Abstract
Background
The clarification of cutaneous dendritic cell subset and the role of thymic stromal lymphopoietin (TSLP) signaling in epicutaneous sensitization with protein antigens, as in the development of atopic dermatitis, is a crucial issue.
Objectives
Because TSLP is highly expressed in the vicinity of Langerhans cells (LCs), we sought to clarify our hypothesis that LCs play an essential role in epicutaneous sensitization with protein antigens through TSLP signaling.
Methods
By using Langerin-diphtheria toxin receptor knock-in mice and human Langerin-diphtheria toxin A transgenic mice, we prepared mice deficient in LCs. We also prepared mice deficient in TSLP receptors in LCs by using TSLP receptor–deficient mice with bone marrow chimeric technique. We applied these mice to an ovalbumin (OVA)-induced epicutaneous sensitization model.
Results
Upon the epicutaneous application of OVA, conditional LC depletion attenuated the development of clinical manifestations as well as serum OVA-specific IgE increase, OVA-specific T-cell proliferation, and IL-4 mRNA expression in the draining lymph nodes. Consistently, even in the steady state, permanent LC depletion resulted in decreased serum IgE levels, suggesting that LCs mediate the TH2 local environment. In addition, mice deficient in TSLP receptors on LCs abrogated the induction of OVA-specific IgE levels upon epicutaneous OVA sensitization.
Conclusion
LCs initiate epicutaneous sensitization with protein antigens and induce TH2-type immune responses via TSLP signaling.
Keywords: Langerhans cell, TSLP, TSLP receptor, epicutaneous sensitization, protein antigen
Skin plays an important immunologic role by eliciting a wide variety of immune responses to foreign antigens.1 Atopic dermatitis (AD) is a pruritic chronic retractable inflammatory skin disease that is induced by the complex interaction between susceptibility genes encoding skin barrier components and stimulation by protein antigens.2,3 Patients with AD exhibit compromised barrier function that leads to the activation of keratinocytes and immune cells, which favors a TH2 bias. A wide array of cytokines and chemokines interact to yield symptoms that are characteristic of AD. For example, thymus and activation-regulated chemokine (CCL17) and macrophage-derived chemokine (CCL22) both attract TH2 cells through CC chemokine receptor 4,4 levels of which correlate well with the severity of AD.5 Elevation in serum IgE levels is also frequently found in patients with AD, sometimes concomitant with food allergy, allergic rhinitis, and asthma.3 Yet it remains unknown how the elevation in serum IgE levels on epicutaneous sensitization with protein antigens is induced in the pathogenesis of AD.
Upon protein antigen exposure, dendritic cells (DCs) acquire antigens and stimulate the proliferation of T cells to induce distinct T helper cell responses to external pathogens.6 Therefore, it has been suggested that DCs initiate AD in humans7; however, it remains unclear which cutaneous DC subset initiates epicutaneous sensitization to protein antigens. In the mouse skin, there are at least 3 subsets of DCs: Langerhans cells (LCs) in the epidermis and Langerin-positive and Langerin-negative DCs in the dermis (Langerin+ dermal DCs and Langerin− dermal DCs, respectively).8–10 It has been reported that the application of large molecules is localized above the size-selective barrier, tight junction (TJ), and that activated LCs extend their dendrites through the TJ to take up antigens.11 Therefore, it can be hypothesized that it is not dermal DCs but rather LCs that initiate epicutaneous sensitization with protein antigens, as in the development of AD.
In humans, polymorphisms in the gene encoding the cytokine thymic stromal lymphopoietin (TSLP) are associated with the development of multiple allergic disorders through the TSLP receptor (TSLPR), which is expressed in several cell types, such as DCs, T cells, B cells, basophils, and eosinophils.12,13 Thus, TSLP seems to be a critical regulator of TH2 cytokine-associated inflammatory diseases.
