8, 9). The emergence of carbapenem resistance in A. baumannii is a significant public health concern (3). One of the major mechanisms of carbapenem resistance in this pathogen is production of carbapenem hydrolyzing beta- lactamases mostly Oxacillinase (OXA) types carbapenemases (10). Carbapenem resistance in A. baumannii is mediated by acquisition of a class B or class D beta-lactamase genes, such as oxacillinase genes (11). OXA-type carbapenamases have been divided into eight subgroups, four of which have been identified in A. baumannii: OXA-23-like, OXA-24-like, OXA-58-like and OXA-51-like (12). Recently, it has been suggested that enzymes belonging to the OXA-51-like subgroup are intrinsic to A. baumannii, occurring in most or all strains, although they are very variably expressed (13).
To control and prevent dissemination of resistant isolates, molecular method for typing of MDR A. baumannii is essential (14). The significant contribution of oxacillinase genes to carbapenem resistance has been emphasized, particularly when they are accompanied by ISAba1 and ISAba3 in the naturally occurring plasmids (2, 15). Several studies on these genes have been performed in the world and in some parts of Iran; however, there is no data on the distribution of blaOXA-type genes in A. baumannii isolates in Shiraz. Therefore, this study was performed to provide information in this regard. This study can help us to know the prevalence of blaOXA-type genes in A. baumannii isolates in our hospital settings for better infection control and treatment in hospitals in Shiraz.
2. Objectives
The aim of this study was to determine antimicrobial susceptibility patterns and distribution of blaOXA-type carbapenemase genes among A. baumannii isolates from hospitalized patients in four teaching hospitals, in Shiraz, Southwest Iran.
3. Materials and Methods
3.1. Bacterial Strains
From December 2013 to May 2013, 200 A. baumannii were isolated from different clinical specimens, including urine, wound, blood, sputum, endotracheal tube (ETT), body fluid, nose, throat and eye from four Shiraz teaching hospitals (Faghihi, Aliasghar, Ghotebedin and Nemazee) in Shiraz, southwest Iran. Some relevant information including sex, sample type and ward name was recorded. The study was approved by the Ethics Committee of Shiraz University of Medical Sciences. The specimens were cultured on MacConkey agar (Merck, Germany) and blood agar (Merck, Germany), then incubated at 37°C for 24 - 48 hours. These isolates were identified using standard biochemical tests, including Oxidase test, TSI (Triple Sugar Iron agar) medium (Merck, Germany) and SIM (Sulfide, Indole, Motility) medium (Merck, Germany). Negative result for oxidase test, no motility and non-fermentation and growth in temperature of 42 - 44°C was considered as the elementary criteria for A. baumannii detection. They were confirmed by Microgen kits (Mast, UK) according to the manufacturer instructions (www.microgenbioproducts.com).
3.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed by disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) (4, 16). Disk diffusion method was performed on Muller-Hinton agar (Merck, Germany), using an inoculum of 105 CFU. Antibiotic disks (Mast, UK) containing ampicillin (10 μg), ampicillin-sulbactam (10 μg), piperacillin (100 μg), piperacillin-tazobactam (110 μg), amikacin (30 μg), gentamicin (120 μg), imipenem (10 μg), meropenem (10 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), ceftazidime (30 μg), cefoxitine (30 μg), aztreonam (30 μg), colistin (25 μg), polymyxin B (300 unit) and tigecycline (15 μg). These antibiotic disks were then placed on agar plates and incubated at 37°C for 24 hours. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality controls strain in each susceptibility determination (6).
3.3. DNA Extraction
2 - 3 grown bacterial colonies on Muller-Hinton agar plates were suspended in 180 μL PBS. Then DNA of the bacteria was extracted by phenol-chloroform (CinnaGen, Iran) method with some modifications as described before (14). They were used as template in Polymerase Chain Reaction (PCR) assay to amplify blaOXA carbapenemase genes (17).
