Abstract
Background
Clinically and biologically, ASD is heterogeneous. Unusual patterns of visual preference as indexed by eye-tracking are hallmarks, yet whether they can be used to define an early biomarker of ASD as a whole, or leveraged to define a subtype is unclear. To begin to examine this issue, large cohorts are required.
Methods
A sample of 334 toddlers from 6 distinct groups (115 ASD, 20 ASD-Features, 57 DD, 53 Other, 64 TD, and 25 Typ SIB) participated. Toddlers watched a movie containing both geometric and social images. Fixation duration and number of saccades within each AOI and validation statistics for this independent sample computed. Next, to maximize power, data from our previous study (N=110) was added totaling 444 subjects. A subset of toddlers repeated the eye-tracking procedure.
Results
As in the original study, a subset of toddlers with ASD fixated on geometric images greater than 69%. Using this cutoff, sensitivity for ASD was 21%, specificity 98%, and PPV 86%. Toddlers with ASD who strongly preferred geometric images had (a) worse cognitive, language, and social skills relative to toddlers with ASD who strongly preferred social images and (b) fewer saccades when viewing geometric images. Unaffected siblings of ASD probands did not show evidence of heightened preference for geometric images. Test-retest reliability was good. Examination of age effects suggest that this test may not be appropriate with children > 4 years.
Conclusions
Enhanced visual preference for geometric repetition may be an early developmental biomarker of an ASD subtype with more severe symptoms.
Keywords: autism spectrum disorder, eye-tracking, early detection, visual attention, eye gaze, geometric preference
INTRODUCTION
Robust biomarkers of autism spectrum disorder (ASD) in infants and toddlers have yet to be discovered, perhaps due to the considerable clinical, and likely etiological, heterogeneity associated with the disorder (1). For example, some children with ASD have high verbal competency, while others may not speak at all; some excel in response to treatment, while others do not (2, 3). Complicating this further is the fact that symptom onset is quite variable in ASD - some toddlers may show signs at or even before the 1st birthday while others may not show signs until the 2nd birthday or beyond (4). Biomarkers are generally conceptualized as measurable indicators of normal or pathologic biological processes (5) and here we use the term to refer to any objective indicator that accurately and reliably identifies ASD or a subtype of ASD.
At a diagnostic level, the urgency to discover early developmental biomarkers is mediated by the belief that a valid early biomarker might hasten the pace of diagnosis, as well as the interval between 1st diagnosis and eventual treatment (6). In theory, early treatment can impact functional connections in the developing brain and lead to improved outcome for children (7, 8). For example, a recent study showed that toddlers who began behavioral treatment prior to age 3 years, several by age 18 months, experienced a gain of 15 points on a standardized IQ test following treatment (9).
At a prognostic level, biomarkers might be able to act as a specifier to diagnosis, generating a more in-depth characterization of a child’s overall clinical profile that may relate to his/her long-term outcome. Relatedly, at the treatment level, biomarkers might be able to identify subgroups of toddlers with ASD who could be matched to specific interventions tailored for that specific subtype. Thus, while identifying toddlers with ASD as early as possible is critical, the heterogeneity of ASD suggests that studies might productively target discovery of clearly definable subtypes of toddlers with ASD.
Biomarker tests of ASD need to be developmentally appropriate, while having the potential to detect pre-symptomatic features. Unusual patterns of visual attention, for example, are emerging as preclinical markers in ASD. In general, toddlers with ASD fail to attend to social attention cues (10) or may display “sticky attention” (11). Eye-tracking technology may be ideally suited to tap into such abnormalities because it is easily implemented, objective, and can be used from infancy to adulthood. Eye-tracking studies with toddlers with ASD under age 3 years have highlighted a range of social visual attention deficits such as a reduced preference for biological motion (12), reduced fixation to eye (13, 14) and head regions (15), difficulties in joint attention (16), and scene monitoring during explicit dyadic cues (17). Although some of these findings have been questioned (18), collectively these studies point to very early developmental origins of social dysfunction in ASD.
Despite their promise, effects reported in eye-tracking studies are often subtle and mainly applicable at the group, not individual subject, level. Further, validation statistics needed to translate eye-tracking metrics into usable biomarkers, such as specificity or positive and negative predictive values (PPV and NPV), are generally not reported. One eye-tracking study, however, did attempt individual-level classification based on the hypothesis that reduced fixation towards the eye region at 6-months would predict diagnostic status at 24–36 months, but found that this measure did not accurately classify individual toddlers as ASD (19). However, a recent baby-sibling design study that intensively tracked 11 ASD infants from 2 to 24 months, suggests that at 6 months in age fixations towards the eye region are just beginning to decline, with maximal reductions in eye region fixation not occurring until 2 years (14). As such, it may be the case that only after the 6-month age point will diagnostic classification efforts be maximally successful.
In order to discover the utility of potential biomarkers using eye-tracking, large samples need to be examined so that validation statistics such as sensitivity and specificity can be computed and potential subgroups identified across a wide age range. In taking this approach, we previously developed a novel implementation of a traditional preferential looking paradigm using eye-tracking technology (20). We quantified ASD and typically developing toddlers’ visual attention preferences towards dynamic (i.e., moving and changing) geometric images (DGI) vs. dynamic social images (DSI) and identified a unique subgroup of toddlers with ASD who strongly preferred to look at geometric rather than social images. Using this “GeoPref Test”, individual ASD subject behavior could predict diagnostic classification (20). Specifically, the study found that toddlers who visually fixated on DGI greater than 69% of the time had a 100% probability of having ASD. However, sensitivity was modest capturing 20–40% of toddlers with ASD depending on the threshold used. Thus, the GeoPref Test showed initial signs of promise at detecting specific ASD subgroups.
