Abstract
Introduction
Magnetic resonance imaging (MRI) was used to monitor changes in the transverse relaxation time constant (T2) in lower hindlimb muscles of mdx mice at different ages.
Methods
Young (5 wks), adult (44 wks), old mdx (96 wks), and age-matched control mice were studied. Young mdx mice were imaged longitudinally, whereas adult and old mdx mice were imaged at a single time point.
Results
Mean muscle T2 and percent of pixels with elevated T2 were significantly different between mdx and control mice at all ages. In young mdx mice, mean muscle T2 peaked at 7–8 weeks and declined at 9–11 weeks. In old mdx mice, mean muscle T2 was decreased compared to young and adult, which could be attributed to fibrosis.
Conclusions
MRI captured longitudinal changes in skeletal muscle integrity of mdx mice. This information will be valuable for pre-clinical testing of potential therapeutic interventions for muscular dystrophy.
Keywords: magnetic resonance imaging, mdx mouse, Duchenne muscular dystrophy, muscle T2, inflammation, fibrosis
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive neuromuscular disease with an incidence of 1 in 3,600 to 6,000 male births1. DMD is caused by a mutation in the dystrophin gene, which encodes for dystrophin, a 427 KDa cytoskeletal protein2. Dystrophin is hypothesized to participate in cytoskeletal organization, stability, and membrane integrity3. The mdx mouse, the most commonly used animal model of DMD, offers an economical way for pre-clincial testing of possible therapeutic interventions. mdx mice are similar to DMD patients in that they lack dystrophin and share some biochemical and histopathological features4. Despite the similarities, the phenotype of mdx mice is less severe than DMD patients. Skeletal muscles of mdx mice undergo repeated cycles of degeneration and regeneration even during adult life5,6 whereas the regenerative capacity appears to be exhausted at an early age in humans with DMD. Many histological studies have confirmed that hindlimb muscles7 and diaphragm8 of mdx mice show progressive weakness and deterioration with age. Furthermore, the phenotype exhibited by old mdx mice more closely resembles changes observed in DMD boys (i.e increased fibrosis and fatty tissue deposition)9.
Histological markers of muscle damage, such as Evans Blue dye10,11 and Procion orange dye12 have been used extensively to measure efficacy of restoring sarcolemmal integrity in pre-clinical trials. The major drawback of these ex vivo measurements is that they can be done only at the end point or when mice are sacrificed. In addition to developing effective therapeutic agents, there have been increasing efforts to develop non-invasive diagnostic biomarkers to understand the progression of disease and effects of therapy13,14. One method, magnetic resonance imaging (MRI), provides the ability to monitor disease progression non-invasively and may provide valuable information on understanding the progression of the disease in both animal models and humans.
Proton transverse relaxation time constant (T2) is a quantitative MR measure used to detect changes in healthy and pathological muscle. Previous studies have used muscle T2 to monitor muscle damage and repair in animal models of spinal cord injury15, cast immobilization,16 and muscular dystrophy17–19. Alterations in muscle T2 have been attributed to muscle damage20, edema21,22, fatty tissue infiltration23, and fibrosis24. In this study we utilized T2 MRI to monitor the pathological changes in mdx mice lower hindlimb muscles with age. Furthermore, changes in muscle T2 were compared to standard histological markers of muscle damage and fibrosis.
Methods
Animals
C57BL/10ScSn-DMDmdx (mdx; n=18) and C57BL/10ScSn (Ctrl; n=12) male mice were included in the study. mdx and Ctrl mice were obtained from Jackson laboratories (Bar Harbor, ME), and were thereafter maintained in-house. Animals were housed in an AAALAC approved facility with 12-hour light:dark cycle (72°F, 42% humidity) and free access to food and water. The University’s Institutional Animal Care and Use Committee approved the experimental protocol.
Experimental protocol
Three age groups of mdx and age-matched Ctrl mice were studied: 1) young mice aged 5 wks, mdx (n=6) and Ctrl (n=4); 2) adult mice aged 44 wks, mdx (n=6) and Ctrl (n=4); and 3) old mice aged 96 wks, mdx (n=6) and Ctrl (n=4). Young Ctrl mice were imaged at a single time point, whereas young mdx mice were imaged longitudinally at the following time points: 5, 6, 7, 8, 9, 10, 11, 13, 14, 17, 21, and 24 weeks of age. Adult and old mdx and age-matched Ctrl mice were imaged at single time points (44 and 96 weeks of age, respectively, see Figure 1).
Figure 1.
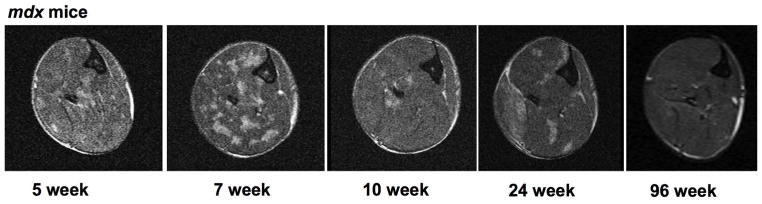
Representative transaxial T2 weighted images of lower hindlimb muscles at the mid-belly region of mdx mice across time points.