3.4. Detection of blaOXA-51-like Gene
Identification of A. baumannii was confirmed using blaOXA-51-like PCR assay by specific primers (Gene Fanavaran, Iran) (18). To amplify the blaOXA-51-like gene, each reaction final volume of 25 μL was considered, containing 1x PCR buffer (CinnaGen, Iran), 1 U Taq polymerase (CinnaGen, Iran), 1.5 mM Mgcl2 (CinnaGen, Iran), 200 μM of dNTP (CinnaGen, Iran), 10 pmol of each primer and 1 μL of extracted DNA. PCR conditions were programed in an Eppendorf Mastercycler as follows: Initial denaturation at 94°C for 3 min, 35 cycles of 94°C for 45 s, 57°C for 45 s, 72°C for 1 min and 5 minute final extension of 72°C. PCR products were electrophoresed (Bio Rad, USA) on 1.2% agarose gel (CinnaGen, Iran), stained with ethidium bromide solution (1 mg/mL) (CinnaGen, Iran) and placed under UV gel transilluminator. A. baumannii reference strain ATCC 19606 was used as positive control for blaOXA-51-like. Negative control was also included in each PCR reaction, containing all components except the DNA template which was replaced by distilled water (6, 14).
3.5. Multiplex PCR Assay for Detection of Oxacillinase Genes
A Multiplex PCR targeting blaOXA-23-like, blaOXA-24-like and blaOXA-58-like genes was used to screen the isolates as previously described (17). All the isolates were subjected to Multiplex PCR performed to detect blaOXA-23-like, blaOXA-24-like and blaOXA-58-like using specific primers (Gene Fanavaran, Iran), as listed in Table 1 (19). Final volume of 25 μL included 1x PCR buffer, 1 U Taq polymerase, 2 mM MgCl2, 200 μM of dNTP, 0.2 μM of each primer and 1 μL of template DNA. Amplification protocol was initial denaturation at 94°C for 5 min, 94°C for 30 s, 53°C for 40 s and 72°C for 50 s and final extension at 72°C for 6 min. The PCR products were run in 2.5% agarose gel electrophoresis, stained with ethidium bromide (CinnaGene, Iran) and visualized under UV gel transilluminator. A. baumannii reference strains NCTC 13304, NCTC 13302 and NCTC 13305 were used as positive control for blaOXA-23-like, blaOXA-24-like and blaOXA-58-like, respectively (6, 14).
Table 1. Primer Sequences Used in This Study to Detect Oxacillinase Genes.
| Primer Sequence (5’ - 3’) | Amplicon Size, bp |
|---|---|
| bla OXA-23-like | 501 |
| GATCGGATTGGAGAACCAGA | |
| ATTTCTGACCGCATTTCCAT | |
| bla OXA-24-like | 246 |
| GGTTAGTTGGCCCCCTAAAA | |
| AGTTGAGCGAAAAGGGGATT | |
| bla OXA-51-like | 353 |
| TAATGCTTTGATCGGCCTTG | |
| TGGATTGCACTTCATCTTGG | |
| bla OXA-58-like | 599 |
| AAGTATTGGGGCTTGTGCTG | |
| CCCCTCTGCGCTCTACATAC |
3.6. Statistical Analyses
To determine the association between variables, data was analyzed by Chi-square and Fisher’s exact test using SPSS, version 21 (SPSS Inc. Chicago, IL, USA). P values < 0.05 were considered as statistically significant.
4. Results
4.1. Bacterial Isolates
Of two hundred isolates, 109 (54.5%) were from male and 91 (45.5%) from female patients. Isolates were collected from four hospitals; 82 (41%) from Nemazee hospital, 53 (26.5%) from Aliasghar hospital, 48 (24%) from Faghihi hospital and 17 (8.5%) from Ghotbedin Hospital. The rates of isolation from different wards were as follows; ICU 143 (71.5%), surgeries ward 16 (8%), neurosurgical ICU 14 (7%), neonates 8 (4%), female internal ward 7 (3.5%), male internal ward 7 (3.5%) and organ transplantation ward 5 (2.5%). Of two hundred isolates, 81 (40.5%) were from sputum, 43 (21.5%) from endotracheal tube, 22 (11%) from wound, 16 (8%) from urine, 12 (6%) from blood, 12 (6%) from body fluids, 4 (2%) from nose, 3 (1.5%) from throat, 2 (1%) from CSF, 1 (0.5%) from eye specimens and 4 (2%) other samples. All A. baumannii isolates (n = 200) in this study had positive results for blaOXA-51-like by PCR (Figure 1).
Figure 1. Agarose Gel Electrophoresis of blaOXA-51-like Gene in Acinetobacter baumannii.