That original study did not completely address whether or not the geometric preference effect was specific to ASD or might also be found in toddlers with other language and cognitive delays. It also was too small to fully address the question of whether this ASD subgroup might have distinctive clinical characteristics or whether the geometric preference effect varied with age or with repeat testing at short or longer intervals. Lastly, it did not include information relevant to the search for ASD endophenotypes, that is, whether this biomarker is shared by non-affected siblings and so could be used in future studies of underlying genetic factors.
In the present study we addressed these issues by first testing and replicating the effect in 334 toddlers completely independent from the original study and including multiple contrast groups including unaffected siblings of ASD probands in our sample. We next combined data from the original study of 110 toddlers with this sample to arrive at an overall dataset of 444 toddlers, producing the largest eye-tracking study of ASD to date.
METHODS and MATERIALS
Participants
Identical to the approach used in our earlier study (20), toddlers were recruited through two mechanisms; community referrals (e.g., website) or a general population-based screening method called the 1-Year Well-Baby Check-Up Approach (21) that allowed for the prospective study of ASD beginning at 12 months based on a toddler’s failure of the CSBS-DP-IT Checklist (22, 23).
Overall, 424 toddlers aged 10 to 49 months attempted participation. Ninety toddlers (21.2%) were excluded for multiple reasons such as a failure to attend to at least 50% of the video, a criterion that was adopted in our previous study (20). See Supplement and Figures S1 and S2. The final sample consisted of 334 toddlers and was non-overlapping and independent from the sample included in our prior study (20).
All assessments were administered by licensed, Ph.D.-level psychologists blind to eye-tracking results. All toddlers, including normal controls, participated in a series of tests that included the Autism Diagnostic Observation Schedule (ADOS; Module T, 1 or 2) (24), the Mullen Scales of Early Learning (25), the Vineland Adaptive Behavior Scales (26), as well as other tests as part of a larger study (see www.autism-center.ucsd.edu) at every test visit. See Table 1 for characteristics of the independent sample. All testing occurred at the University of California San Diego Autism Center.
Table 1.
Subject Characteristic Table for the Independent Sample (N=334).
| Characteristic | Mean (SD) [Range] | P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD (n=115) |
ASD Features (n=20) |
TD (n=64) |
DD (n=57) |
Typ Sib ASD (n=25) |
Other (n=53) |
ASD vs ASD Features |
ASD vs TD |
ASD vs LD |
ASD vs TypSib |
ASD vs Other |
|
| Sex, M/F | 88/27 | 15/5 | 35/27 | 45/12 | 12/13 | 26/27 | 0.884 | 0.004 | 0.723 | 0.014 | 0.001 |
| Age, mo | 28.0 (8.4) [12–49] |
22.2 (9.3) [11–42] |
23.6 (9.9) [12–44] |
22.0 (8.3) [10–46] |
19.1 (6.0) [12–31] |
22.0 (8.7) [12–43] |
0.006 | 0.004 | <0.001 | <0.001 | <0.001 |
|
Mullen Scales (t- scores) |
|||||||||||
| Visual Reception |
42.3 (12.9) | 49.7 (11.7) | 59.0 (9.1) | 50.6 (9.5) | 57.3 (9.4) | 55.0 (11.3) | 0.020 | <0.001 | <0.001 | <0.001 | <0.001 |
| Fine Motor | 39.4 (12.8) | 47.4 (13.1) | 57.7 (8.3) | 51.5 (8.9) | 58.6 (7.4) | 55.5 (10.7) | 0.012 | <0.001 | <0.001 | <0.001 | <0.001 |
| Receptive Language |
32.3 (13.2) | 46.2 (11.8) | 54.6 (9.2) | 43.8 (11.4) | 51.6 (8.6) | 51.7 (11.9) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Expressive Language |
32.0 (13.0) | 42.0 (12.7) | 52.8 (8.4) | 39.4 (9.9) | 53.2 (10.1) | 46.7 (10.8) | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| ELC* | 77.0 (19.8) | 93.3 (18.2) | 112.5 (11.3) | 93.7 (13.9) | 111.6 (12.5) | 104.5 (15.9) | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
Vineland (Standard Scores) |
|||||||||||
| Communication | 81.2 (13.4) | 90.1 (12.4) | 102.4 (9.8) | 90.3 (10.7) | 99.0 (9.8) | 94.9 (11.5) | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 |
| Daily Living | 86.5 (11.6) | 87.5 (16.5) | 100.7 (9.2) | 92.5 (12.1) | 99.1 (11.7) | 96.6 (11.8) | 0.731 | <0.001 | 0.002 | <0.001 | <0.001 |
| Socialization | 84.3 (12.1) | 91.8 (9.9) | 103.3 (8.5) | 96.8 (7.5) | 102.2 (8.1) | 98.6 (9.9) | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 |
| Motor Skills | 91.0 (11.1) | 93.6 (12.2) | 101.1 (7.5) | 96.88 (9.3) | 101.8 (9.4) | 95.9 (11.8) | 0.350 | <0.001 | 0.001 | <0.001 | <0.001 |
| Adaptive Behavior Composite |
82.4 (13.4) | 87.0 (15.1) | 102.0 (8.5) | 92.9 (8.1) | 100.4 (8.7) | 95.5 (10.9) | 0.170 | <0.001 | <0.001 | <0.001 | <0.001 |
|
ADOS ∧ (Module T, 1, or 2) |
|||||||||||
| ADOS SA/CoSo Score |
13.6 (4.7) | 7.7 (4.6) | 1.6 (1.6) | 3.6 (2.9) | 2.2 (2.5) | 4.0 (3.5) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| ADOS RRB Score |
3.6 (1.9) | 2.0 (1.5) | 0.1 (0.4) | 0.6 (0.8) | 0.4 (0.8) | 0.7 (1.0) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| ADOS Total Score |
17.2 (5.9) | 9.65 (4.81) | 1.8 (1.7) | 4.4 (3.1) | 2.6 (2.7) | 4.7 (3.9) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Administered ADOS module depended on the age and language ability of the toddler at the time of testing.
