Abstract
Objectives
Predictive factors for efficacy of bevacizumab in advanced ovarian cancer have remained elusive. We investigated ascites both as a prognostic factor and as a predictor of efficacy for bevacizumab.
Methods
Using data from GOG 0218, patients receiving cytotoxic therapy plus concurrent and maintenance bevacizumab were compared to those receiving cytotoxic therapy plus placebo. Presence of ascites was determined prospectively. Chi-square and Wilcoxon-Mann-Whitney tests compared baseline variables between subgroups. Survival was estimated by Kaplan-Meier method, and Cox proportional hazard models were used to evaluate independent prognostic factors and estimate their covariate-adjusted effects on survival.
Results
Treatment arms were balanced with respect to ascites and other prognostic factors. Overall, 886 (80%) women had ascites, 221 (20%) did not. Those with ascites were more likely to have: poorer performance status (p<0.001); serous histology (p=0.012); higher baseline CA125 (p<0.001); and suboptimal cytoreduction (p=0.004). In multivariate survival analysis, ascites was prognostic of poor OS (Adjusted HR 1.22, 95% CI 1.00-1.48, p=0.045), but not PFS. In predictive analysis, patients without ascites treated with bevacizumab had no significant improvement in either PFS (AHR 0.81, 95% CI 0.59-1.10, p=0.18) or OS (AHR 0.94, 95% CI 0.65-1.36, p=0.76). Patients with ascites treated with bevacizumab had significantly improved PFS (AHR 0.71, 95% CI 0.62-0.81, p<0.001) and OS (AHR 0.82, 95% CI 0.70-0.96, p=0.014).
Conclusions
Ascites in women with advanced ovarian cancer is prognostic of poor overall survival. Ascites may predict the population of women more likely to derive long-term benefit from bevacizumab.
INTRODUCTION
Despite initial success with surgery and cytotoxic chemotherapy, the majority of women with advanced epithelial ovarian, fallopian tube and primary peritoneal cancer will experience recurrence, chemotherapy resistance, and disease-related mortality [1]. The incorporation of agents targeting tumor angiogenesis has improved progression-free survival, but identification of predictive markers to select patients for anti-angiogenic therapy has remained elusive.
Bevacizumab is a humanized monoclonal antibody that neutralizes vascular endothelial growth factor (VEGF), a central promoter of angiogenesis which has been associated with the progression of epithelial ovarian cancers [2-4]. The level of VEGF in serum and ascites is directly related to disease burden, and inversely related to survival, often independent of other established prognostic factors [5-7]. Pre-clinical studies of anti-VEGF antibodies confirmed that blocking VEGF inhibits angiogenesis and the formation of ascites [8,9]. In phase II ovarian cancer trials for patients with recurrent ovarian cancer, bevacizumab has demonstrated anti-tumor activity as a single agent [10,11]. Despite the fact that four positive randomized controlled trials evaluating bevacizumab in combination with and/or following cytotoxic chemotherapy in both front-line and recurrent disease settings have demonstrated significant benefit in terms of progression-free survival (PFS), the intent-to-treat analyses have yet to establish an impact on overall survival (OS) [12-15]. Additionally, bevacizumab has been associated with serious (but rare) side effects and the use of bevacizumab remains significantly more expensive than cytotoxic therapies. Therefore, the identification of predictive clinical, pathologic and biologic factors that could be utilized to select patients with a greater likelihood of clinical benefit, remains a high priority.
GOG 0218 was a randomized, placebo-controlled trial in which 1,873 women with advanced (Stage III-IV) ovarian cancer underwent a maximal attempt at pre-treatment cytoreductive surgery followed by one of three treatment regimens. Women were then randomly assigned to either: standard cytotoxic chemotherapy plus concurrent placebo followed by maintenance placebo (Arm 1), standard chemotherapy plus concurrent bevacizumab followed by maintenance placebo (Arm 2), or standard chemotherapy plus concurrent bevacizumab followed by maintenance bevacizumab for a maximum of 10 months (Arm 3) [14]. Those randomly assigned to Arm 3 demonstrated a four-month improvement in median PFS (HR for progression, 0.717, 95% CI 0.625-0.824, p<0.001) compared with those assigned to Arm 1. The significant PFS benefit was consistently demonstrated in planned subset analyses based on the combination of stage and residual disease, histologic cell type, tumor grade, performance status and age. No significant improvement in OS was demonstrated in the intent-to-treat analysis. A subsequent unplanned analysis by Randall et al. demonstrated a benefit in OS among patients with stage IV disease [16], similar to the subset analysis of high-risk patients from ICON7 [15]. These studies illustrate the potential success that could be achieved when a predictive marker is utilized to select a more responsive patient population. Given that cancer staging is imprecise, it is appealing to develop more robust predictive markers with a rationale related to VEGF biology and tumor angiogenesis. Ascites is a common prognostic factor in advanced ovarian cancer that is associated with VEGF, but has not been evaluated as a predictive marker for response to anti-VEGF therapy [17].