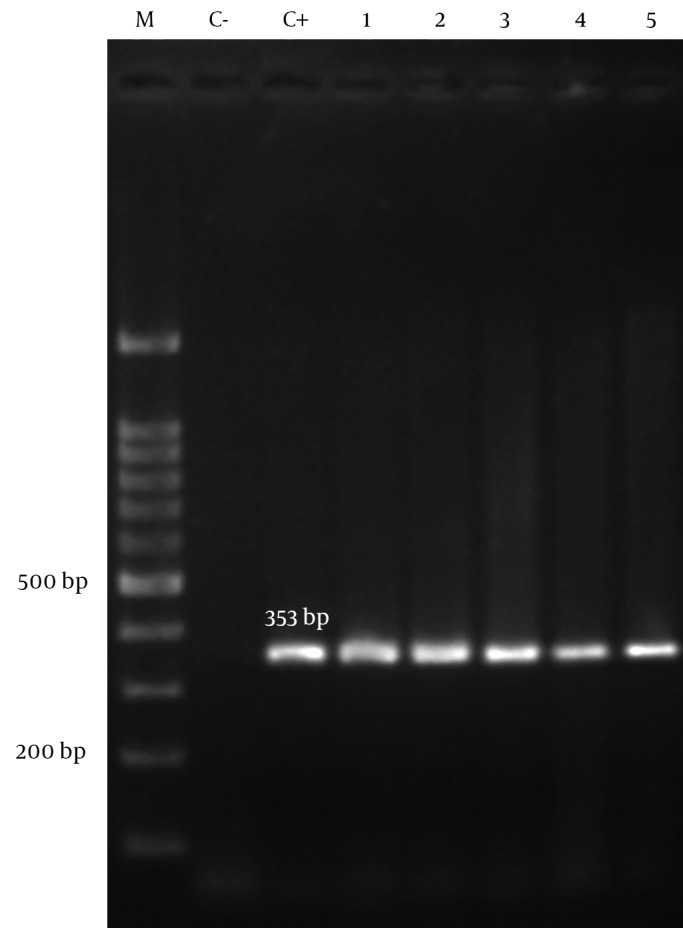
1, 2, 3, 4, 5, clinical isolates of A. baumannii with blaOXA-51-like gene; C-, negative control; C+, positive control; M, 100 bp DNA ladder.
4.2. Antimicrobial Susceptibility Testing
All the isolates were susceptible to colistin and polymyxin B and all were MDR as defined before, they were totally resistant to piperacillin, piperacillin-tazobactam, ampicillin, ceftazidime, cefoxitin and aztreonam (Table 2). The association between source of isolates and antibiotic resistance pattern was not statistically significant (P > 0.05).
Table 2. The Results of Antibiogram for Studied A. baumannii Isolates a.
| Antibiotics | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Imipenem | 2 (1) | 1 (0.5) | 197 (98.5) |
| Meropenem | 1 (0.5) | - | 199 (99.5) |
| Piperacillin | - | - | 200 (100) |
| Piperacillin-tazobactam | - | - | 200 (100) |
| Ampicillin | - | - | 200 (100) |
| Ampicillin-sulbactam | 6 (3) | 3 (1.5) | 191 (95.5) |
| Ceftazidime | - | 1 (0.5) | 199 (99.5) |
| Cefoxitine | - | - | 200 (100) |
| Gentamicin | 27 (13.5) | 4 (2) | 169 (84.5) |
| Amikacin | 17 (8.5) | 10 (5) | 173 (86.5) |
| Ciprofloxacin | 1 (0.5) | - | 199 (99.5) |
| Levofloxacin | 1 (0.5) | - | 199 (99.5) |
| Aztreonam | - | - | 200 (100) |
| Colistin | 200 (100) | - | - |
| Polymyxin B | 200 (100) | - | - |
| Tigecycline | 175 (87.5) | 21 (10.5) | 4 (2) |
a Values are presented as No (%).