SA=Social Affect; CoSo= Communication Social Score; RRB=Restricted and Repetitive Behavior.
6% of the sample received a WPPSI instead of a Mullen.
Based on diagnoses given at the final diagnosis age, the independent sample of 334 toddlers consisted of 6 discrete diagnostic groups (115 ASD, 20 ASD-Feat, 57 DD, 53 Other, 64 TD, and 25 Typ SIB). Briefly, the ASD group included toddlers with a final diagnosis of ASD, ASD-Feat included toddlers with ASD symptoms but did not meet full diagnostic criteria, DD included toddlers with either a language or global developmental delay, Other included a wide array of toddlers such as premature or prenatal drug exposed, TD included typical toddlers and Typ Sib included unaffected toddlers with ASD siblings. See Supplement for more detailed information.
Following replication tests of the original finding, the current sample (n=334) was combined with the original sample (n=110; (20) to capitalize on increased power to detect eye-tracking behavior effects (combined n=444 subjects; 152 ASD, 20 ASD-Feat, 79 DD, 53 Other, 115 TD, and 25 Typ SIB).
Apparatus, Movie, and Eye-tracking Procedure
Apparatus and Movie
A TOBII T120 eye-tracker (www.tobii.com, screen size 17” TFT) was used to measure toddlers’ fixations and number of saccades in response to a visual stimulus. Toddlers were presented with a movie consisting of two rectangular areas of interest (AOIs) that contained DGI and DSI images that were placed side-by-side in which scenes changed in a simultaneous, time-linked fashion identical to our previous experiment (20). Audio information was not presented. The final movie contained a total of 28 scenes with single-scene duration varying from 2–4 seconds for a total presentation time of 60 seconds. The side (left/right) of presentation of DGI and DSI scenes was randomized across subject and diagnosis. See Supplement for more information.
Statistical Analyses
Visual Attention as Indexed by Fixation Time and Relation to Clinical Characteristics and Age
Using Tobii software, fixation data were calculated using a 35-pixel radius filter. Percent time spent fixating within each AOI (i.e., DGI or DSI) was tabulated for each subject. To compare percent fixation time within DGI between groups a one-way analysis of covariance (ANCOVA) was performed with 6 levels (diagnostic groups) using the age of the child at testing as a covariate. Significant effects were followed by planned contrasts with Bonferroni correction for multiple comparisons, and 95% confidence interval (CI) of the mean difference between groups and effect sizes reported. Examinations of the relationship between percent fixation on DGI and clinical measures were determined using linear regression controlling for the effects of age. In order to examine if clinical symptoms were more or less severe in toddlers with ASD that strongly preferred DGI (i.e., ≥ 69% towards DGI) relative to those that preferred DSI (i.e., ≥ 69% towards DSI), clinical characteristics of toddlers within each subgroup were directly compared using ANCOVA controlling for age.
Sensitivity, Specificity, PPV, NPV, and ROC Curve Analyses
In order to determine the specific percentage of fixation time within DGI that would best discriminate ASD from other toddlers, a receiver operating curve (ROC) was generated that graphically displayed a plot of the true vs. false positives. With respect to PPV and NPV, two methods were used: 1) PPV and NPV were calculated within the study sample, as would be applicable in a 2nd tier screening approach; 2) PPV and NPV were calculated taking into account the ASD prevalence rate of 1.47 percent in the general population (27), as would be applicable in a 1st tier screening approach.
Number of Saccades
The number of saccades per second was determined for each subject by dividing the overall total number of saccades by the total looking time. Differences in saccade data between diagnostic groups were examined utilizing a one-way ANCOVA covarying for age and planned contrasts.
Test-Retest Reliability
To determine the stability of the GeoPref Test, 208 toddlers (61 ASD, 9 ASD-Feat, 37 DD, 28 Other, 63 TD, and 10 TYP SIB) ranging in age from 12–48 months participated in a retest session within 1 hour to 24 months following their first GeoPref Test. For more information, see Supplement
Fixation Patterns Between Sibling Pairs (Exploratory)
Data was available from 36 sibling pairs (11 concordant for ASD, 12 discordant for ASD and 13 typical sibling pairs). An ICC was used as an exploratory analysis to determine the degree to which sibling pairs resembled each other in terms of their preference for DGI. See Supplement.