Recently, it has been reported that basophils induce TH2 through TSLPR.13 On the other hand, it is also known that skin DCs elicit a TH2 response in the presence of mechanical injury by inducing cutaneous TSLP14 and that LCs are critical in the development of skin lesions induced by the topical application of vitamin D3 analogues through TSLP signaling.15 However, these skin inflammation models are induced in an antigen-independent manner; therefore, it is important to address the degree to which TSLP is essential in TH2 shifting and to identify the cells that are essential for TSLP signaling transduction upon epicutaneous sensitization, which is relevant to inflammatory skin diseases, such as AD. This will lead to the understanding of the underlying mechanism and to developing new therapeutic targets for inflammatory skin diseases.
It is known that TSLP activates human epidermal LCs and DCs in vitro16–18 and that TSLP is highly expressed in the epidermis of the lesional skin of patients with AD. Since LCs are localized in the epidermis, we hypothesized that LCs initiate epicutaneous sensitization through TSLP signaling. By applying an LC ablation system, we found that LCs are crucial for TH2 induction and IgE production upon epicutaneous protein exposure through TSLP signaling.
METHODS
Animals and bone marrow chimera
C57BL6 (B6) and BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). OT-II T-cell receptor transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, Me). Langerin-diphtheria toxin subunit A (DTA) mice were generated by Dr Daniel Kaplan,19 and Langerin-enhanced green fluorescent protein (eGFP)-diphtheria toxin receptor (DTR) knock-in mice were kindly provided by Dr Bernard Mallissen (CIML, Institut National de la Santé et de la Recherche Médicale, Marseille, France).
TSLPR−/− mice (BALB/c or B6 background) were generated by Dr Steven Ziegler.20 Seven- to 12-week-old female mice bred in specific pathogen-free facilities at Kyoto University were used for all experiments.
For LC depletion specifically, Langerin-eGFP-DTR mice were used. Intraperitoneal injection of 1 μg of DT (Sigma-Aldrich, St Louis, Mo) in 500 μL of PBS depleted Langerin+ DC subsets, including LCs and Langerin+ dermal DCs. Langerin+ dermal DCs in the dermis recover 1 week after DT injection, but LCs remain undetectable for 4 weeks after depletion.21 Since only LCs are depleted between 1 and 3 weeks after DT injection, we can evaluate the role of LCs in epicutaneous sensitization by applying ovalbumin (OVA) between 1 and 3 weeks after DT injection. Therefore, we injected DT 7 days before epicutaneous sensitization. Control mice were intraperitoneally injected with 500 μL of PBS on the same day.
To generate bone marrow (BM) chimeric (BMC) mice, 6-week-old mice were irradiated (9 Gy) and transplanted with BM cells (1 × 107 cells per recipient). All experimental procedures were approved by the institutional Animal Care and Use Committee of Kyoto University Graduate School of Medicine.
Epicutaneous sensitization
Mice were anesthetized with diethylethel (Nacalai Tesque, Kyoto, Japan) and then shaved with an electric razor (THRIVE Co Ltd, Osaka, Japan). A single skin site on each mouse was tape-stripped at least 5 times with adhesive cellophane tape (Nichiban, Tokyo, Japan). One hundred microgram of OVA in 100 μL of normal saline or placebo was placed on patch-test tape (Torii Pharmaceutical Co, Ltd, Tokyo, Japan). Each mouse had a total of three 2-day exposures to the patch, separated by 1-day intervals. Mice were euthanized at the end of the third cycle of sensitization (day 9).
Antigen-specific T-cell proliferation
To assess the OVA-specific T-cell priming capacity of cutaneous LCs, 100 μL of normal saline with or without 100 μg of OVA was placed on the shaved and tape-stripped mouse back skin. CD4 T cells were isolated from OT-II mice by using magnetic bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany) and labeled with 8 μM of carboxyfluorescein succinimidyl ester (CFSE). Forty-eight hours after epicutaneous sensitization, 5 × 106 CFSE-labeled OT-II T cells were transferred to naive mice via the tail vein. An additional 48 hours later, skin draining brachial lymph nodes (LNs) were collected and analyzed by means of flow cytometry.
Statistical analysis
Unless otherwise indicated, data are presented as means ± SDs and each data point is representative of 3 independent experiments. P values were calculated according to the 2-tailed Student t test.
A complete description of the methods, and any associated references, is available in this article’s Online Repository at www.jacionline.org.