4.3. Detection of Oxacillinase Genes
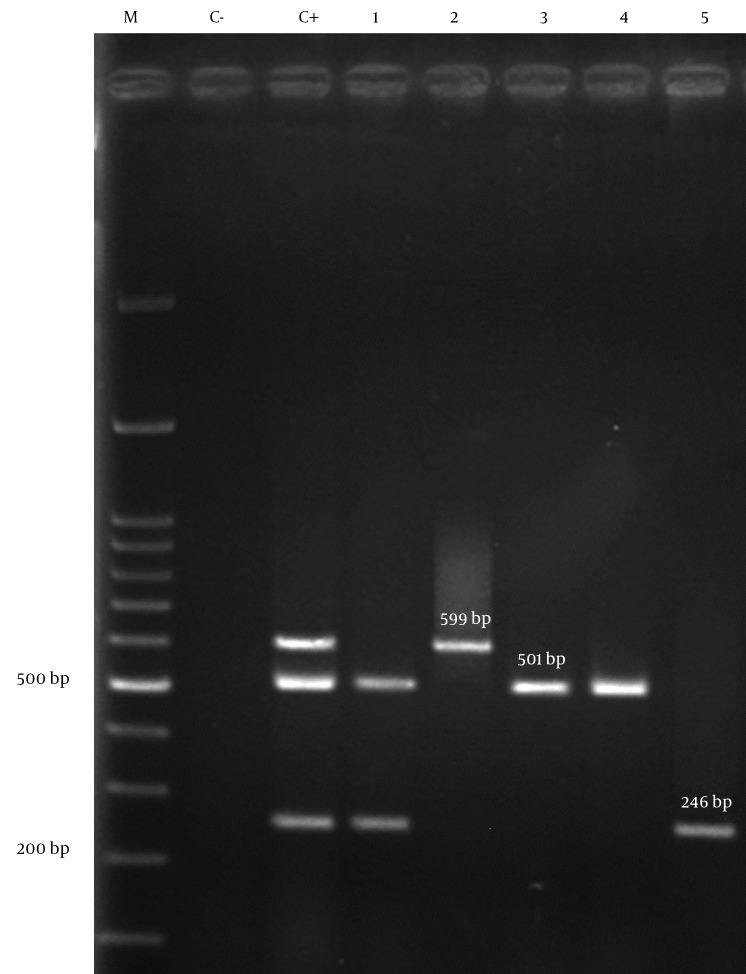
All isolates of A. baumannii were tested by Multiplex PCR for the presence of oxacillinase genes. Eighty (40%) of 200 isolates had positive results for blaOXA-23-like, 14 (7%) for blaOXA-24-like and 1 (0.5%) isolate for blaOXA-58-like. The co-existence of two different blaOXA genes in the samples was detected for blaOXA-23-like plus blaOXA-24-like in nine (4.5%) isolates (Figure 2). The association between source of isolates and presence of genes was not statistically significant.
Figure 2. Agarose Gel Electrophoresis of blaOXA-type Carbapenemases by Multiplex PCR.
1, clinical isolate of A. baumannii containing blaOXA-23-like and blaOXA-24-like genes; 2, clinical isolate of A. baumannii having blaOXA-58-like gene; 3,4, clinical isolates of A. baumannii having blaOXA-23-like genes; 5, clinical isolate of A. baumannii with blaOXA-24-like gene; C-, negative control; C+, positive control (A. baumannii NCTC 13304, NCTC 13302, NCTC 13305 for blaOXA-23-like, blaOXA-24-like, blaOXA-58-like ); M, 100 bp DNA ladder.
5. Discussion
Acinetobacter baumannii is an emerging nosocomial pathogen which is in part due to its capacity of acquiring resistance to multiple antimicrobial agents (20). Occurrence of multidrug-resistance and Pandrug-Resistant (PDR) in A. baumannii is a growing concern (7). All of the isolates in this study were multidrug-resistant and resistant to most of the antibiotics (Table 2). This pathogen is an important Gram-negative bacterium involved in nosocomial infections, especially in ICU wards (18). As shown in our study, 71.5% of the isolates were obtained from hospitalized patients in ICU. This result confirms the fact that A. baumannii is often an important cause of infection in hospitalized patients in ICU.