RESULTS
Differences in Visual Preference Patterns towards DGI between Diagnostic Groups
Independent Sample (N=334)
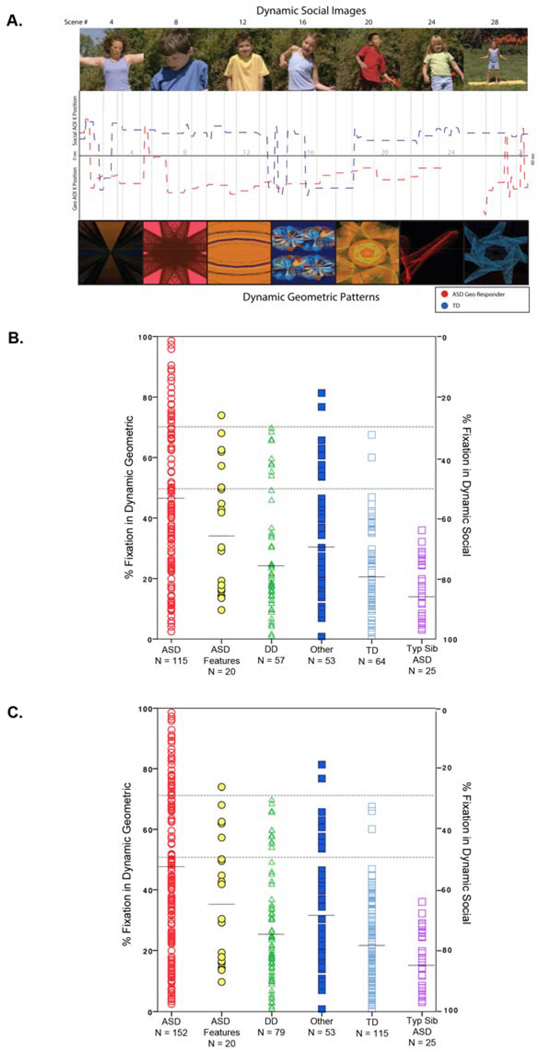
Within the independent sample, differences in percent fixation towards DGI was found using an ANCOVA controlling for the age of participants (F6,327 = 16.39, P<0.001, partial η2=0.23), replicating our previous findings (20). Follow up contrasts comparing the ASD group to each other group, with Bonferroni correction and examination of the confidence interval of the difference in percent fixation between groups, revealed that toddlers with ASD had significantly greater percent fixation on DGI than all other diagnostic groups (ASD vs. DD, P<0.001, CI: 9.32%–22.33% fixation, cohen’s d=0.77; ASD vs. Other, P=0.004, CI: 3.09%–16.39% fixation, cohen’s d=0.50; ASD vs. TD, P<0.001, CI: 13.46%–25.84% fixation, cohen’s d=0.97; ASD vs. TYP SIB, P<0.001, CI: 14.19%–31.99% fixation, cohen’s d=0.92), except the ASD-Feat Group (ASD vs. ASD-Feat, P=0.33, CI: −4.83%–14.32% fixation, cohen’s d=0.23). See Figure 1 and supplemental on-line videos for examples and Supplement for total looking time within each group.
Figure 1.
(A) Sample social and geometric scenes from the 1-minute 28-scene) GeoPref Test. Visual scanning data (X-axis fixation points) from a typically developing toddler (blue line) and an ASD Geometric Responder toddler (red line) are plotted across time. Breaks in the line represent a lack of fixation towards the movie. (B) Scatterplot of the independent group (N=334) illustrating the percentage of fixation time to both DGI and DSI for each toddler across each diagnostic group. Total percent time viewing DGI and DSI sums to 100% for each toddler. For example, a toddler who spends 80% viewing time on geometric images (as noted on the Y axis on the left) thus spends 20% viewing time on social images (as noted on the Y axis on the right). A toddler who spends >50% viewing geometric images is considered a “geometric responder” and a toddler who spends >50% viewing social images is considered a “social responder.” (C) Scatter plot of the combined group (N=444).
Combined Sample (N=444)
Within the combined sample, the exact same pattern was observed in differences to DGI fixation percentages controlling for the age of participants (F6,437 = 20.23, P<0.001, partial η2=0.22). See Supplement for follow-up contrasts.
Sensitivity, Specificity, PPV, NPV and ROC Curves
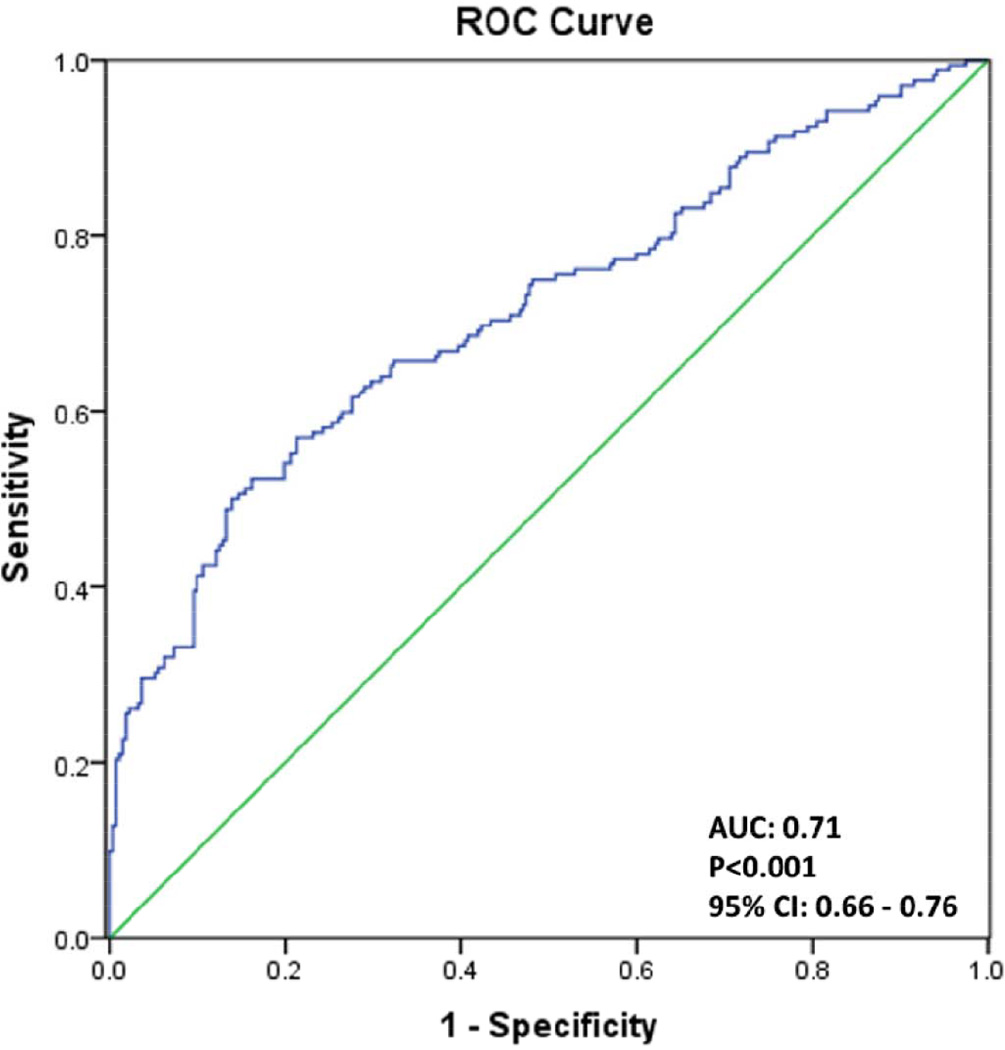
Using the 69% fixation threshold (i.e., a toddler fixated on geometric shapes 69% of the time or greater), validation statistics found within the independent sample were almost identical to the previous study with sensitivity of 21%, specificity 98%, PPV 86% and NPV 70%. This almost identical replication suggests that the GeoPref Test accurately identifies a select and highly stable subtype within the autism spectrum.