RESULTS
LC depletion impaired the development of OVA-induced allergic skin dermatitis model
To assess the role of LCs in epicutaneous sensitization with protein antigens and induction of IgE, we applied OVA to mice epicutaneously.22 In this model, we observed a rise in OVA-specific serum IgE and IgG1 levels, both of which are induced in a TH2-dependent manner, as well as the development of dermatitis characterized by the infiltration of CD3+ T cells, eosinophils, and neutrophils and local expression of mRNA for the cytokines IL-4, IL-5, and IFN-γ.22 These findings exhibited characteristics of allergic skin inflammation such as AD. To evaluate the roles of LCs, we used knock-in mice expressing eGFP and DTR under the control of the Langerin gene, called Langerin-eGFP-DTR mice.23
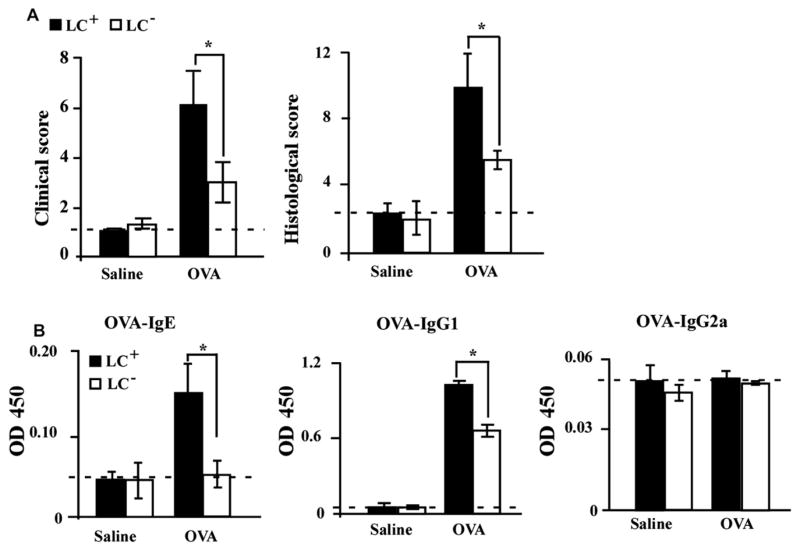
In the OVA-induced allergic skin dermatitis model, LC-depleted mice showed milder clinical manifestations than did LC–non-depleted mice (Fig 1, A, left panel). Histology of the patched skin area showed pronounced lymphocyte infiltration and edema in the dermis of sensitized LC–non-depleted mice, which was less apparent in sensitized LC-depleted mice (see Fig E1, A and B, in this article’s Online Repository at www.jacionline.org). The histologic score of LC-depleted mice was also lower than that of LC–non-depleted mice (Fig 1, A, right panel). In addition, serum OVA-specific IgE and IgG1 levels in LC-depleted mice were significantly lower than those in wild-type (WT) mice (Fig 1, B). On the other hand, the TH1-dependent immunoglobulin IgG2a was not induced by the application of OVA (Fig 1, B). These data suggest that LCs are involved in the development of OVA-induced AD-like skin inflammation and induction of IgE.
FIG 1.
LCs are crucial for epicutaneous sensitization with OVA. A, Total clinical severity scores (left panel) and total histology scores (right panel) of LC–non-depleted (LC+) and LC-depleted (LC−) mice (n = 5 mice per group). B, Serum OVA-specific antibodies as determined by ELISA. OD values for IgE, IgG1, and IgG2a levels were measured at a wavelength of 450 nm. *P < .05.