Magnetic Resonance Imaging
MRI was performed in a 4.7 T horizontal bore magnet (Bruker Avance). The animals were anesthetized using an oxygen and isoflurane mixture (3% isoflurane) and maintained under 0.5–1% isoflurane for the duration of the MR procedure. Respiratory rate of the mouse was monitored throughout the scan. The lower hindlimbs of the mouse were inserted up to the knee into a 2.0 cm internal diameter, custom-built solenoid 1H coil (200 MHz). T2-weighted MR images were acquired with the following parameters: multiple slice, single spin-echo images were acquired with repetition time (TR) =2,000ms, echo time (TE)=14ms and 40ms, FOV 10–20mm, slice thickness 0.5–1mm, acquisition matrix = 128 × 256, and 2 signal averages10. Diffusion weighting was fixed at both TEs (diffusion weighting 3 mm2/s at both 14 and 40 ms). Hahn spin echoes were implemented to avoid the contribution of stimulated echoes in the T2 measurement10,25. The T2 decay was fit to a single exponential decay curve10. Based on our previous work, we find that calculating T2 from 2 echoes is sufficient to distinguish between healthy and damaged muscle. This method also has high between-day reproducibility in control mice (n=10, coefficient of variation of 2.3 % for anterior and 3.4% for posterior compartments). Signal-to-noise ratios were 25:1 at TE = 14 ms and 9:1 at TE = 40 ms.
Muscle T2 Analysis from MRI
Muscle T2 values of the middle 6–8 slices from anterior and posterior compartments of hindlimbs (Figure 2) were computed and analyzed from T2 maps created from 2 echo times (TEs: 14 and 40 ms) using in-house software as described previously16. This was done in order to improve the coverage and to increase the reliability. T2 was calculated using the following equation: T2 = (26 ms)/ln (SI14/SI40), where SI 14 and SI 40 are the pixel intensities at TE of 14 ms and 40 ms, respectively26. The percent of muscle damage detected by MRI was defined as the percentage of pixels in a region of interest (ROI) which had T2 values over 2 standard deviations above the mean muscle T2 found in control mice (> 29ms) up to a maximum value of 100 ms.
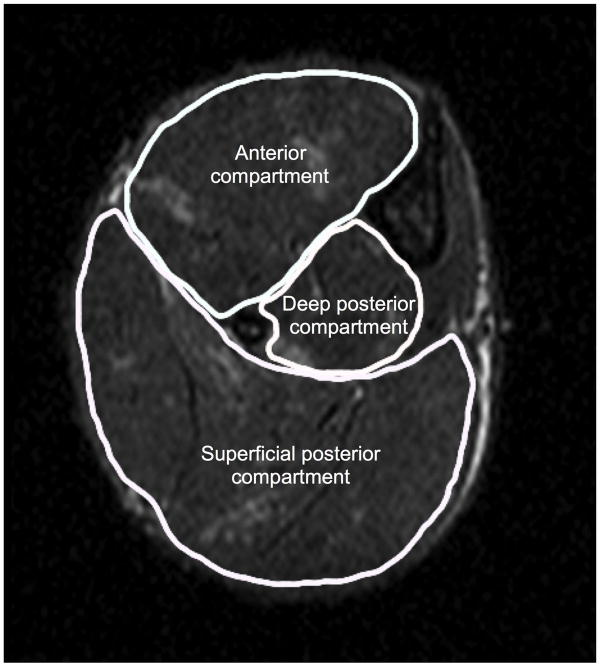
Figure 2.
Transaxial MR image taken at the mid-belly region of mdx lower hindlimb showing anterior and posterior compartments for muscle T2 analysis. The anterior compartment includes tibilais anterior and extensor digitorum longus; the posterior compartment includes gastrocnemius, soleus, and plantaris.
Histology
We performed a qualitative histological evaluation of hind limb muscles in 24 weeks and 96-week old mdx (n = 3) and Ctrl (n = 3) mice. Different lower limb muscles, including tibialis anterior (TA), extensor digitorum longus (EDL), gastrocnemius (Gastr), and soleus (SOL) were dissected carefully from the hindlimbs, fixed at resting length, frozen immediately in melting isopentane precooled in liquid nitrogen, and then stored at −80°C for further analysis. Ten-micron slices of the samples were taken from the mid-belly region, then stained with hematoxylin and eosin (H&E) and Trichrome. H&E stained cross sections were evaluated visually for morphological characteristics, such as the presence of inflammatory infiltrates and centrally nucleated fibers (CNFs). CNFs were quantified in mdx and Ctrl mice. The samples were also stained with Masson trichrome to determine collagen tissue deposition (i.e. fibrotic area). Percent CNFs and fibrotic area was quantified using ImageJ software (NIH, 1.47v), and the fibrosis index was defined as fibrotic area divided by total area × 10027.