Ascites is a hallmark of many advanced ovarian cancers, and VEGF expression has been implicated in the pathogenesis of ascites [18-20]. The accumulation of ascites also contributes significantly to the morbidity experienced by patients with ovarian cancers, and it is a poor prognostic indicator [17]. Given the clear association between ascites and VEGF, there is a plausible biologic rationale for selective benefit in this population. Given that VEGF induces microvascular permeability, advanced ovarian cancers expressing VEGF (and tumor microenvironments permitting initiation of angiogenesis through VEGF response) may be those associated with ascites formation. We hypothesized that ascites is a poor prognostic factor and could be used to predict response to anti-angiogenic therapy with bevacizumab.
METHODS
Patients & Study Design
The primary analysis of GOG 0218 has been previously reported [14]. Of note, the original inclusion criteria specified patients with residual disease <1 cm and the primary endpoint of the study was OS. During the course of the study, protocol amendments were approved to permit enrollment of patients with residual disease >1 cm, and to change the primary endpoint to PFS. Our post hoc analysis of GOG protocol 0218 was performed comparing patients with and without ascites. Patients treated on Arm 1 were compared to patients treated on Arm 3. Arm 2 was excluded from the current investigation given the lack of significant PFS prolongation in the primary analysis for bevacizumab received only during the chemotherapy phase of treatment. Each patient's baseline characteristics, including the presence of ascites (defined as peritoneal fluid > 50 cm3), were reported by their institution and recorded prior to randomization. Each patient provided informed consent upon enrollment in GOG 0218. For this secondary analysis, a waiver of authorization was obtained from the Temple University School of Medicine IRB (#21818) and no additional consents were required.
Statistical Analysis
Before data analysis, we used an acceptance sampling procedure to gauge how accurately ascites was recorded by participating sites, taking the operative report as the standard. The sample matched perfectly, leading us to conclude that there was less than 0.05 probability that even only 5% of the transcribed values of ascites might be discordant with the operative reports. Data related to patient demographics, clinical and pathologic factors, chemotherapy administration, and outcomes of progression-free and overall survival were abstracted from the clinical trial database (updated January 2015) and analyzed. Categorical variables were compared between those with and without ascites by the Pearson chi-square test and continuous variables were compared using the Wilcoxon–Mann–Whitney test [21,22]. Progression free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method [23]. The Cox proportional hazards model was used to evaluate independent prognostic factors and to estimate their covariate-adjusted effects on PFS and OS [24]. Covariates used in the model included: Presence of ascites, age, body mass index (BMI), performance status, stage, histology, CA-125 value at diagnosis, tumor residual following cytoreductive surgery and protocol therapy. The nonlinearity of the effect of continuous variables was assessed using restricted cubic splines [25]. All statistical tests were two-tailed with the significance level set at α = 0.05. Statistical analyses were performed using the R programming language and environment [26].
RESULTS
We included a total of 1107 patients (treatment Arms 1 and 3). The baseline demographic and clinical information is summarized in Table 1. There were 886 (80%) patients with ascites and 221 (20%) patients without. Patients with and without ascites differed in predictable ways (Table 1). Patients with ascites were more likely to have a poor performance status (PS =2, 8.5% vs 4.1%, p<0.001), high grade serous histology (89.1% vs 80.5%, p=0.012), higher median pre-treatment CA-125 (397.0 IU/ml vs 162 IU/ml, p<0.001), and sub-optimal surgical cytoreduction with tumor > 1 cm remaining (56.7% vs 44.8%, p=0.004), compared to patients without ascites.
Table 1.
Patient Demographics and Characteristics by Presence of Ascites (N = 1107)
| Ascites Present | No N = 221 | Yes N = 886 | P |
|---|---|---|---|
| Age years | 59.2 (IQR: 51.7-65.8) | 59.8 (IQR: 52-67.3) | 0.5361 |
| BMI kg/m2 | 24.7 (IQR: 22.2-28.7) | 25.6 (IQR: 22.2-30.7) | 0.111 |
| Baseline CA-125 IU/ml | 162.0 (IQR: 72.4-468.1) | 397 (IQR: 166.6-1220) | < 0.0011 |
| Race/Ethnicity | 0.52 | ||
| White | 192 (86.9%) | 737 (83.2%) | |
| Asian | 10 (4.5%) | 63 (7.1%) | |
| Black | 6 (2.7%) | 38 (4.3%) | |
| Hispanic | 9 (4.1%) | 31 (3.5%) | |
| Other | 4 (1.8%) | 17 (1.9%) | |
| Performance status | < 0.0012 | ||
| 0 | 132 (59.7%) | 412 (46.5%) | |
| 1 | 80 (36.2%) | 399 (45.0%) | |
| 2 | 9 (4.1%) | 75 (8.5%) | |
| Surgical stage (FIGO) | 0.0672 | ||
| III | 174 (78.7%) | 644 (72.7%) | |
| IV | 47 (21.3%) | 242 (27.3%) | |
| Histology | 0.0122 | ||
| Serous | 178 (80.5%) | 789 (89.1%) | |
| Mixed epithelial | 12 (5.4%) | 31 (3.5%) | |
| Endometrioid | 11 (5.0%) | 29 (3.3%) | |
| Clear-cell/mucinous | 11 (5.0%) | 21 (2.4%) | |
| Other | 9 (4.1%) | 16 (1.8%) | |
| Tumor residual | 0.0042 | ||
| Microscopic | 10 (4.5%) | 42 (4.7%) | |
| Optimal (≤ 1 cm) | 112 (50.7%) | 342 (38.6%) | |
| Suboptimal (> 1 cm) | 99 (44.8%) | 502 (56.7%) | |
| Treatment arm | 0.6962 | ||
| I (standard chemo.) | 109 (49.3%) | 450 (50.8%) | |
| III (extended bev.) | 112 (50.7%) | 436 (49.2%) |
Continuous variables are presented as median (IQR = interquartile range). N is the number of non–missing values. Bev = bevacizumab
Tests used:
Wilcoxon test
Pearson test
Ascites as a prognostic factor
In comparisons of unadjusted survival rates, median PFS was shorter for patients with ascites: 12.6 months (95% CI, 11.8–13.1 months) compared to 15.8 months (95% CI, 14.5–18.2 months; p<0.001) for those without. The covariate-adjusted multivariate model for PFS is summarized in Table 2. Ascites was not prognostic of worse PFS in this model with an adjusted hazard ratio (AHR) of 1.17 (95% CI, 0.99-1.39, p=0.063). Unadjusted median OS was significantly worse for patients with ascites: 41.3 months (95% CI, 39.4-45.8) compared to 52.7 months (95% CI, 45.8-63.7), p<0.001, for those without. The multivariate model for OS is summarized in Table 3. Ascites was prognostic of OS: AHR 1.22 (95% CI, 1.00-1.48, p=0.045).