Impaired T-cell proliferation and TH2 induction by LC depletion
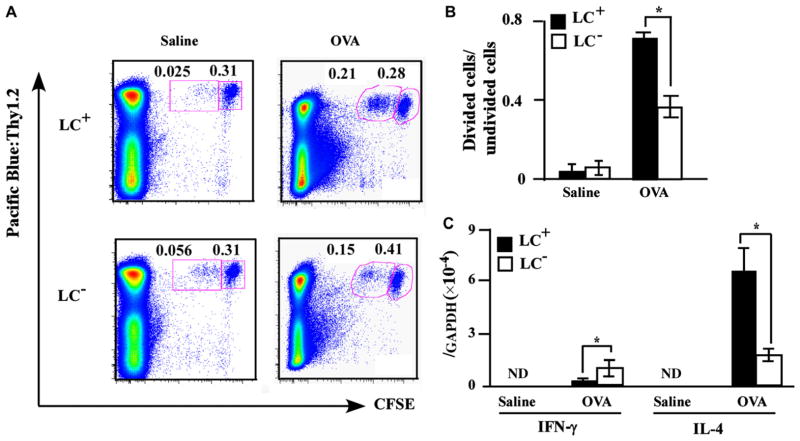
Priming of antigen-specific TH2 cells and proliferation is an important step in the development of this model. To assess the T-cell priming capacity of cutaneous LCs upon protein allergen exposure, LC-depleted and LC–non-depleted mice were percutaneously sensitized with OVA on the back and transferred with CFSE-labeled OT-II T cells, which express an OVA-specific T-cell antigen receptor. Next, single-cell suspensions prepared from the skin-draining brachial LNs were analyzed by means of flow cytometry to evaluate T-cell division by LCs in the draining LNs. LC-depleted mice showed impaired T-cell division after OVA sensitization compared with LC–non-depleted mice, suggesting that LCs stimulate T-cell proliferation, at least to some degree, in this model (Fig 2, A and B).
FIG 2.
LCs are critical for antigen-specific T-cell proliferation. Mice in the presence or absence of LCs (LC+ and LC−, respectively) were treated with OVA and transplanted with CFSE-labeled OT-II T cells (n = 5 mice per group). Skin-draining LNs were analyzed for OVA-specific T-cell proliferation (A and B) and mRNA expression levels for IFN-γ and IL-4 (C). Boxes in (A) demarcate divided cells (left) and undivided cells (right). *P < .05. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; ND, not detected.
To evaluate the role of LCs in T-cell priming, we examined the mRNA expression of TH2 cytokine IL-4 and TH1 cytokine IFN-γ in draining LNs after OVA sensitization. The IL-4 mRNA expression level of draining LNs was significantly decreased in LC-depleted mice, while the IFN-γ mRNA expression level was significantly higher in LC-depleted mice than in LC–non-depleted mice (Fig 2, C). These results suggest that LCs are crucial for stimulating T-cell proliferation to a certain extent and TH2 induction pronouncedly in skin-draining LNs in this model.
LCs are responsible for initiating epicutaneous sensitization to protein antigens
It has been reported that LCs are dispensable for initiating contact hypersensitivity to haptens, which may cause a discrepancy in our findings on the necessity of LCs for protein antigen sensitization.21,24 To evaluate the extent of skin penetration by protein antigens and haptens, we patched fluorescein isothiocyanate (FITC)-conjugated OVA or painted FITC on the back skin of B6 mice, and performed immunohistochemical analysis. FITC-conjugated OVA retained above the TJ was indicated by staining with anti–claudin-1 antibody (see Fig E2, left panel, in this article’s Online Repository at www.jacionline.org). On the other hand, when we painted FITC on the skin of the mouse back skin, it readily penetrated into the dermis where dermal DCs locate (Fig E2, right panel).
LCs are critical for IgE production
To further assess the role of LCs in IgE production, we used gene-targeted Langerin-DTA mice, which constitutively lack LCs throughout life.19 WT and Langerin-DTA mice were bred under super pathogen free conditions for 6 to 10 weeks, and serum IgE levels were measured by means of ELISA. On the FVB background, the serum IgE level was lower in Langerin-DTA mice than in WT controls (Fig 3, A, left panel), while no significant difference was seen on the C57BL/6 (B6) background (Fig 3, A, right panel). We also found that the expression level of IgE on peritoneal mast cells was decreased in LC-deficient mice in both the FVB and B6 backgrounds (Fig 3, B). Preincubation of mast cells with IgE in vitro did not change the data, arguing that surface expression of FcεRI on mast cells was decreased in LC-deficient mice, which is an indicator of lower serum IgE level. Therefore, the above data strongly suggest that LCs are crucial for IgE production, which is consistent with the findings in the OVA-induced skin inflammation model (Figs 1 and 2).
FIG 3.