The tradeoffs between sensitivity and specificity of the GeoPref Test are further illustrated in Figure 2 and associated tables that contain validation statistics for the independent sample (N=334) as well as the combined sample (N=444), See Table 2. Validation statistics of the GeoPref Test can change based on the specific cut-off threshold used and whether or not toddlers with ASD features are considered true positives. For example, the sensitivity of the test can be improved by lowering the fixation threshold to 50%. Results of the GeoPref Test only slightly change if ASD-Feat toddlers were included as true positives. For more information regarding validation statistics within narrow age bins (e.g., 24–30 months), see Supplement Table S3 and Supplement Figure S5.
Figure 2.
Top: Receiver Operating Characteristic Curve plot graphically illustrating the true positive rate (sensitivity) versus the false positive rate (1-specificity) of the combined (n=444) sample. Bottom: Validation Statistics associated with Geo Fixation levels at 50% and 69% and with and without considering toddlers with ASD features as true positives for both the independent and combined samples.
Table 2.
Validation Statistics for the Independent (Top) and Combined (Bottom) Samples
| Independent Validation Sample |
Using 50% Geo Fixation Cutoff (ASD only as True Pos) |
Using 50% Geo Fixation Cutoff (ASD + ASD Features as True Pos) |
Using 69% Geo Fixation Cutoff (ASD only as True Pos) |
Using 69% Geo Fixation Cutoff (ASD + ASD Features as True Pos) |
|---|---|---|---|---|
| Validation Statistics | N | N | N | N |
| True Positives (TP) | 43 | 49 | 24 | 25 |
| True Negatives (TN) | 190 | 176 | 215 | 196 |
| False Positives (FP) | 29 | 23 | 4 | 3 |
| False Negatives (FN) | 72 | 86 | 91 | 110 |
| Total Sample Size | 334 | 334 | 334 | 334 |
| Sensitivity | 37% | 36% | 21% | 19% |
| Specificity | 87% | 88% | 98% | 99% |
| PPV | 60% | 68% | 86% | 89% |
| NPV | 73% | 67% | 70% | 64% |
| Combined Sample | ||||
| Validation Statistics | N | N | N | N |
| True Positives (TP) | 58 | 64 | 35 | 36 |
| True Negatives (TN) | 260 | 246 | 288 | 269 |
| False Positives (FP) | 32 | 26 | 4 | 3 |
| False Negatives (FN) | 94 | 108 | 117 | 136 |
| Total Sample Size | 444 | 444 | 444 | 444 |
| Sensitivity | 38% | 37% | 23% | 21% |
| Specificity | 89% | 90% | 99% | 99% |
| PPV | 64% | 71% | 90% | 92% |
| NPV | 73% | 70% | 71% | 66% |
Relationship between DGI Fixation, Age, and ASD Symptoms
Combined Sample (N=444)
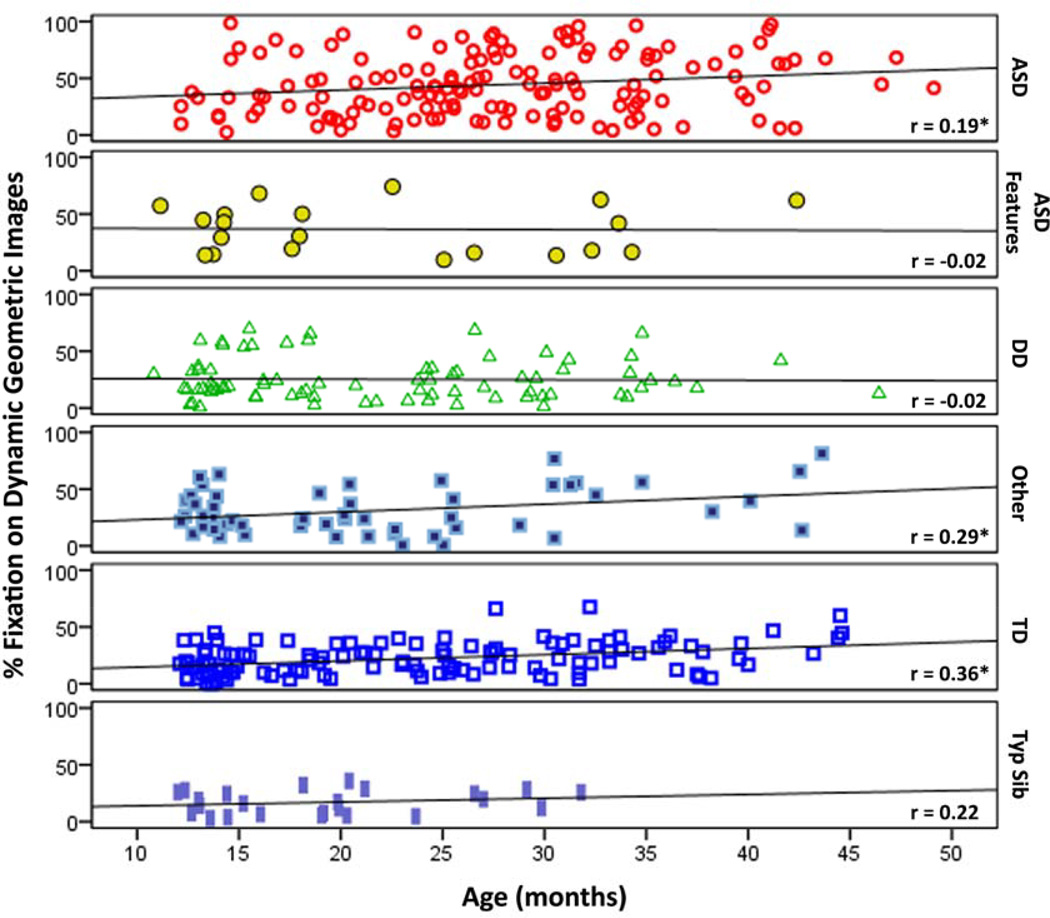
Given that the pattern of effects and effect sizes from the independent and combined samples were very similar we examined further relationships with the combined sample to increase power (though the relationships do not change if examined only in the independent sample). Overall, among all participants a significant relationship was found between DGI percent fixation and age (r=.26, p<.001), justifying age as a covariate in our main analyses. Due to different age distributions between each diagnostic group age effects were examined for each group separately. As indicated in Figure 3, toddlers from the ASD, Other, and TD groups tended to fixate more on DGI with increasing age, suggesting that the GeoPref Test may be less effective with older children (i.e., > 4 years old).