Statistical Analysis
All statistical analysis was performed using SPSS for Mac (version 20.0). The coefficient of variance was computed to examine the variability between repeated measures in Ctrl mice. Results were expressed as mean ± standard deviation (SD) unless specified as mean ± standard error of the mean (SEM). Research hypotheses were tested at an alpha level of 0.05. One-way analysis of variance (ANOVA) for repeated measurements was performed to monitor the changes in hindlimb muscles of young mdx mice. Independent-sample t-tests were used to make comparisons between control and mdx groups at each time point (α=0.05, adjusted for multiple comparisons using a modified Bonferonni correction).
Results
T2 variability
MR T2 values were measured in Ctrl mice and were found to be highly reproducible. The average day-to-day reliability (CV) for the anterior compartment was 2.3% (25th percentile, 0.65%; 75th percentile, 3.8%), and for the posterior compartment it was 3.4%. (25th percentile, 2.3%; 75th percentile 3.9%).
Comparisons among age groups of mdx mice and controls
Mean muscle T2 in the anterior compartments of young (5 – 24 weeks), adult (44 weeks), and old mdx mice (96 weeks) were significantly higher than age-matched control mice (Figure 3). However, in the posterior compartment, significant differences were observed only in the young and adult groups compared to controls. Old mdx mice showed lower T2 compared to young and adult mdx mice (Figure 3).
Figure 3.
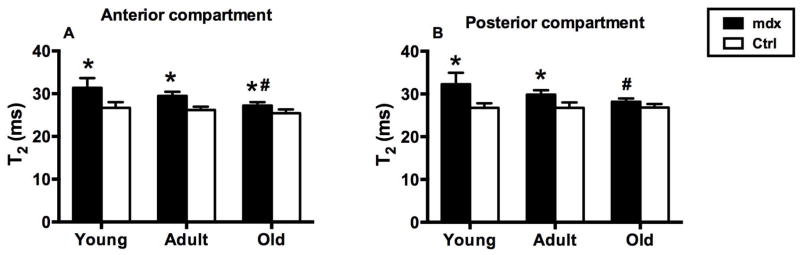
Muscle T2 values in (A) anterior and (B) posterior compartments in young (5 – 24 weeks), adult (44 weeks), and old mdx (96 weeks) and control mice. (A) In anterior compartment, the mdx mice had higher T2 values in all age groups than control mice (* P<0.05). T2 values in old mdx mice were significantly lower than young and adult mdx mice. (# P<0.05). (B) In posterior compartment, the mdx mice had higher T2 values in young and adult groups than age-matched controls (*P<0.05). T2 values of old mdx mice were significantly lower than young and adult mdx mice.
The percentage of pixels with elevated T2 in mdx mice, both in the anterior and posterior compartments were significantly different from age-matched control mice (Figure 4). In addition, the percentage of pixels with elevated T2 in young mdx mice was significantly higher than in adult and old mdx mice.
Figure 4.
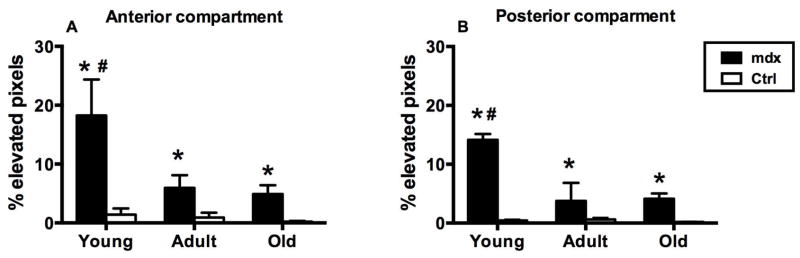
Percent elevated pixels in (A) anterior and (B) posterior compartments in young, adult, and old mdx and control mice. In anterior and posterior compartments, the mdx mice had higher T2 values in all age groups than control mice (* P<0.05). Young mdx mice had significantly higher % elevated pixels than adult and old mdx mice. (# P<0.05).