Table 2.
Multivariate Progression-Free Survival Analysis
| Covariate | AHR | 95% CI | P * |
|---|---|---|---|
| Ascites | 0.063 | ||
| No | 1.00 | referent | |
| Yes | 1.17 | 0.99–1.39 | |
| Age (years)† | 1.01 | 1.01–1.02 | < 0.001 |
| BMI (kg/m2)‡ | 1.01 | 1.00–1.02 | 0.131 |
| Race/Ethnicity | 0.158 | ||
| White | 1.00 | referent | |
| Asian | 0.85 | 0.65–1.12 | |
| Black | 1.33 | 0.97–1.82 | |
| Hispanic | 0.98 | 0.70–1.38 | |
| Other | 0.69 | 0.42–1.14 | |
| Performance status | 0.002 | ||
| 0 | 1.00 | referent | |
| 1 | 1.10 | 0.96–1.26 | |
| 2 | 1.55 | 1.21–1.98 | |
| FIGO Stage | 0.042 | ||
| III | 1.00 | referent | |
| IV | 1.17 | 1.01–1.37 | |
| Histology | < 0.001 | ||
| Serous | 1.00 | referent | |
| Mixed epithelial | 0.80 | 0.56–1.12 | |
| Endometrioid | 0.70 | 0.49–1.01 | |
| Clear-cell/mucinous | 3.66 | 2.55–5.27 | |
| Other | 1.03 | 0.66–1.61 | |
| CA-125 (μg/mL)§ | < 0.001 | ||
| < 150 | 1.02 | 1.01–1.03 | |
| ≥ 150 | 1.00 | 0.99–1.00 | |
| Tumor residual | 0.009 | ||
| Microscopic | 1.00 | referent | |
| ≤ 1 cm | 1.05 | 0.75–1.46 | |
| > 1 cm | 1.27 | 0.92–1.76 | |
| Treatment arm | < 0.001 | ||
| I (standard chemo.) | 1.00 | referent | |
| III (extended bev.) | 0.74 | 0.65–0.84 |
The p-values are from the overall test of significance of each covariate in the model.
The AHR denotes the change in risk of progression or death associated with an increase of 1 year in age.
The AHR denotes the change in risk of progression or death associated with an increase of 1kg/m2 in BMI.
The AHR denotes the change in risk of progression or death associated with a 10% increase in CA-125 (μg/mL) over the given ranges.
Bev = bevacizumab
Table 3.
Multivariate Overall Survival Analysis
| Covariate | AHR | 95% CI | P * |
|---|---|---|---|
| Ascites | 0.045 | ||
| No | 1.00 | referent | |
| Yes | 1.22 | 1.00–1.48 | |
| Age (years)† | 1.01 | 1.01–1.02 | < 0.001 |
| BMI (kg/m2)‡ | 1.01 | 1.00–1.02 | 0.071 |
| Race/Ethnicity | 0.802 | ||
| White | 1.00 | referent | |
| Asian | 0.94 | 0.68–1.28 | |
| Black | 1.19 | 0.83–1.71 | |
| Hispanic | 0.91 | 0.61–1.37 | |
| Other | 0.85 | 0.48–1.51 | |
| Performance status | < 0.001 | ||
| 0 | 1.00 | referent | |
| 1 | 1.15 | 0.99–1.34 | |
| 2 | 1.82 | 1.40–2.37 | |
| Stage | 0.077 | ||
| III | 1.00 | referent | |
| IV | 1.17 | 0.98–1.38 | |
| Histology | < 0.001 | ||
| Serous | 1.00 | referent | |
| Mixed epithelial | 0.92 | 0.62–1.35 | |
| Endometrioid | 0.75 | 0.49–1.14 | |
| Clear-cell/mucinous | 3.78 | 2.61–5.48 | |
| Other | 1.06 | 0.63–1.77 | |
| CA-125 (μg/mL)§ | < 0.001 | ||
| < 150 | 1.02 | 1.01–1.03 | |
| ≥ 150 | 1.00 | 0.99–1.01 | |
| Tumor residual | 0.753 | ||
| Microscopic | 1.00 | referent | |
| ≤ 1 cm | 0.96 | 0.67–1.38 | |
| > 1 cm | 1.02 | 0.72–1.44 | |
| Treatment arm | 0.053 | ||
| I (standard chemo.) | 1.00 | referent | |
| III (extended bev.) | 0.87 | 0.75–1.00 |
The p-values are from the overall test of significance of each covariate in the model.