LCs are essential for IgE production. The serum IgE levels (A) and IgE expression levels (B) on peritoneal mast cells (indicated by MFI) of WT and Langerin-DTA mice on FVB (left panel) and B6 (right panel) backgrounds. Mast cells were also preincubated with IgE (labeled with pre-IgE) in vitro before the measurement of IgE expression (Fig 3, B). Each symbol represents an individual animal. *P < .05. MFI, Mean fluorescence intensity.
TSLP receptor on LCs is upregulated by protein antigen exposure
It has been reported that TSLP is involved in the exacerbation of mouse TH2-mediated allergic inflammation through the direct stimulation of TH2 effector cells.25 However, it remains unknown which cells initiate TH2 induction via TSLP signaling under the epicutaneous sensitization of protein antigens. TSLP is highly expressed in the skin lesions of human AD,17,18,26,27 and the major cells in proximity to keratinocytes are LCs; therefore, we evaluated the effect of TSLPR expression on LCs. We found that LCs expressed TSLPR, but the expression level was low under the steady state. On the other hand, the expression level of TSLPR on LCs was pronouncedly enhanced by the topical application of OVA (Fig 4).
FIG 4.
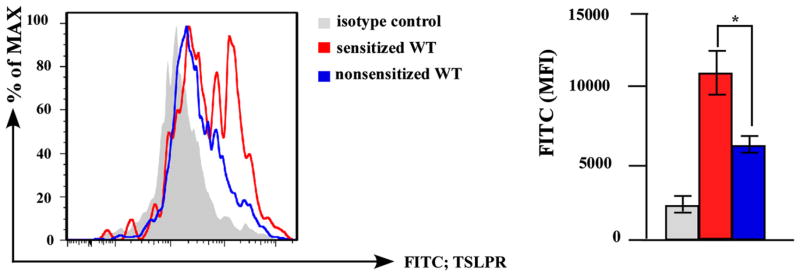
TSLPR on LCs is a responsible target of TSLP upon epicutaneous OVA sensitization. Epidermal cell suspensions from B6 (WT) mice with (sensitized) or without (nonsensitized) epidermal application of OVA were stained with TSLPR antibody. TSLPR expressions of MHC class II+ CD11c+ LCs was analyzed by flow cytometry (left, histogram; right, average ± SD of MFI). n = 3 per group. *P < .05. MFI, Mean fluorescence intensity.
Establishment of BMC mice deficient in TSLPR on LCs
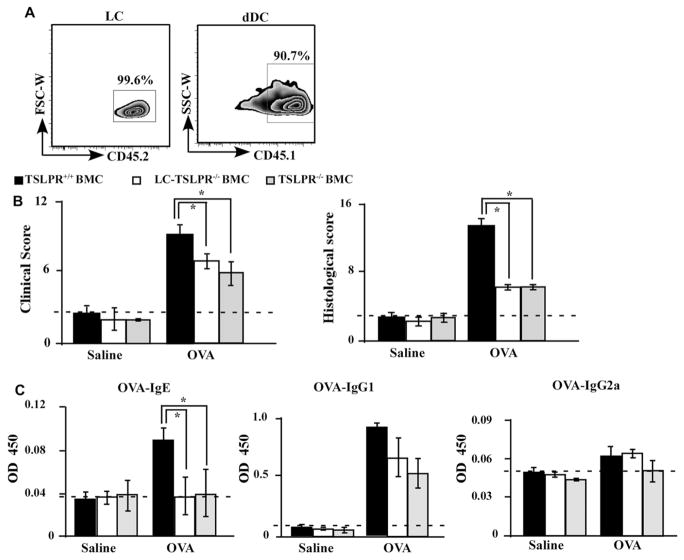
Next, we sought to clarify the significance of TSLP in epicutaneous sensitization with protein antigens and to identify responsible cells mediating TSLP signaling. Since cells ensuring epidermal LC renewal are radioresistant, LCs and their derivatives found in skin-draining LNs are of host origin.28 We irradiated B6 mice and B6 background TSLPR-deficient (TSLPR−/−) mice, and then transferred BM cells from B6 mice into the irradiated mice. TSLPR is expressed on not only LCs but also T cells, B cells, basophils, eosinophils, and dermal DCs. Of note, LCs are radioresistant while T cells, B cells, basophils, eosinophils, and dermal DCs are radiosensitive. When mice were irradiated and transplanted with BM cells, more than 95% of the blood cells in the recipient mice had been replaced with donor-derived cells within 2 months after the transfer, whereas almost 100% of LCs were derived from the host, unlike the vast majority of dermal DCs that were donor-derived at this point (Fig 5, A). Therefore, given that TSLPR−/− mice were reconstituted with BM cells from B6 mice, these mice were deficient in TSLPR on LCs, but other BM-derived cells expressing TSLPR were present. Accordingly, by using a hematopoietic BMC system, we generated mice in which TSLPRs were lacking in LCs (LC-TSLPR−/− BMC mice) (see Fig E3 in this article’s Online Repository at www.jacionline.org).