T2 changes in hindlimb muscles of young mdx mice
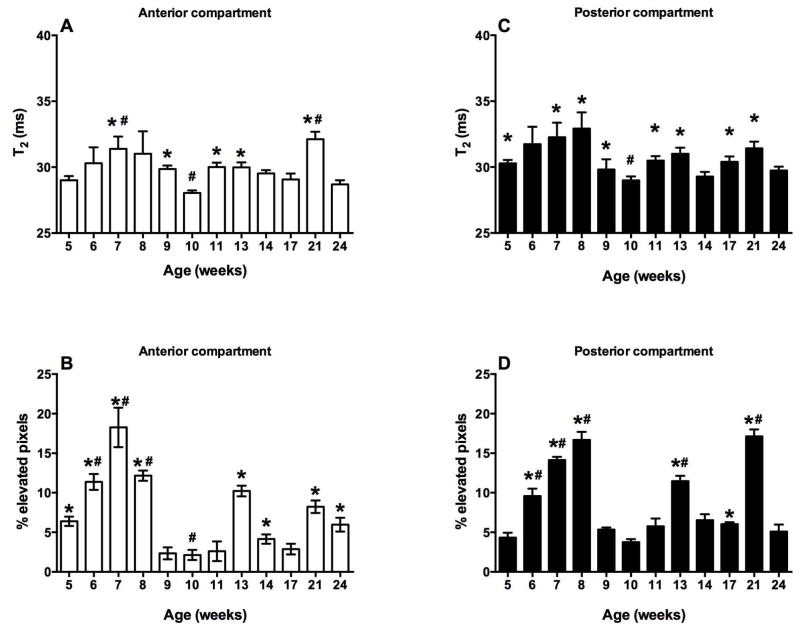
Young mdx mice presented with asynchronous, cyclical changes in both anterior and posterior compartments with age (Figure 5). In the anterior compartment, there was an increase in the mean muscle T2 from 5 to 7 weeks, followed by a significant decrease at 10 weeks. Muscle T2 values were higher at 11, 13, 17, and 21 weeks as compared to the 10-week time point (Figure 5A). The number of pixels with elevated T2 in the anterior compartment was 3-fold higher at the 7-week time point than the 5-week time point and remained elevated at the 8-week time point. Thereafter, the percentage of pixels with elevated T2 significantly decreased at 9 weeks and remained below 4% until 11 weeks. The percent pixels with elevated T2 values increased at the 13-week time point, thereafter dropping down at 17, 21, and 24-weeks (Figure 5B).
Figure 5.
Mean muscle T2 and % pixels with elevated T2 in anterior (A, B) and posterior (C, D) compartments of mdx mice (mean ± SEM). Mean T2 and % pixels with elevated T2 of anterior and posterior compartment are highest between 7–8 weeks and lowest at 10-weeks. * represents significant difference from 10 week time point (P<0.05) and # represents significant difference from 5 week time point (P<0.05).
Similar to the anterior compartment, the T2 values in the posterior compartment showed cyclical changes. Mean muscle T2 values of the posterior compartment at 8 weeks were significantly higher than at 10 weeks. Moreover, T2 values at 10 weeks were reduced as compared to 5, 7, 11, 13, 17, and 21 weeks (Figure 5C). Variation in the percent pixels with elevated T2 showed a similar cyclical pattern. There was a 2-fold increase in percent pixels with elevated T2 from 5 to 6 weeks. This increase continued at 7 and 8 weeks. Following the 8-week time point, there was a significant decrease in percent pixels with elevated T2 at 9 weeks. It remained less than 7% until 11 weeks and thereafter increasing at the 13-week time point. Furthermore, following 13 weeks, the percent pixels with elevated T2 dropped to 6% at 17 and 18 weeks, respectively and increased at 21 weeks. However, at 24 weeks there was a decline in percent pixels with elevated T2 (Figure 5D).
Histological analyses
Histological analysis of anterior (TA and EDL) and posterior hindlimb muscles (Gastr and SOL) of mdx mice showed a significant increase in number of CNFs compared to age-matched controls (Figure 6A). In addition, Masson trichrome staining of hindlimb muscles revealed greater fibrotic tissue accumulation in mdx mice at 96 weeks of age than at younger ages and controls (Supplementary Material, Figure S1). Old mdx muscles showed approximately 2-fold higher percentage of fibrotic area than adult mdx muscles (Figure 6B).
Figure 6.
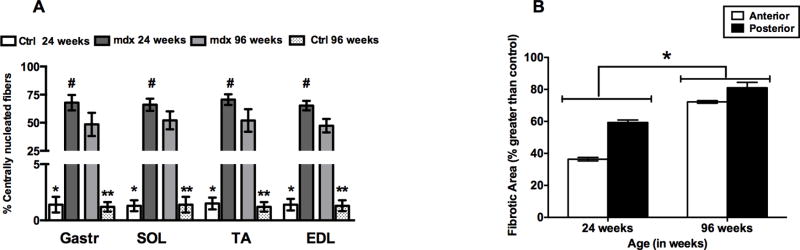
(A) Quantification of CNF of mdx and age-matched Ctrl mice. * represents significant difference between Ctrl and mdx mice at age 24 weeks, ** represents significant difference between Ctrl and mdx mice at age 96 weeks, # represents significant difference between young and old mdx mice (P<0.05). (B) Quantification of percent fibrotic/necrotic tissue in the hindlimb muscles of mdx mice at 24 and 96 weeks. Significant difference was found between hindlimb muscles of mdx mice at the 24- and 96-week time points (* P<0.05).