The AHR denotes the change in risk of progression or death associated with an increase of 1 year in age.
The AHR denotes the change in risk of progression or death associated with an increase of 1kg/m2 in BMI.
The AHR denotes the change in risk of progression or death associated with a 10% increase in CA-125 (μg/mL) over the given ranges.
Bev = bevacizumab
Treatment arm as a prognostic factor
The multivariate model for PFS (Table 2) demonstrated that the adjusted hazard ratio for PFS was significantly improved in all patients treated with bevacizumab compared to those treated on the control arm: AHR for progression 0.74 (95% CI, 0.65-0.84), p<0.001. However, the multivariate OS model (Table 3) did not show a significant difference in OS for patients treated with bevacizumab compared to controls: AHR 0.87 (95% CI, 0.75-1.00), p=0.053. This finding was similar to the original analysis of GOG 0218.
Ascites as a predictive factor
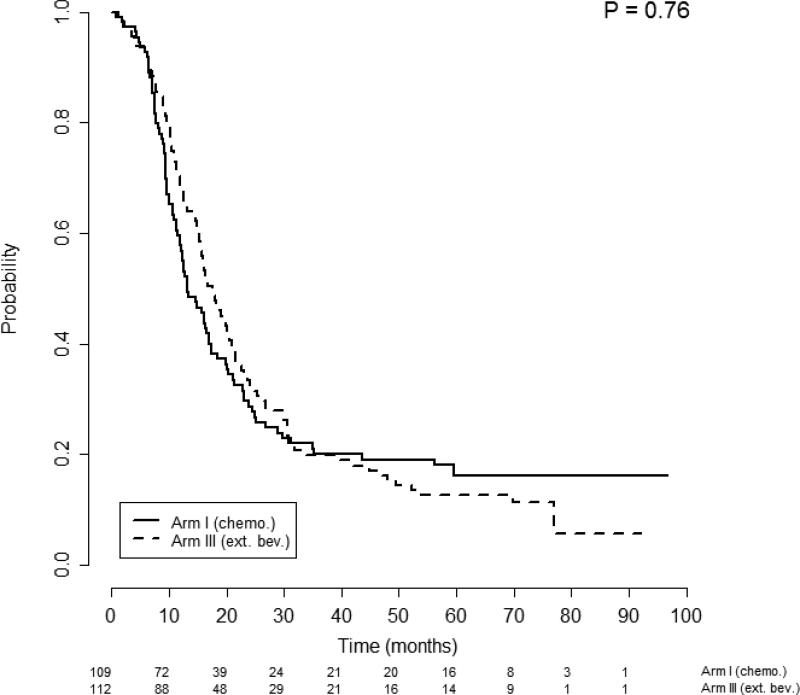
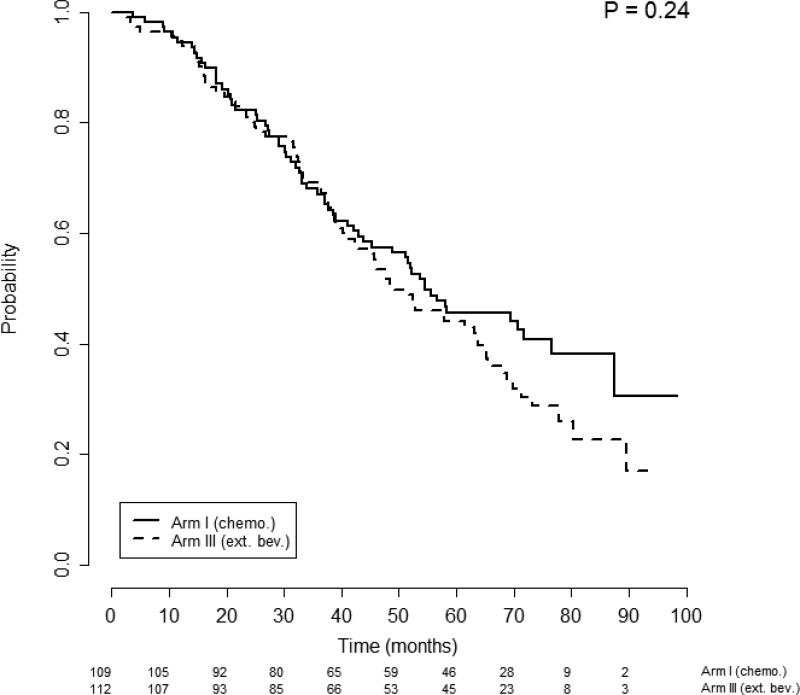
Given that the log-rank test of survival equality among the 4 possible ascites-by-treatment patient subgroups was significant, we conducted further analyses to determine whether ascites was predictive of response to bevacizumab. Survival differences were investigated separately for patients with or without ascites at randomization and stratified by treatment arm. Patients without ascites (n=221) had PFS that was not significantly different between treatment Arm 1: median of 13.1 months (95% CI, 12.0-17.4); and Arm 3: median of 17.5 months (95% CI, 15.4-21.0); p=0.76, (Figure 1). Multivariate analysis confirmed no significant difference in the risk of progression among patients without ascites between those that did and did not receive bevacizumab: AHR 0.81 (95% CI, 0.59-1.10), p=0.18. Similarly, OS among patients without ascites was not significantly different between Arm 1: Median of 54.5 months (95% CI, 43.7- —); and Arm 3: median of 48.5 months (95% CI, 42.3-64.8), p=0.24 (Figure 2). Once again, multivariate analysis confirmed no significant difference in the hazard of death by receipt of bevacizumab for patients without ascites: AHR 0.94 (95% CI, 0.65-1.36), p=0.76.