To detect antibiotic resistant patterns, the isolates were investigated primarily by phenotypic methods (21). Our results revealed that 98.5% and 99.5% of the isolates were resistant to imipenem and meropenem, respectively. In some studies in Tehran, 50.9%, 52.5%, 62% and 67.5% of the isolates were resistant to imipenem and 51.8%, 52.5%, 62% and 84.5% to meropenem in 2008, 2009, 2011 and 2013, respectively (6, 12, 18, 20). In another study in Tehran in 2013, resistance rates of 99% and 91.5% were reported to imipenem and meropenem, respectively (22). Mirnejad and Vafaei, reported 76% resistance to imipenem and 69% resistance to meropenem among tested isolates in Tehran in 2013 (23). In two separate studies, one in Ahvaz in 2013, 96.1% resistance to imipenem and meropenem (14), and another in Kermanshah in 2013, 79.8% resistance to imipenem and 75% resistance to meropenem were reported (15). Therefore, our results showed that resistance to carbapenems has an increasing trend which is probably because of dissemination of highly resistant lineages of A. baumannii in our area. Increased resistance to carbapenem causes a real concern over an imminent threat of untreatable A. baumannii infections (3).
The current study showed low susceptibility rates to most of available antimicrobial agents for the treatment of infections caused by A. baumannii, except for polymyxin B and colistin. In our study, all of the isolates were sensitive to colistin and polymyxin B, while other studies conducted in Tehran demonstrated 12% resistance to colistin and 3% resistance to polymyxin B in 2011 (17). Moreover, in two studies in Tehran and Ahvaz in 2013, all of the isolates were sensitive to these antibiotics (12, 14). In a study in Kermanshah in 2013, all isolates were sensitive to polymyxin B and colistin (15). Sensitivity of all A. baumannii isolates to colistin was also reported in Tehran in 2013 (22). Also in a study in Tehran in 2013, among all antibiotic tested, the lowest resistance rate to polymyxin B (3%) was observed (23). The current study also described the important role of class D carbapenem hydrolyzing beta-lactamases. Production of class D oxacillinase by A. baumannii distributed worldwide is the main mechanism of resistance to carbapenems in this organism. The major carbapenemase genes involved in carbapenem resistance in A. baumannii are blaOXA-23-like, blaOXA-24-like and blaOXA-58-like (8, 14). Alleles encoding OXA-23-like, OXA-24-like and OXA-58-like enzymes were consistently associated with resistance or at least reduced susceptibility to carbapenemases (24).
Our PCR results revealed that all A. baumannii isolates had blaOXA-51-like gene. The blaOXA-51-like genes are located intrinsically in chromosome of all A. baumannii strains (8). This result provides evidence that detection of blaOXA-51-like can be used as a simple and reliable way of identifying A. baumannii (13). GenBank submissions describing variants from isolates of A. baumannii from many different countries distributed over four continents clearly suggest that blaOXA-51-like is ubiquitous in A. baumannii (25). Distribution of other blaOXA-type genes in A. baumannii is variable. OXA-23-like was the first OXA-type beta-lactamase identified in A. baumannii (26). The results obtained in this study indicated that most of our isolates (40%) carried the blaOXA-23-like gene. In other studies investigating outbreaks of OXA-23-producing A. baumannii strains, the rate of blaOXA-23-like ranged 31% to 94% in different parts of the world (14). So our results for blaOXA-23-like gene are in the reported ranges. In both separate studies in Tehran, in 2008 and 2009, 25% of the isolates had positive results for blaOXA-23-like gene (6, 20). Moreover, in other two studies by Karmostaji et al. (12) and Goudarzi et al. (22), in Tehran in 2013, 81.3% and 55.7% had positive findings for this gene, respectively. 85% and 55.7% positivity rates were reported for blaOXA-23-like gene in studied A. baumannii isolates in Ahvaz and Kermanshah, respectively (14, 15).
In our study, 7% of the A. baumannii isolates had positive results for blaOXA-24-like gene. The reported frequency rate of this gene has been previously reported as 0 - 85.4% in different parts of the world (14). In a study in Tehran in 2008, 17.9% of isolates contained blaOXA-24-like gene (6); In addition, in another study in Tehran in 2009, 15% had positive results for this gene (20). Three studies in 2013 in Tehran, Ahvaz and Kermanshah showed frequencies of 8.13%, 8.7% and 19.2% for this gene in the studied isolates, respectively (12, 14, 15). Some authors reported blaOXA-58-like frequency as 2 - 84.9% in A. baumannii isolates in different parts of the world (14). In several studies in Tehran in 2008, 2009 and 2013, 9%, 21.2% and 0.8% of the isolates had positive results for blaOXA-58-like, respectively (6, 12, 20). Moreover, a study conducted in Ahvaz and Kermanshah, in 2013, revealed that blaOXA-58-like gene was not detected (14, 15). In our study, we identified blaOXA-58-like gene only in one A. baumannii isolate (0.5%). This frequency was similar to the rate previously reported from central Iran.