Discussion
These results show that T2 is highest in young mice followed by asynchronous, cyclical alterations in muscle T2 up to at least 6 months of age. Furthermore, we observed a decrease in percent pixels with elevated T2, indicative of less muscle edema, damage, and inflammation, in adult and old mdx mice than in young mdx mice. Similar findings have been reported by Pratt et al28 in a case study of an mdx mouse using T2-weighted images. In this study we extend those findings and compare quantitative T2 MR measurements in a group of mice with histological measurements.
mdx versus Controls
Muscle T2 was higher in hindlimb muscles of mdx mice than in controls in all age groups. Elevated muscle T2 has been reported in canine models of muscular dystrophy and in children with DMD29,30. Although the exact mechanism for this T2 change is not completely understood, T2 changes have been attributed to accumulation of fatty tissue30, edematous tissue22, and fibrotic tissue24. Previous studies have demonstrated shifts in muscle T2 following muscle damage and regeneration in rats as well as in mdx mice10,31,32. A study by Cabello et al32 observed changes in signal intensity on T2-weighted images after inducing inflammatory processes using Candida albicans and confirming MR alterations by histological measurements. Similar studies have been conducted by Does et al22 using λ-carrageenan inducing edema in rat muscles. Furthermore, Mcintosh et al31 observed foci of high signal intensity in mdx muscles that corresponded to dystrophic lesions in histological sections.
Changes in mdx muscle T2 over lifespan
Despite the absence of dystrophin protein, mdx mice show minimal functional signs of dystrophic pathology during the majority of their lifespan. However, the mdx mouse model has been shown to display signs of pathology during early and late stages of life. The disease progression in mdx mice has been generally divided into 3 stages: young, adult, and old age.
Young
Muscles of young mdx mice have been reported to undergo cycles of degeneration/regeneration33,34. Although the cause of these cycles remains elusive, it has been suggested that increased degeneration may occur due to increased exploratory behavior and locomotor activity of young mice4. Correspondingly, in this study, T2 in young mdx mice was significantly elevated as compared to old mdx mice. Furthermore, we found that there was more variation in mean muscle T2 in young mdx mice than in older mice. Although the factors contributing to elevated T2 are not well established, T2 has also been correlated with the size of the extracellular fluid in denervated muscle35. Additionally, T2 has also been shown to be sensitive to fiber type and fiber size change36,37. In this study we observed that mdx mice at 24 weeks of age had increased extravascular space as compared to age-matched controls as well as an increased number of fibers with central nuclei.
In young mdx mice, we found that mean muscle T2 is elevated between 5–8 weeks and peaks at 7–8 weeks of age. Furthermore, it has been reported that muscle degeneration and regeneration in mdx mice becomes more pronounced between 3–8 weeks of age, decreases significantly thereafter, and is believed to continue at a lower rate after this period38–40. For example, the tibialis anterior muscle has been shown to demonstrate signs of necrosis from 3 weeks after birth41,42. Furthermore, Passaquin et al observed greater necrosis in the SOL than the EDL at 3 weeks with greater muscle damage in SOL (~86%) than in EDL (~36%) at about 5 weeks of age43. On the other hand, satellite cell replication has been reported to peak between weeks 4 and 8 and continues at a slower pace until at least 44 weeks of age40. The reason for the acute onset of pathological changes and cyclical degeneration and regeneration processes at this age is still unresolved. It has been postulated to be due to: 1) an increase of in-cage locomotor activity; and 2) downregulation of various genes, including utrophin44. In addition, Yokota et al 45 observed an increase in CNFs at 10 weeks of age, which correlated well with the increase in revertant fibers. Similarly, we observed that muscle T2 and % pixels with elevated T2 starts increasing from 5 weeks and continue to increase until 7–8 weeks of age. Thereafter, both parameters decreased significantly at 10 weeks of age. After the initial bout, hindlimb muscles displayed age-dependent cyclical alterations, most prominent until 24 weeks of age.
Adult
During the adult lifespan, muscles of adult mdx mice, despite being bigger and heavier than control mice, have reduced specific force. Skeletal muscles from mdx mice show low levels of necrotic and regenerated myofibers4. Similarly, we observed the presence of central nuclei in young as well as adult mdx muscles. Furthermore, it has been reported that skeletal muscles of adult mdx mice do not show extensive fibrosis, except of the diaphragm8. The extent of involvement of different muscle groups varies and may be related to position or use of a muscle group in the body20,46,47.
Old age
Older mdx mice show a more involved progressive dystrophic phenotype. Extensive fibrosis has been reported in skeletal muscles of old mdx mice48,49. Furthermore, T2 has been shown to decrease with accumulation of fibrous connective tissue in old mdx mice24. Similarly, we observed a lower mean muscle T2 in 96-week-old mdx mice compared to young mdx mice. The decrease in muscle T2 may be attributed to accumulation of fibrotic tissue in muscles of old mdx mice as shown by an increase in collagenous tissue deposition. Previous studies have shown a significant correlation between T2 and histological measurements of fibrosis in the diabetic heart50. Furthermore, MRI studies have established a correlation between shortened T2 values with increasing degree of fibrosis51,52.