Figure 1.
Progression free survival of patients without ascites stratified by treatment arm. Figures below months indicate the numbers of patients at risk. The p-value is from the log-rank test of survival differences between the treatment subgroups. Arm 1 median PFS was 13.1 months (95% CI 12-17.4) compared with Arm 3 median PFS of 17.5 months (95% CI 15.4 - 21), p=0.76.
Figure 2.
Overall survival of patients without ascites stratified by treatment arm. Figures below months indicate the numbers of patients at risk. The p-value is from the log-rank test of survival differences between the treatment subgroups. Arm 1 median OS was 54.5 months (95% CI 43.7 - —) compared with Arm 3 median OS of 48.5 months (95% CI 42.3 – 64.8), p=0.24.
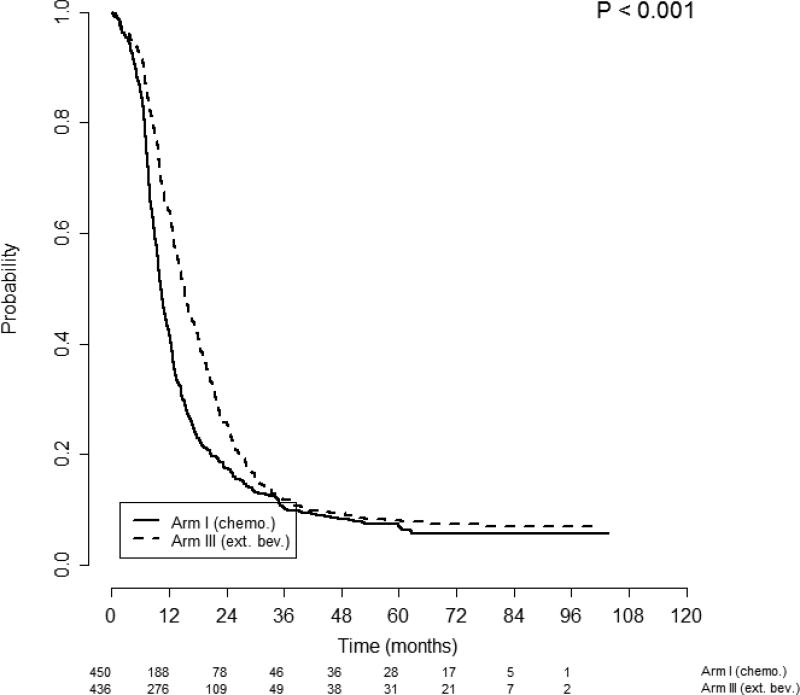
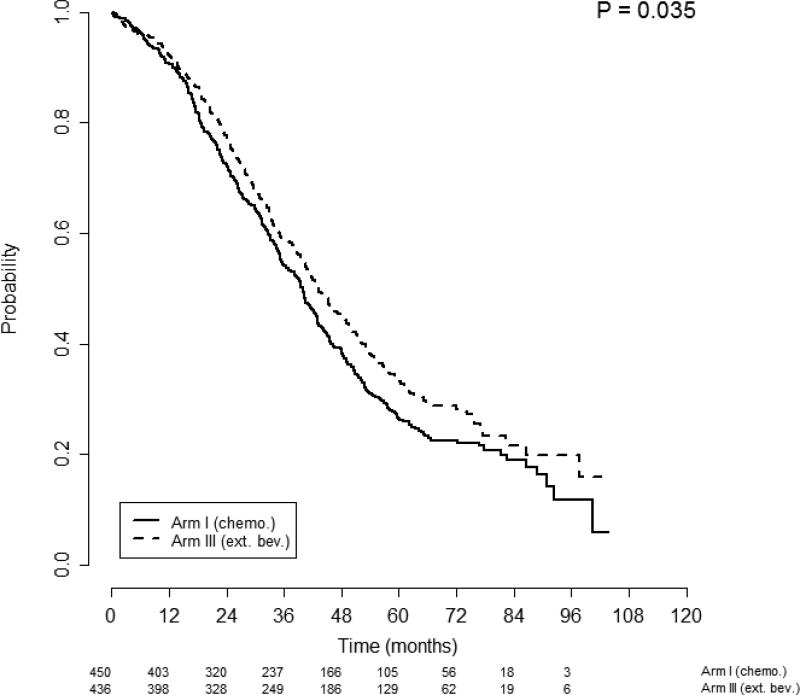
When patients with ascites (n=886) were analyzed by randomization to bevacizumab, improvements in both PFS and OS were observed. Patients with ascites in treatment Arm 1 had shorter PFS than those in Arm 3: median of 10.4 months (95% CI, 9.7–11.2 months) vs. 15.2 months (95% CI, 14.1–16.2 months), p<0.001, respectively (Figure 3). This finding was supported by an adjusted multivariate analysis: AHR 0.71 (95% CI, 0.62-0.81), p<0.001. Patients with ascites in treatment Arm 1 also had shorter OS than those in arm 3: median of 39.9 months (95% CI, 35.7–42.8) vs. 43.3 months (95% CI, 40.4–48.3), p=0.035 (Figure 4). The adjusted multivariate analysis confirmed this finding: AHR for OS 0.82 (95% CI, 0.70-0.96), p=0.014.