FIG 5.
An essential target of TSLP for IgE induction is TSLPR on LCs. A, B6 (Ly45.2) mice were irradiated and transplanted with BM cells from B6 (Ly45.1) mice. The epidermis and the dermis of BMC mice were separated, and single-cell suspensions were stained and analyzed by flow cytometry. B, Total clinical severity scores (left panel) and histology scores (right panel) of TSLPR+/+ BMC, LC-TSLPR−/− BMC, and TSLPR−/− BMC mice (n = 5 mice per group). C, Serum OVA-specific antibodies as determined by ELISA. OD values for IgE, IgG1, and IgG2a levels were measured at a wavelength of 450 nm. *P < .05.
Essential target of TSLP is TSLPR on LCs in OVA-induced allergic skin dermatitis model
In the context of OVA-induced AD-like skin inflammation, LC-TSLPR−/− BMC mice showed milder clinical and histologic findings than did TSLPR+/+ BMC mice, but these findings were nearly comparable with those of TSLPR−/− BMC mice (Fig 5, B; see Fig E4 in this article’s Online Repository at www.jacionline.org). Consistently, OVA-specific IgE levels in the serum after OVA challenge were significantly lower in LC-TSLPR−/− BMC mice than in TSLPR+/+ BMC mice (Fig 5, C). These data indicate that LCs play an important role in epicutaneous sensitization upon protein antigens in accord with IgE induction through TSLP-TSLPR signaling.
TSLPR on LCs are dispensable for antigen-specific T-cell proliferation but vital for TH2 induction
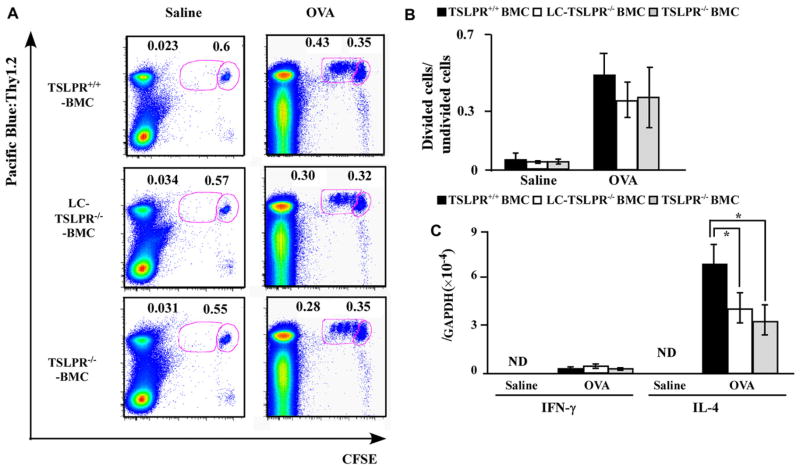
The above results suggest that LCs stimulate T cells to differentiate into TH2, resulting in IgE induction. To clarify this issue, we assessed the T-cell proliferation and differentiation capacity of LCs in the presence or absence of TSLPR. We transferred CFSE-labeled OT-II T cells into mice topically treated with OVA, and dividing cells in the draining LNs were measured by means of flow cytometry (Fig 6, A). The ratio of dividing OT-II CD4+ T cells to undivided OT-II CD4+ T cells was comparable among LC-TSLPR−/− BMC, TSLPR+/+ BMC, and TSLPR−/− BMC mice (Fig 6, B). In addition, IFN-γ mRNA level in the draining LNs 96 hours after OVA application was similar among these 3 groups (Fig 6, C). On the other hand, the IL-4 mRNA expression level in skin-draining LNs was significantly lower in LC-TSLPR−/− BMC mice than in the other 2 groups (Fig 6, C). These results indicate that TSLPRs on LCs are dispensable for antigen-specific T-cell proliferation but vital for inducing TH2 differentiation.