It is important to consider the limitations of this study. First, the experiment may have benefited from performing analysis on the individual muscles, but we were not able to do this due to lack of resolution. Improvements would include analyzing individual muscles in anterior and posterior compartments. Second, T2 in muscle has been reported to be multicomponent when using highly sampled echo trains22,53–56. In our analysis we used mono-exponential analysis based on the signal decay between 2 echo times. This dual-echo approach has been used previously10,20,57 but has limitations due to an inability to resolve multiple components and poor performance at low SNR values. Conversely, using multi-exponential analysis it might have been possible to differentiate between fibrosis, muscle damage, and inflammation22. However, obtaining images at multiple TEs during in vivo experiments without the impact of simulated echoes on the primary echo decay can prove quite challenging.
In summary, our findings suggest that: 1) the age of mdx mice is important when assessing response to different therapeutic strategies; and 2) MR T2 is a sensitive biomarker that can be used to monitor age-related changes in skeletal muscles of mdx mice.
Supplementary Material
Acknowledgments
The authors would like to thank Sean Germain for assistance with acquiring MR images. The study was supported by Muscular Dystrophy Association (MDA 4176), the National High Magnetic Field Lab (NHMFL), The Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center (U54AR052646), Department of Defense (MD 110050), and the National Institutes of Health (R01HL78670).
Abbreviations
- ANOVA
analysis of variance
- DMD
Duchenne muscular dystrophy
- FOV
field of view
- Gastr
gastrocnemius
- MRI
magnetic resonance imaging
- ROI
region of interest
- SOL
soleus
- T2
muscle transverse relaxation time
- TA
tibilais anterior
- TE
echo time
- TR
repetition time
Footnotes
Financial disclosure: The authors have no financial relationship with the manufacturers of any product or equipment discussed in this manuscript.
Conflict of interest: The authors of this manuscript declare that there is no conflict of interest.
References
- 1.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C Group DMDCCW. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet neurology. 2010;9(1):77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 4.Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiology of disease. 2008;31(1):1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JE, Bressler BH, Ovalle WK. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. Journal of muscle research and cell motility. 1988;9(6):499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- 6.Dangain J, Vrbova G. Muscle development in mdx mutant mice. Muscle Nerve. 1984;7(9):700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- 7.Pastoret C, Sebille A. mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci. 1995;129(2):97–105. doi: 10.1016/0022-510x(94)00276-t. [DOI] [PubMed] [Google Scholar]
- 8.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352(6335):536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 9.Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. Journal of neuropathology and experimental neurology. 2009;68(7):762–773. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- 10.Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve. 2005;32(5):605–612. doi: 10.1002/mus.20398. [DOI] [PubMed] [Google Scholar]
- 11.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. Journal of anatomy. 2002;200(Pt 1):69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Consolino CM, Brooks SV. Susceptibility to sarcomere injury induced by single stretches of maximally activated muscles of mdx mice. Journal of applied physiology (Bethesda, Md: 1985) 2004;96(2):633–638. doi: 10.1152/japplphysiol.00587.2003. [DOI] [PubMed] [Google Scholar]
- 13.Heckmatt JZ, Dubowitz V. Detecting the Duchenne carrier by ultrasound and computerized tomography. Lancet. 1983;2(8363):1364. doi: 10.1016/s0140-6736(83)91119-4. [DOI] [PubMed] [Google Scholar]
- 14.Heckmatt JZ, Dubowitz V, Leeman S. Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet. 1980;1(8183):1389–1390. doi: 10.1016/s0140-6736(80)92656-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Bose P, Walter GA, Thompson FJ, Vandenborne K. A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal cord. 2008;46(7):488–493. doi: 10.1038/sj.sc.3102169. [DOI] [PubMed] [Google Scholar]
- 16.Frimel TN, Kapadia F, Gaidosh GS, Li Y, Walter GA, Vandenborne K. A model of muscle atrophy using cast immobilization in mice. Muscle Nerve. 2005;32(5):672–674. doi: 10.1002/mus.20399. [DOI] [PubMed] [Google Scholar]