Figure 3.
Progression free survival of patients with ascites stratified by treatment arm. Figures below months indicate the numbers of patients at risk. The p-value is from the log-rank test of survival differences between the treatment subgroups. Arm 1 median PFS was 10.4 months (95% CI 9.7 – 11.2) compared with Arm 3 median PFS of 15.2 months (95% CI 14.1 – 16.2), p<0.001.
Figure 4.
Overall survival of patients with ascites stratified by treatment arm. Figures below months indicate the numbers of patients at risk. The p-value is from the log-rank test of survival differences between the treatment subgroups. Arm 1 median OS was 39.9 months (95% CI 35.7 – 42.8) compared with Arm 3 median OS of 43.3 months (95% CI 40.4 – 48.3), p=0.035.
DISCUSSION
This secondary analysis of GOG 0218 has confirmed that ascites is a negative prognostic factor for overall survival in a prospective cohort of advanced epithelial ovarian cancer patients. Further, we have identified a sub-group of patients, based on the presence of ascites, for which treatment with cytotoxic chemotherapy with bevacizumab followed by extended bevacizumab was associated with significant improvements in both progression free survival and overall survival. In addition, our findings support the plausible biologic rationale that patients with malignant ascites have cancers with a phenotype representative of the initiation phase of angiogenesis, and therefore are more likely to respond to anti-VEGF therapy. Moreover, this treatment effect was not observed among the patients without ascites. Therefore, we propose that ascites is a clinical biomarker predictive of response to anti-angiogenic therapy.
The optimal timing for the use of bevacizumab in advanced ovarian cancers remains a pressing clinical issue. In the primary setting, two large randomized trials (GOG 0218 and ICON 7) have demonstrated the combination of bevacizumab with standard therapy is associated with improved survival without progression. A subset analysis of ICON 7 patients with large volume macroscopic residual disease at completion of primary surgery or stage IV disease deemed “high risk” demonstrated a greater than 7 month median OS benefit: HR 0.64 (95% CI, 0.48-0.85; p=0.002). The intent-to-treat analysis of GOG 0218 failed to demonstrate a significant OS benefit; potential explanations for lack of a statistically significant effect include long post-progression survival times in this study population, the utilization of multiple regimens to manage progressive or recurrent disease, including a high frequency of cross-over to commercial bevacizumab or other anti-angiogenic agents. Similar to the post hoc analysis identifying a “high-risk” subgroup appearing to benefit significantly in terms of OS in ICON 7, our secondary analysis of GOG 0218 demonstrated a significant impact on OS when considering a selected group (patients with ascites) at higher risk for recurrence and death from disease.
Predictive markers that accurately identify sub-groups of patients that would derive maximum benefit from a given targeted therapy have been eagerly sought. Recent papers highlight differing approaches toward this goal. Wimberger et al, studied total VEGF receptor expression by immunohistochemistry in the primary tumors of 73 patients, and noted a significant correlation between total receptor expression and sub-optimal cytoreduction [27]. Specifically, the expression of VEGF receptor 1 was prognostic and significantly associated with a worse PFS in this cohort. As this investigation was exploratory in nature, no data regarding any association between VEGF receptor expression and clinical response to anti-angiogenic therapy were described. Chan, et al, reported a preliminary biomarker study with exploratory clinical outcomes based on data from The Cancer Genome Atlas (TCGA) project [28]. This study focused on the micro-RNA, miR-378, which has been implicated in metastasis. The authors found that miR-378 was overexpressed in ovarian cancers and that downstream targets of this molecule may serve as predictive markers of response to anti-angiogenic agents. Specifically, the overexpression of the target gene, ALCAM, was predictive of improved PFS in the TCGA cohort, while overexpression of EHD1 was predictive of a worse PFS. Their results are intriguing but have yet to be validated. A biomarker signature predictive of efficacy for bevacizumab has been reported by Collinson, et al. [29]. The authors used clinical specimens from ICON 7 to identify three candidate serum markers: mesothelin, fms-like tyrosine kinase-4, and α1-acid glycoprotein. These markers, along with CA-125, established a proteomic signature that was predictive of both PFS and OS in patients treated with bevacizumab in the first validation cohort, and of PFS in the second validation cohort. Additional validation studies on larger cohorts of patients are needed to better understand the clinical utility of this proteomic signature.