We identified nine isolates (4.5%) with co-existence of two different blaOXA-23-like plus blaOXA-24-like. In two studies in 2013, in Tehran and Kermanshah, 5.7% and 16.4% of A. baumannii isolates had such a co-existence (12, 15). In another study in 2013 in Ahvaz, no co-existence between these genes was reported (14). Of note, 105 A. baumannii isolates resistant to imipenem and meropenem in our study possessed only the intrinsic blaOXA-51-like, but they had negative result for other investigated genes. Resistance to carbapenems in those isolates may be due to other mechanisms other than oxacillinase production (14, 27).
In conclusion, our study confirmed that MDR A. baumannii strains were disseminated in Shiraz, Iran. Such A. baumannii with different blaOXA-carbapenemase genes were isolated from hospitalized patients at Shiraz teaching hospitals (Nemazee, Faghihi, Aliasghar, Ghotbedin) in different wards. The blaOXA-51-like genes were the most prevalent subgroup, as they are intrinsic to A. baumannii. Moreover, blaOXA-23-like gene is another most prevalent resistance gene among MDR A. baumannii isolates. The distribution of blaOXA-58-like gene was low in our study. Controlling infections of MDR A. baumannii in hospitals needs a common strategy issued by decision makers and health-care authorities to make hospitals a safer place for patients.
Acknowledgments
The authors would like to express their sincere appreciation to Dr. Saeed Shoja from Ahvaz Jundishapur University of Medical Sciences for his provision of positive controls for PCR.
Footnotes
Authors’ Contributions:Study idea, design and the protocol: Mohammad Motamedifar and Sara Kooti; sample collection, experiments, possession of raw data: Sara Kooti; data analysis, critical revision of the manuscript for important intellectual content: Mohammad Motamedifar and Jamal Sarvari; final approval of the entire study: Mohammad Motamedifar and Jamal Sarvari. Sara Kooti, and Mohammad Motamedifar wrote the manuscript.
Funding/Support:This study was financially supported by Shiraz University of Medical Sciences, grant No.92-6488. This paper was extracted from MSc thesis of Sara Kooti, supervised by Mohammad Motamedifar.
References
- 1.Opazo A, Dominguez M, Bello H, Amyes SG, Gonzalez-Rocha G. OXA-type carbapenemases in Acinetobacter baumannii in South America. J Infect Dev Ctries. 2012;6(4):311–6. doi: 10.3855/jidc.2310. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–63. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 4.Peymani A, Higgins PG, Nahaei MR, Farajnia S, Seifert H. Characterisation and clonal dissemination of OXA-23-producing Acinetobacter baumannii in Tabriz, northwest Iran. Int J Antimicrob Agents. 2012;39(6):526–8. doi: 10.1016/j.ijantimicag.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 6.Feizabadi MM, Fathollahzadeh B, Taherikalani M, Rasoolinejad M, Sadeghifard N, Aligholi M, et al. Antimicrobial susceptibility patterns and distribution of blaOXA genes among Acinetobacter spp. Isolated from patients at Tehran hospitals. Jpn J Infect Dis. 2008;61(4):274–8. [PubMed] [Google Scholar]
- 7.Gaynes R, Edwards JR, National Nosocomial Infections Surveillance S. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 8.Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39(2):105–14. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Shete VB, Ghadage DP, Muley VA, Bhore AV. Multi-drug resistant Acinetobacter ventilator-associated pneumonia. Lung India. 2010;27(4):217–20. doi: 10.4103/0970-2113.71952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irfan S, Turton JF, Mehraj J, Siddiqui SZ, Haider S, Zafar A, et al. Molecular and epidemiological characterisation of clinical isolates of carbapenem-resistant Acinetobacter baumannii from public and private sector intensive care units in Karachi, Pakistan. J Hosp Infect. 2011;78(2):143–8. doi: 10.1016/j.jhin.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Lin MF, Chang KC, Lan CY, Chou J, Kuo JW, Chang CK, et al. Molecular epidemiology and antimicrobial resistance determinants of multidrug-resistant Acinetobacter baumannii in five proximal hospitals in Taiwan. Jpn J Infect Dis. 2011;64(3):222–7. [PubMed] [Google Scholar]