FIG 6.
TSLPR on LCs are vital for TH2 induction. TSLPR+/+ BMC, LC-TSLPR−/− BMC, and TSLPR−/− BMC mice were treated with OVA or saline and transplanted with CFSE-labeled OT-II T cells. Skin-draining LNs were analyzed for OVA-specific T-cell proliferation (A and B) and cytokine mRNA expression levels for IFN-γ and IL-4 (C). Boxes in (A) demarcate divided cells (left) and undivided cells (right). n = 5 mice per group. *P < .05. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; ND, not detected.
TSLP promotes the expression of OX40L and the production of TH2 chemokines by DCs
We next sought to elucidate the mechanism underlying TH2 induction of LCs via TSLP-TSLPR signaling. Modulation of costimulatory molecule expression was among the candidates, as it has been demonstrated that the interaction between membrane OX40L on DCs and OX40 on naive T cells results in the induction of IL-4 production by T cells in humans,26 and that treating mice with OX40L-blocking antibodies substantially inhibit the TH2 immune responses induced by TSLP in the lung and the skin.29
Therefore, it is important to evaluate the expression levels of costimulatory molecules on LCs in OVA-sensitized skin by means of flow cytometry. TSLPR−/− (BALB/c background) and WT control BALB/c mice were percutaneously sensitized with OVA. Seventy-two hours later, epidermal cell suspensions were prepared and stained with anti-OX40L, CD80, and CD40 antibodies. The mean fluorescence intensity of OX40L expressed by LCs from OVA-sensitized TSLPR−/− mice was significantly lower than that in WT control mice. On the other hand, expression levels of CD40 and CD80 on LCs were comparable between WT control and TSLPR−/− mice (see Fig E5, A, in this article’s Online Repository at www.jacionline.org).
It is known that serum levels of CCL17 and CCL22 correlate with the severity of AD.5 We incubated BM-derived DCs from BALB/c mice with recombinant mouse TSLP and found that TSLP induced DCs to express CCL17 and CCL22 mRNA (Fig E5, B), while the expression level of the TH1 chemokine CXCL10 was suppressed by TSLP (Fig E5, C). These results suggest that TSLP instructs cutaneous DCs to create a TH2-permissive microenvironment by modulating the expression levels of chemokines.
DISCUSSION
In this study, we have demonstrated that LCs are the essential cutaneous DC subset in the induction of IgE upon epicutaneous sensitization with protein antigens. We also found that TSLPR expression on LCs is enhanced upon protein antigen exposure to the skin and that LCs plays an important role in this process through TSLP-TSLPR signaling. In addition, we have demonstrated that TSLP stimulation causes LCs to express OX40L as shown previously in human studies and that BM-derived DCs induce TH2 chemokines while suppressing TH1 chemokines, which may shift the immune environment to a TH2 milieu.
While a previous report suggests the significance of LCs in the induction of TH2 immune responses in humans,30 other studies have reported that dermal DCs, but not LCs, are essential for murine epicutaneous sensitization with hapten, as in contact hyper-sensitivity that is mediated by TH1.19,21,31,32 In our study, we have demonstrated that LCs seem to be indispensable for TH2 induction upon protein antigen sensitization. Therefore, dermal DCs and LCs may play an important role for TH1- and TH2-type immune reactions, respectively.
While protein antigens remain above the TJ, haptens can readily penetrate into the dermis as shown in Fig E2; therefore, LCs may not be essential for sensitization to hapten as reported previously.21,24 Upon protein antigen exposure to the skin, however, LCs are vital in the induction of antigen-specific IgE. It is still an intriguing issue how clinical and histologic scores, T-cell proliferation, and IL-4 production were only partially suppressed by the deficiency of LCs. These results suggest that other antigen-presenting cells, such as dermal DCs, might be able to induce antigen-specific T-cell proliferation in the draining LNs and that other TH2-inducing cells, such as basophils and mast cells, may contribute to produce IL-4 in the draining LNs. These issues need to be answered in the future.