Evaluating sub-sets of patients from ICON7, Winterhoff, et al. suggested that the molecular classification system proposed by The Cancer Genome Atlas (TCGA) project could be used to predict response to bevacizumab [30]. The authors obtained 380 specimens from patients enrolled in ICON7, and used gene expression data to stratify them into one of the four TCGA classifications: Differentiated, immunoreactive, mesenchymal and proliferative. Only patients with the mesenchymal tumor type appeared to derive a progression free survival benefit from treatment with bevacizumab.
Strengths of this investigation include the large number of patients available for review from a prospective, randomized, placebo controlled, multi-institutional clinical trial. Patients were classified as with or without ascites by their treating institution prior to randomization, thus limiting selection bias. All patients had pre-specified evaluation and follow up, and standard definitions of disease progression or recurrence were used. Finally, the outcomes data are mature, with a median follow up of 73.2 months (95% CI 71.8-74).
However, this study is limited in several ways. The post hoc nature of the analysis renders the results hypothesis generating rather than conclusive. Also, given that 20% of patients were classified as not having ascites, there may have been insufficient power to demonstrate a statistically significant impact of bevacizumab on survival in this subset. Additionally, it is possible that volume of ascites could be a more robust predictor of degree of benefit from VEGF targeted therapy. Unfortunately the classification of ascites for patients enrolled onto GOG 0218 was semi-quantitative, and thus we were unable to determine the relationship between ascites volume per se and outcome measures in those treated with or without bevacizumab. Finally, it would be premature and ill advised to incorporate these findings into clinical practice based on a single study, and the results are unlikely to be confirmed in a prospective manner. However, if these findings were to be validated through a similar analysis of data from one or more of the independent randomized phase III trials, the clinical determination of malignant ascites could be a simple and cost-effective way of selecting patients with the greatest probability of long term benefit from bevacizumab.
Highlights.
We analyzed data from GOG 0218 to determine if ascites predicts response to bevacizumab.
Ascites was shown to be a negative prognostic factor in epithelial ovarian cancers.
Ascites is a significant clinical factor that may predict response to bevacizumab.
ACKNOWLEDGEMENTS
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), the NRG Oncology SDMC grant U10 CA180822 and the NRG Oncology Operations grant U10CA 180868. This investigation was also supported by the GOG Young Investigator Award. The clinical trial upon which this manuscript is based was sponsored by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI), under the Collaborative Research and Development Agreement (CRADA) for bevacizumab between NCI and Genentech, Inc.
The following Gynecologic Oncology Group member institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Mount Sinai School of Medicine, Northwestern Memorial Hospital, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Rush-Presbyterian-St. Luke's Medical Center, Magee Women's Hospital, SUNY Downstate Medical Center, University of Kentucky, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Yale University, GOG Japan-Saitama Medical University International Medical Center, University of Wisconsin Hospital, Cancer Trials Support Unit, University of Texas - Galveston, Women and Infants Hospital, Korean Gynecologic Oncology Group, The Hospital of Central Connecticut, Georgia Core, GYN Oncology of West Michigan, PLLC, Aurora Women's Pavilion of West Allis Memorial Hospital, and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentations: This manuscript was presented March 23, 2014, during a scientific plenary session at the Society of Gynecologic Oncology's Annual Meeting on Women's Cancer, Tampa, Florida.
CONFLICT OF INTEREST
Dr. Robert Burger has served in a limited capacity as a consultant to Genentech at Advisory Board meetings. In addition, Dr. Michael Bookman who would like to disclose that he is a member of the Independent Data Monitoring Committee for phase III trials in ovarian cancer for Genentech-Roche as well as a member of Boehringer-Inghelheim's Independent Data Monitoring Committee for phase III trials in ovarian cancer. Additionally, Dr. Bookman serves on the Ad-hoc advisory board for AbbVie which serves to develop clinical trials in ovarian cancer with investigational (non-marketed) agents. Dr. Bookman also reports that he serves on numerous Ad-hoc advisory boards and has received personal fees from each of the following entities: AstraZeneca, Genentech-Roche, Novartis, Endocyte, Gradalis, Cerulean, GlaxoSmithKline, Clovis Oncology, Daiichi Sankyo, OxiGene, and Sanofi Aventi. His position on these boards assists in the development of clinical trials in ovarian cancer with investigational (non-marketed) agents. Further, Dr. Gini Fleming reports that she is the Institutional PI of a different Genentech-Roche sponsored trial while Dr. Monk discloses that he has received honorariums from Roche/Genentech for speaker bureaus and consulting fees from Roche/Genentech. Dr. Monk also reports that his institution has received research grants from Genentech. Finally, Dr. Matthew Boente discloses that he is a member of the Genentech Speakers Bureau and has received personal fees. There are no other conflicts of interest to report.