Figure 3.
Scatterplots with a best fit line illustrating the relationship between percent fixation towards DGI and age across each diagnostic group. * p<.05.
Linear regressions within each group with symptom rating scales as the dependent variables, DGI percent fixation and age as explanatory variables demonstrated that the degree to which a toddler fixated on DGI, independent of age, was significantly related to his/her symptom severity across a range of areas such as receptive and expressive language ability, cognition, autism symptom profile and adaptive functioning, controlling for age. For example, as illustrated in Table 3, a 1 percent increase in an ASD child’s fixation towards geometric images was associated with a 0.29 reduction in his/her expressive language ability. In contrast, no significant relationships were found between DGI percent fixation and test scores within any other group, both when accounting for the age and not accounting for the age of the child.
Table 3.
Coefficient and r values based on linear regression demonstrate the relationship between percent fixation towards dynamic geometric images and various social, cognitive, and language skills within the ASD group. All regression models include percent geometric fixation and age as variables. Partial r expresses the unique variance accounted for in clinical measures by percent fixation to geometric images among ASD children.
| TEST NAME | COEFFICIENT | STD. ERROR |
partial r | P-VALUE |
|---|---|---|---|---|
| Mullen Scales Of Early Learning | ||||
| Visual Reception | −0.229 | 0.036 | −0.237 | 0.004 |
| Receptive Language | −0.299 | 0.039 | −0.295 | < 0.001 |
| Expressive Language | −0.291 | 0.038 | −0.290 | < 0.001 |
| Early Learning Composite | −0.219 | 0.056 | −0.306 | < 0.001 |
| Vineland Adaptive Behavior Scales | ||||
| Communication | −0.140 | 0.040 | −0.273 | 0.001 |
| Daily Living | −0.083 | 0.035 | −0.193 | 0.018 |
| Socialization | −0.112 | 0.033 | −0.266 | 0.001 |
| Adaptive Behavior Composite | −0.114 | 0.038 | −0.238 | 0.003 |
| Autism Diagnostic Observation Schedule (ADOS) | ||||
| ADOS SA/CoSo Score | 0.353 | 0.014 | 0.349 | < 0.001 |
| ADOS RRB Score | 0.46 | 0.006 | 0.046 | 0.578 |
| ADOS Total Score | 0.306 | 0.017 | 0.302 | < 0.001 |
ASD “Geometric Responders” vs. ASD “Social Responders.”
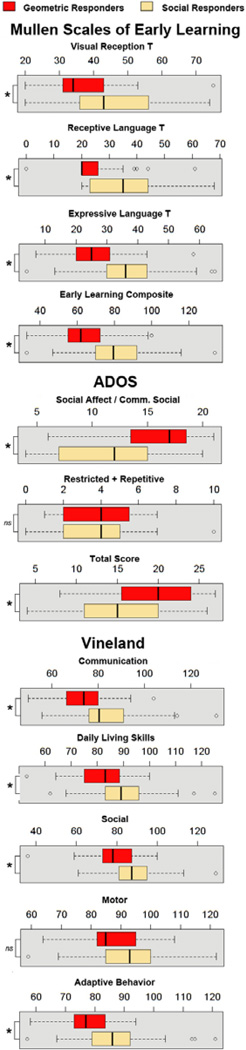
The toddlers with ASD in our sample reflected a wide range of visual preference patterns; some toddlers strongly preferring DGI (defined as DGI fixation levels ≥69%), while others strongly preferred DSI (defined as DSI fixation levels ≥69%). Using an ANCOVA with age as a covariate, direct comparisons between these two subgroups revealed differences in visual reception, (F2,84=12.26, P<0.001, partial η2=0.23), receptive language (F2,84=9.70, P<0.001, partial η2=0.19), expressive language (F2,84=6.60, P=0.002, partial η2=0.14), and the early learning composite score (F2,84=9.17, P<0.001, partial η2=0.18) based on scores from the Mullen Scales of Early Learning. Similarly, significant differences were found in the Daily Living subscale (F2,86=10.86, P<0.001, partial η2=0.20), Socialization subscale (F2,84=18.23, P<0.001, partial η2=0.30), Communication subscale (F2,84=5.61, P=0.005, partial η2=0.12), and the Adaptive Behavior Composite (F2,84=13.97, P<0.001, partial η2=0.25) between ASD Geometric and ASD social responders. Finally, significant differences were also found between these two ASD subytpes on the ADOS Social Affect / Communication scale (F2,86=8.20, P=0.001, partial η2=0.16) and total ADOS score (F2,86=5.76, P=0.004, partial η2=0.12). Collectively, results showed that toddlers with the ASD Geometric responder profile had worse scores on every test compared to toddlers with ASD with the ASD social responder profile. See Figure 4.