- 12.Karmostaji A, Najar Peerayeh S, Hatef Salmanian A. Distribution of OXA-Type Class D β-Lactamase Genes Among Nosocomial Multi Drug Resistant Acinetobacter baumannii Isolated in Tehran Hospitals. Jundishapur J Microbiol. 2013;6(5):1–5. doi: 10.5812/jjm.8219. [DOI] [Google Scholar]
- 13.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44(8):2974–6. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoja S, Moosavian M, Peymani A, Tabatabaiefar MA, Rostami S, Ebrahimi N. Genotyping of carbapenem resistant Acinetobacter baumannii isolated from tracheal tube discharge of hospitalized patients in intensive care units, Ahvaz, Iran. Iran J Microbiol. 2013;5(4):315–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Mohajeri P, Farahani A, Feizabadi MM, Ketabi H, Abiri R, Najafi F. Antimicrobial susceptibility profiling and genomic diversity of Acinetobacter baumannii isolates: A study in western Iran. Iran J Microbiol. 2013;5(3):195–202. [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institude (CLSI). Performance standards for antimicrobial susceptibility testing. 12 ed. Clinical and Laboratory Standards Institude (CLSI); 2010. [Google Scholar]
- 17.Shahcheraghi F, Abbasalipour M, Feizabadi M, Ebrahimipour G, Akbari N. Isolation and genetic characterization of metallo-beta-lactamase and carbapenamase producing strains of Acinetobacter baumannii from patients at Tehran hospitals. Iran J Microbiol. 2011;3(2):68–74. [PMC free article] [PubMed] [Google Scholar]
- 18.Sohrabi N, Farajnia S, Akhi MT, Nahaei MR, Naghili B, Peymani A, et al. Prevalence of OXA-type beta-lactamases among Acinetobacter baumannii isolates from Northwest of Iran. Microb Drug Resist. 2012;18(4):385–9. doi: 10.1089/mdr.2011.0077. [DOI] [PubMed] [Google Scholar]
- 19.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–23. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Taherikalani M, Fatolahzadeh B, Emaneini M, Soroush S, Feizabadi MM. Distribution of different carbapenem resistant clones of Acinetobacter baumannii in Tehran hospitals. New Microbiol. 2009;32(3):265–71. [PubMed] [Google Scholar]
- 21.Nowak P, Paluchowska P, Budak A. Distribution of blaOXA genes among carbapenem-resistant Acinetobacter baumannii nosocomial strains in Poland. New Microbiol. 2012;35(3):317–25. [PubMed] [Google Scholar]
- 22.Goudarzi H, Douraghi M, Ghalavand Z, Goudarzi M. Assessment of antibiotic resistance pattern in Acinetobacter bumannii carrying bla oxA type genes isolated from hospitalized patients. Novel Biomed. 2013;1(2):54–61. [Google Scholar]
- 23.Mirnejad R, Vafaei S. Antibiotic resistance patterns and the prevalence of ESBLs among strains of Acinetobacter baumannii isolated from clinical specimens. The Journal of Genes, Microbes and Immunity. 2013;2013:1–8. doi: 10.5899/2013/jgmi-00002. [DOI] [Google Scholar]
- 24.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27(4):351–3. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Brown S, Amyes SG. The sequences of seven class D beta-lactamases isolated from carbapenem-resistant Acinetobacter baumannii from four continents. Clin Microbiol Infect. 2005;11(4):326–9. doi: 10.1111/j.1469-0691.2005.01096.x. [DOI] [PubMed] [Google Scholar]
- 26.Paton R, Miles RS, Hood J, Amyes SG, Miles RS, Amyes SG. ARI 1: beta-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int J Antimicrob Agents. 1993;2(2):81–7. doi: 10.1016/0924-8579(93)90045-7. [DOI] [PubMed] [Google Scholar]
- 27.Kulah C, Mooij MJ, Comert F, Aktas E, Celebi G, Ozlu N, et al. Characterisation of carbapenem-resistant Acinetobacter baumannii outbreak strains producing OXA-58 in Turkey. Int J Antimicrob Agents. 2010;36(2):114–8. doi: 10.1016/j.ijantimicag.2010.03.017. [DOI] [PubMed] [Google Scholar]