It has been reported that basophils induce TH2 through TSLPR and that LCs are essential in the vitamin D3-induced skin lesions through TSLP signaling.13,15 In this study, we have demonstrated the significance of TSLP-TSLPR signaling on LCs under epicutaneous sensitization with protein antigens, which is clinically relevant to AD. Our findings will lead to the understanding of the underlying mechanism and developing new therapeutic targets for inflammatory skin diseases.
Supplementary Material
Clinical implications.
TSLPRs on LCs can be a therapeutic target of skin inflammatory reactions induced by epicutaneous sensitization with protein antigens, such as in the development of AD.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministries of Education, Culture, Sports, Science and Technology (to K.K.) and by a Grant-in-Aid from the Japan Society for the Promotion of Science Fellows (to S.N.).
This work was also supported by a grant from the NIH (AR056632 to D.H.K.) and the American Skin Association. B.Z.I. was supported by a grant from the Dermatology Foundation.
Abbreviations used
- AD
Atopic dermatitis
- BM
Bone marrow
- BMC
Bone marrow chimeric
- CFSE
Carboxyfluorescein succinimidyl ester
- DCs
Dendritic cells
- DTA
Diphtheria toxin subunit A
- DTR
Diphtheria toxin receptor
- eGFP
Enhanced green fluorescent protein
- FITC
Fluorescein isothiocyanate
- LCs
Langerhans cells
- LN
Lymph node
- OVA
Ovalbumin
- TSLP
Thymic stromal lymphopoietin
- TSLPR
TSLP receptor
- TJ
Tight junction
- WT
Wild type
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Egawa G, Kabashima K. Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Invest Dermatol. 2011;131:2178–85. doi: 10.1038/jid.2011.198. [DOI] [PubMed] [Google Scholar]
- 2.Moniaga CS, Kabashima K. Filaggrin in atopic dermatitis: flaky tail mice as a novel model for developing drug targets in atopic dermatitis. Inflamm Allergy Drug Targets. 2011;10:477–85. doi: 10.2174/187152811798104881. [DOI] [PubMed] [Google Scholar]
- 3.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009;129:1878–91. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 4.Nickel R, Beck LA, Stellato C, Schleimer RP. Chemokines and allergic disease. J Allergy Clin Immunol. 1999;104:723–42. doi: 10.1016/s0091-6749(99)70281-2. [DOI] [PubMed] [Google Scholar]
- 5.Shimada Y, Takehara K, Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004;34:201–8. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Novak N, Peng W, Yu C. Network of myeloid and plasmacytoid dendritic cells in atopic dermatitis. Adv Exp Med Biol. 2007;601:97–104. doi: 10.1007/978-0-387-72005-0_10. [DOI] [PubMed] [Google Scholar]
- 8.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains Langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–31. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, et al. Blood-derived dermal Langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–46. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206:2937–46. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–33. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–84. 984.e1–5. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elentner A, Finke D, Schmuth M, Chappaz S, Ebner S, Malissen B, et al. Langer-hans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009;13:2658–72. doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu YJ, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol. 2007;119:982–90. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–92. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda T, Nakajima S, Egawa G, Ogasawara K, Malissen B, Miyachi Y, et al. Compensatory role of Langerhans cells and Langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol. 2010;125:1154–6. e2. doi: 10.1016/j.jaci.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–51. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41:1862–71. doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on in-filtrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–80. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–41. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–78. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–76. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 32.Mori T, Kabashima K, Yoshiki R, Sugita K, Shiraishi N, Onoue A, et al. Cutaneous hypersensitivities to hapten are controlled by IFN-gamma-upregulated keratinocyte Th1 chemokines and IFN-gamma-downregulated Langerhans cell Th2 chemokines. J Invest Dermatol. 2008;128:1719–27. doi: 10.1038/jid.2008.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.