Figure 4.
Box and whiskers plot representing distribution of Mullen, ADOS, and Vineland scores for toddlers with ≥ 69% fixation towards DGI (i.e., Geometric Responders, N=35, red box) and ≥ 69% fixation towards DSI (i.e., Social Responders, N=54, tan box). The black line in the center of each box represents the median, the top and bottom of each box represent the 1st and 3rd quartiles respectively and the whiskers represent the minimum and maximum of the data. Outliars are represented as circles. Scores were significantly different between ASD Geo and ASD Social responders for all test subscales, with the exception of motor on the Vineland and the restricted and repetitive on the ADOS.
Unique Saccade Pattern in Toddlers with an ASD who Preferred Geometric Images
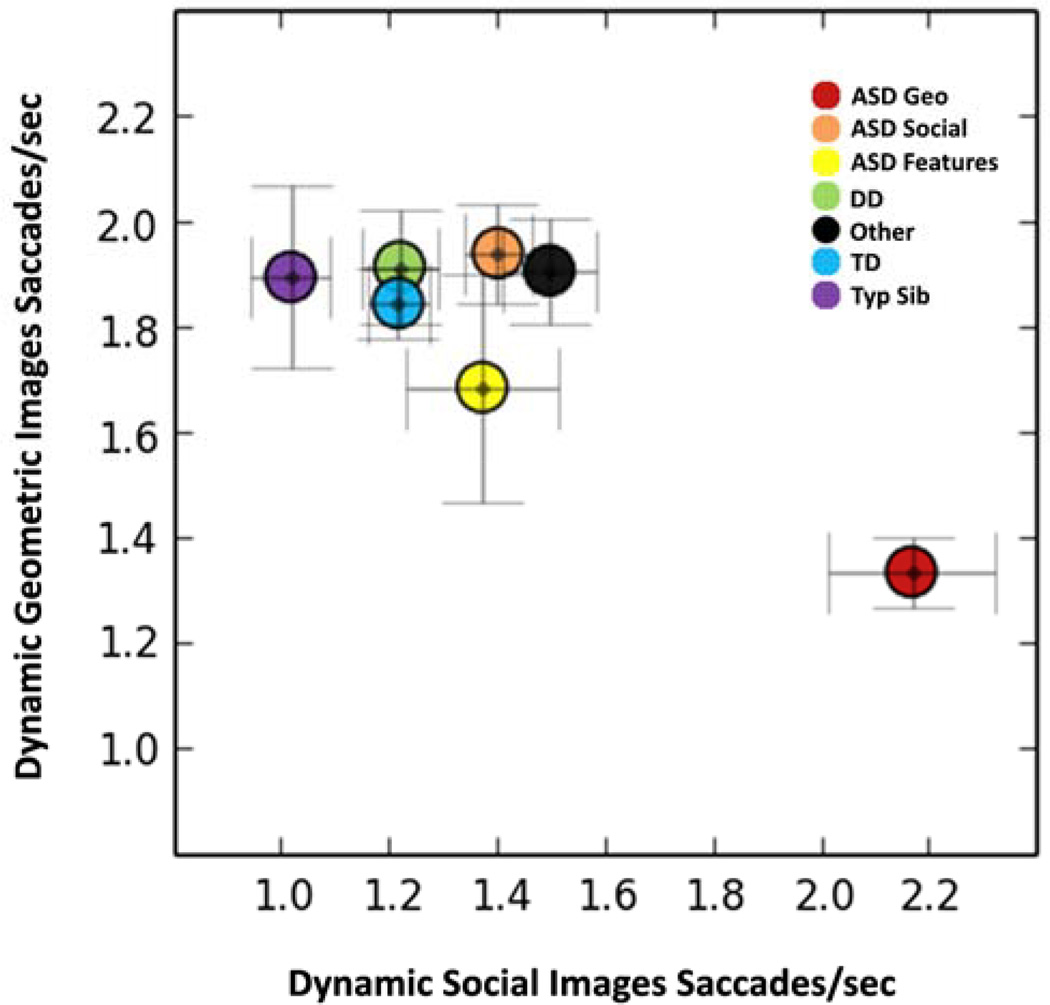
Our previous study split toddlers with ASD into two groups based on the middle point of the GeoPref Test (i.e., 50% fixation) and demonstrated that toddlers with ASD that preferred DGI (defined as DGI fixation levels >50%) exhibited fewer saccades when looking at geometric images than all other toddlers (20). To replicate these findings we performed similar analyses here. Similar to percent time fixating on DGI, an ANCOVA controlling for age demonstrated a main effect of diagnosis in the number of saccades per seconds while viewing DGI (F7,433=4.12, P<0.001, partial η2=0.06). Contrasts revealed that ASD geometric responders exhibited significantly fewer saccades (i.e., 1.33 saccades/sec) and thus longer bouts of sustained attention when they were viewing DGI relative to ASD social responders (1.9 saccades/sec, P<0.001, cohen’s d=0.76); TD toddlers (1.84 saccades/sec, P<0.001, cohen’s d=0.71), DD toddlers (1.91 saccades/sec, P<0.001, cohen’s d=0.70), TypSibs (1.89 saccades/sec, P=0.002, cohen’s d=0.82), and Other (1.91 saccades/sec, P<0.001, cohen’s d=0.91). However, there were no significant differences between ASD and ASD-Feat toddlers (1.68 saccades/sec; P=0.068, cohen’s d=0.47). In contrast, when the ASD geometric responders viewed their non-preferred stimuli, namely the social stimuli, they exhibited a significantly greater number of saccades relative to other contrast groups, all p-values < 0.001 (See Figure 5).