- 17.Dunn JF, Zaim-Wadghiri Y. Quantitative magnetic resonance imaging of the mdx mouse model of Duchenne muscular dystrophy. Muscle Nerve. 1999;22(10):1367–1371. doi: 10.1002/(sici)1097-4598(199910)22:10<1367::aid-mus5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456(7221):511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter G, Cordier L, Bloy D, Sweeney HL. Noninvasive monitoring of gene correction in dystrophic muscle. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2005;54(6):1369–1376. doi: 10.1002/mrm.20721. [DOI] [PubMed] [Google Scholar]
- 20.Mathur S, Vohra RS, Germain SA, Forbes S, Bryant ND, Vandenborne K, Walter GA. Changes in muscle T2 and tissue damage after downhill running in mdx mice. Muscle Nerve. 2011;43(6):878–886. doi: 10.1002/mus.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant ND, Li K, Does MD, Barnes S, Gochberg DF, Yankeelov TE, Park JH, Damon BM. Multi-parametric MRI characterization of inflammation in murine skeletal muscle. NMR in biomedicine. 2014 doi: 10.1002/nbm.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan RH, Does MD. Compartmental relaxation and diffusion tensor imaging measurements in vivo in lambda-carrageenan-induced edema in rat skeletal muscle. NMR in biomedicine. 2008;21(6):566–573. doi: 10.1002/nbm.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal cord. 2004;42(12):711–716. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 24.Bo Li Z, Zhang J, Wagner KR. Inhibition of myostatin reverses muscle fibrosis through apoptosis. Journal of cell science. 2012;125(Pt 17):3957–3965. doi: 10.1242/jcs.090365. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Bose P, Walter GA, Anderson DK, Thompson FJ, Vandenborne K. Changes in muscle T2 relaxation properties following spinal cord injury and locomotor training. European journal of applied physiology. 2006;97(3):355–361. doi: 10.1007/s00421-006-0199-4. [DOI] [PubMed] [Google Scholar]
- 26.Prior BM, Ploutz-Snyder LL, Cooper TG, Meyer RA. Fiber type and metabolic dependence of T2 increases in stimulated rat muscles. Journal of applied physiology (Bethesda, Md: 1985) 2001;90(2):615–623. doi: 10.1152/jappl.2001.90.2.615. [DOI] [PubMed] [Google Scholar]
- 27.van Putten M, de Winter C, van Roon-Mom W, van Ommen GJ, t Hoen PA, Aartsma-Rus A. A 3 months mild functional test regime does not affect disease parameters in young mdx mice. Neuromuscular disorders: NMD. 2010;20(4):273–280. doi: 10.1016/j.nmd.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Pratt SJ, Xu S, Mullins RJ, Lovering RM. Temporal changes in magnetic resonance imaging in the mdx mouse. BMC research notes. 2013;6(1):262. doi: 10.1186/1756-0500-6-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arpan I, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Triplett WT, Deol JK, Sweeney HL, Walter GA, Vandenborne K. T(2) mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR in biomedicine. 2013;26(3):320–328. doi: 10.1002/nbm.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255(3):899–908. doi: 10.1148/radiol.10091547. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh LM, Baker RE, Anderson JE. Magnetic resonance imaging of regenerating and dystrophic mouse muscle. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1998;76(2–3):532–541. doi: 10.1139/bcb-76-2-3-532. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Cabello J, Carrero-Gonzalez B, Aviles P, Santisteban C, Mendez RJ, Ferreiros J, Malpica N, Santos A, Gargallo-Viola D, Regadera J. Magnetic resonance imaging in the evaluation of inflammatory lesions in muscular and soft tissues: an experimental infection model induced by Candida albicans. Magnetic resonance imaging. 1999;17(9):1327–1334. doi: 10.1016/s0730-725x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 33.DiMario JX, Uzman A, Strohman RC. Fiber regeneration is not persistent in dystrophic (MDX) mouse skeletal muscle. Dev Biol. 1991;148(1):314–321. doi: 10.1016/0012-1606(91)90340-9. [DOI] [PubMed] [Google Scholar]
- 34.Pastoret C, Sebille A. Age-related differences in regeneration of dystrophic (mdx) and normal muscle in the mouse. Muscle Nerve. 1995;18(10):1147–1154. doi: 10.1002/mus.880181011. [DOI] [PubMed] [Google Scholar]
- 35.Polak JF, Jolesz FA, Adams DF. Magnetic resonance imaging of skeletal muscle. Prolongation of T1 and T2 subsequent to denervation Investigative radiology. 1988;23(5):365–369. doi: 10.1097/00004424-198805000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Bonny JM, Zanca M, Boespflug-Tanguy O, Dedieu V, Joandel S, Renou JP. Characterization in vivo of muscle fiber types by magnetic resonance imaging. Magnetic resonance imaging. 1998;16(2):167–173. doi: 10.1016/s0730-725x(97)00249-x. [DOI] [PubMed] [Google Scholar]