REFERENCES
- 1.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of New Platinum-Based Treatment Regimens in Advanced-Stage Ovarian Cancer: A Phase III Trial of the Gynecologic Cancer InterGroup. J.Clin.Oncol. 2009 Mar 20;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akutagawa N, Nishikawa A, Twasaki M, Fujimoto T, Teramoto M, Kitajima Y, et al. Expression of vascular endothelial growth factor and E-cadherin in human ovarian cancer: association with ascites fluid accumulation and peritoneal dissemination in mouse ascites model. Jpn.J.Cancer Res. 2002 Jun;93:644–651. doi: 10.1111/j.1349-7006.2002.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabushita FT, Shimazu M, Noguchi M, Kishida T, Narumiya H, Sawaguchi K, et al. Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer. Oncol.Rep. 2003 Jan-Feb;10:89–95. [PubMed] [Google Scholar]
- 4.Belotti D, Calcagno C, Garofalo A, Caronia D, Riccardi E, Giavazzi R, et al. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol.Cancer.Res. 2008 Apr;6:525–534. doi: 10.1158/1541-7786.MCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 5.Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999 Jan 1;85:178–187. [PubMed] [Google Scholar]
- 6.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am.J.Pathol. 1995 Jul;147:33–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin.Cancer Res. 1999 Mar;5(5):87–591. [PubMed] [Google Scholar]
- 8.Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998 Jun 15;58:2594–2600. [PubMed] [Google Scholar]
- 9.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003 Sep l;63:5224–5229. [PubMed] [Google Scholar]
- 10.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J.Clin.Oncol. 2007 Nov 20;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 11.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J.Clin.Oncol. 2007 Nov 20;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J.Clin.Oncol. 2014 May 1;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 13.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J.Clin.Oncol. 2012 Jun 10;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N.Engl.J.Med. 2011 Dec 29;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 15.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N.Engl.J.Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. 12/29; 2012/03. [DOI] [PubMed] [Google Scholar]
- 16.Randall L, Burger R, Nguyen H. Outcome differences in patients with stage IV epithelial ovarian, primary peritoneal and fallopian tube cancers treated with and without bevacizumab. Annual Meeting of the Society of Gynecology. 2013 Abstracts. [Google Scholar]
- 17.Omura GA, Brady MF, Homesley FID, Yordan E, Major FJ, Buchsbaum HJ, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J.Clin.Oncol. 1991 Jul;9(l):138–1150. doi: 10.1200/JCO.1991.9.7.1138. [DOI] [PubMed] [Google Scholar]
- 18.Luo JC, Yamaguchi S, Shinkai A, Shitara K, Shibuya M. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res. 1998 Jun 15;58:2652–2660. [PubMed] [Google Scholar]
- 19.Herr D, Sallmann A, Bekes I, Konrad R, Holzheu I, Kreienberg R, et al. VEGF induces ascites in ovarian cancer patients via increasing peritoneal permeability by downregulation of Claudin 5. Gynecol.Oncol. 2012 May 8; doi: 10.1016/j.ygyno.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JT, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin.Cancer Res. 2003 Nov 15;9:5721–5728. [PubMed] [Google Scholar]
- 21.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated systme of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine Series 5. 1900;50:157–175. [Google Scholar]
- 22.Mann H, Whitney D. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 23.Kaplan E, Meier P. Nonparametric Estimation from incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 24.Cox D. Regression Models and Life-Tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 25.Molinari N, Daures J, Durand J. Regression splines for threshold selection in survival data analysis. Statistics in Medcine. 2001;20:237–247. doi: 10.1002/1097-0258(20010130)20:2<237::aid-sim654>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2013. [Google Scholar]
- 27.Wimberger P, Chebouti I, Kasimir-Bauer S, Lachmann R, Kuhlisch E, Kimmig R, et al. Explorative investigation of vascular endothelial growth factor receptor expression in primary ovarian cancer and its clinical relevance. Gynecol.Oncol. 2014 Apr 5; doi: 10.1016/j.ygyno.2014.03.574. [DOI] [PubMed] [Google Scholar]
- 28.Chan JK, Kiet TK, Blansit K, Ramasubbaiah R, Hilton JF, Kapp DS, et al. MiR-378 as a biomarker for response to anti-angiogenic treatment in ovarian cancer. Gynecol.Oncol. 2014 Mar 25; doi: 10.1016/j.ygyno.2014.03.564. [DOI] [PubMed] [Google Scholar]
- 29.Collinson F, Hutchinson M, Craven RA, Cairns DA, Zougman A, Wind TC, et al. Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection. Clin.Cancer Res. 2013 Sep 15;19:5227–5239. doi: 10.1158/1078-0432.CCR-13-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winterhoff BJ, Kommoss S, Oberg AL, Wang C, Riska SM, Konecny GE, et al. Bevacizumab and improvement of progression-free survival (PFS) for patients with the mesenchymal molecular subtype of ovarian cancer. J Clin Oncol (Meeting Abstracts) 2014:32. [Google Scholar]
- 31.Gourley C, McCavigan A, Perren T, Paul J, Michie CO, Churchman M, et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J Clin Oncol (Meeting Abstracts) 2014;32:5502. [Google Scholar]