- 37.English AE, Joy ML, Henkelman RM. Pulsed NMR relaxometry of striated muscle fibers. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1991;21(2):264–281. doi: 10.1002/mrm.1910210211. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JE, Kao L, Bressler BH, Gruenstein E. Analysis of dystrophin in fast- and slow-twitch skeletal muscles from mdx and dy2J mice at different ages. Muscle Nerve. 1990;13(1):6–11. doi: 10.1002/mus.880130103. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JE, Ovalle WK, Bressler BH. Electron microscopic and autoradiographic characterization of hindlimb muscle regeneration in the mdx mouse. The Anatomical record. 1987;219(3):243–257. doi: 10.1002/ar.1092190305. [DOI] [PubMed] [Google Scholar]
- 40.McGeachie JK, Grounds MD, Partridge TA, Morgan JE. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci. 1993;119(2):169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- 41.Radley HG, Grounds MD. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiology of disease. 2006;23(2):387–397. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Shavlakadze T, White J, Hoh JF, Rosenthal N, Grounds MD. Targeted expression of insulin-like growth factor-I reduces early myofiber necrosis in dystrophic mdx mice. Molecular therapy: the journal of the American Society of Gene Therapy. 2004;10(5):829–843. doi: 10.1016/j.ymthe.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, Ruegg UT. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscular Disorders. 2002;12(2):174–182. doi: 10.1016/s0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 44.Khurana TS, Watkins SC, Chafey P, Chelly J, Tome FM, Fardeau M, Kaplan JC, Kunkel LM. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscular disorders: NMD. 1991;1(3):185–194. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- 45.Yokota T, Lu QL, Morgan JE, Davies KE, Fisher R, Takeda S, Partridge TA. Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. Journal of cell science. 2006;119(Pt 13):2679–2687. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- 46.Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve. 1998;21(5):567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Wooddell CI, Zhang G, Griffin JB, Hegge JO, Huss T, Wolff JA. Use of Evans blue dye to compare limb muscles in exercised young and old mdx mice. Muscle Nerve. 2010;41(4):487–499. doi: 10.1002/mus.21527. [DOI] [PubMed] [Google Scholar]
- 48.Keeling RM, Golumbek PT, Streif EM, Connolly AM. Weekly oral prednisolone improves survival and strength in male mdx mice. Muscle Nerve. 2007;35(1):43–48. doi: 10.1002/mus.20646. [DOI] [PubMed] [Google Scholar]
- 49.Lefaucheur JP, Pastoret C, Sebille A. PHENOTYPE OF DYSTROPHINOPATHY IN OLD MDX MICE. Anatomical Record. 1995;242(1):70–76. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- 50.Bun SS, Kober F, Jacquier A, Espinosa L, Kalifa J, Bonzi MF, Kopp F, Lalevee N, Zaffran S, Deharo JC, Cozzone PJ, Bernard M. Value of in vivo T2 measurement for myocardial fibrosis assessment in diabetic mice at 11. 75 T. Investigative radiology. 2012;47(5):319–323. doi: 10.1097/RLI.0b013e318243e062. [DOI] [PubMed] [Google Scholar]
- 51.Anderson SW, Jara H, Ozonoff A, O’Brien M, Hamilton JA, Soto JA. Effect of disease progression on liver apparent diffusion coefficient and T2 values in a murine model of hepatic fibrosis at 11. 7 Tesla. MRI Journal of magnetic resonance imaging: JMRI. 2012;35(1):140–146. doi: 10.1002/jmri.22807. [DOI] [PubMed] [Google Scholar]
- 52.Scalera JE, Soto JA, Jara H, Ozonoff A, O’Brien M, Anderson SW. Multiexponential T(2) analyses in a murine model of hepatic fibrosis at 11. 7 T. MRI NMR in biomedicine. 2013;26(1):83–90. doi: 10.1002/nbm.2822. [DOI] [PubMed] [Google Scholar]
- 53.Ebdrup S, Refsgaard HH, Fledelius C, Jacobsen P. Synthesis and structure-activity relationship for a novel class of potent and selective carbamate-based inhibitors of hormone selective lipase with acute in vivo antilipolytic effects. Journal of medicinal chemistry. 2007;50(22):5449–5456. doi: 10.1021/jm0607653. [DOI] [PubMed] [Google Scholar]
- 54.Ababneh Z, Beloeil H, Berde CB, Gambarota G, Maier SE, Mulkern RV. Biexponential parameterization of diffusion and T2 relaxation decay curves in a rat muscle edema model: decay curve components and water compartments. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2005;54(3):524–531. doi: 10.1002/mrm.20610. [DOI] [PubMed] [Google Scholar]
- 55.Cole WC, LeBlanc AD, Jhingran SG. The origin of biexponential T2 relaxation in muscle water. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1993;29(1):19–24. doi: 10.1002/mrm.1910290106. [DOI] [PubMed] [Google Scholar]
- 56.Saab G, Thompson RT, Marsh GD. Effects of exercise on muscle transverse relaxation determined by MR imaging and in vivo relaxometry. Journal of applied physiology (Bethesda, Md: 1985) 2000;88(1):226–233. doi: 10.1152/jappl.2000.88.1.226. [DOI] [PubMed] [Google Scholar]
- 57.Heemskerk AM, Drost MR, van Bochove GS, van Oosterhout MF, Nicolay K, Strijkers GJ. DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2006;56(2):272–281. doi: 10.1002/mrm.20953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.