Figure 5.
Plot illustrating the average number of saccades per second when toddlers were viewing DGI or DSI for each diagnostic group. Toddlers with ASD that displayed a “Geometric Responder” profile were plotted separately from toddlers with ASD that displayed a “Social Responder” profile. The ASD Geometric Responder toddlers (red circle, bottom right of graph) displayed a unique saccade pattern that included fewer saccades when fixating on geometric shapes, but greater saccades when viewing social images. Error bars represent standard error of the mean. Dynamic Geometric Image Saccades/sec means±standard errors for groups are: ASD Geo: 1.33±0.07, ASD Soc: 1.94±0.09, ASD Features: 1.68±0.22, DD: 1.91±0.11, Other: 1.91±0.10, TD: 1.86±0.07, and Typ Sib: 1.89±0.17. Dynamic Social Image Saccades/sec means±standard errors for groups are: ASD Geo: 2.17±0.16, ASD Soc: 1.40±0.06, ASD Features: 1.37±0.14, DD: 1.22±0.07, Other: 1.50±0.09, TD: 1.22±0.06, and Typ Sib: 1.02±0.07.
Test-Retest Reliability
Overall, a visual preference for DGI, or lack thereof, appears to be a largely stable phenomenon. However, test-retest reliability was stronger with immediate retest (i.e., within 1 month of original test session; ICC=0.84, p<0.001) in comparison to later (i.e., > 1 year past original test session; ICC=0.52, p<.01). See supplementary information, Table S1, S2, and Figure S4.
Fixation Patterns Between Sibling Pairs (Exploratory)
Patterns of visual fixation were significantly correlated in siblings concordant for ASD but not in other sibling groups. See Supplement and Table S4 for more information.
DISCUSSION
In infants there is a near imperative to preferentially attend to the human face and social stimuli over non-social stimuli, even within minutes after birth (28). Yet, as investigated here, a subgroup of toddlers with ASD - about 20% - do not show this preference. Instead, this unique subtype of ASD prefers to visually examine dynamic geometric images. A strong preference for moving geometric images over social images was highly specific to this ASD subtype as compared to toddlers with typical development, language delay, global developmental delay as well as to unaffected sibs of toddlers with ASD.
With specificity levels as high as 98%, the GeoPref test was able to signify ASD status in a subset of individual toddlers with very high accuracy and as such may be more powerful than other biomarker attempts including those at the behavioral (29–31), genetic (32–34), and neuroimaging levels (35–37). Eye-tracking technology is attractive as a potential tool in early identification and clinical evaluation efforts because patterns of eye gaze are objective, quantifiable behaviors based on neural systems known to be abnormal in ASD, such as the visual attention system (38–40).
It has been clear since Leo Kanner’s original definition of autism in the 1940’s (41) that children with autism do not visually attend to cues in their environment, both social and non-social, to the same degree or in the same way as typically developing children (41–51). What is less clear, is whether unusual patterns of preferential visual attention in ASD are sequelae resulting from having the disorder across time, or are primary and early emerging. New studies have shown that baby siblings of ASD probands who later test positive for ASD display “sticky attention” or periods of abnormally prolonged visual fixation as young as 12-months in age (11), and that fixations within the eye region begin to abnormally decline sometime after 6 months (14). In the present study, the ASD toddlers with a geometric preference, many between 12–24 months, not only showed abnormalities in what they preferred to look at, but also produced significantly fewer saccades while viewing geometric shapes than all other toddlers. Collectively, our study combined with those of others (11, 14) suggest that abnormalities in visual attention and preference are one of the earliest emerging warning signs of ASD and while this may be reflective of very early neural circuit organization abnormalities, experience dependent mechanisms likely also play a role in the development of abnormal visual attention in ASD across the first years of life (52, 53).
Results of the present study go deeper than the pressing need to discover biomarkers of ASD that will hasten early detection and treatment. Beyond this goal is the knowledge that some toddlers with ASD, no matter how many tests are administered or how early treatment is started, will do well in life, while others may not even learn to speak. Understanding factors relating to outcome and prognosis in ASD children are among the most important goals in the field of autism today (54–58). While research has shown that up to 25% of ASD children may lose their diagnosis at some point (58), very little is known about factors that might predict later outcome at the time of diagnosis, or even prior to that time. In the current study, toddlers with the most intense fixation towards geometric images were also those with the most severe ASD symptoms, worst language, and lowest overall IQ scores. Our initial data thus suggest that what a toddler prefers to visually examine may be a valuable prognostic marker. Such ASD geometric responders not only need to be identified very early, but may require unique, yet to be determined, treatment approaches. Conversely, toddlers with ASD with intense fixation towards social images had better language, higher IQ and fewer symptoms than the ASD geometric responders. It is conceivable, although yet to be empirically verified, that toddlers with ASD who are very strong social responders at early ages will be those who enjoy more positive long term outcomes.
While heightened visual fixation towards geometric images may indeed represent a unique subgroup of toddlers with ASD, this trait is likely not suitable for the label “endophenotype” which, according to current definition, requires the trait to be heritable and present in unaffected family members (59). Interestingly, fixation towards geometric images was actually lower in unaffected siblings of ASD probands relative to normal controls, suggesting a protective mechanism, possibly genetic, for unaffected siblings. One reason to suggest that an intense fixation towards geometric images might be, at least in part, genetically driven is that sibling pairs concordant for ASD showed the highest correlation in visual attention towards geometric images, while no significant correlation was found between typically developing sibling pairs or in sibling pairs where only one had ASD.
Although the GeoPref Test was highly accurate in identifying a subset of true positive ASD cases, and had good test-retest reliability performance, overall the test’s sensitivity to detect all ASD cases fell within the range of 20–40%, depending on the cut off used. It would be a misunderstanding of ASD, however, to assume any single test will detect all ASD cases. Single biomarkers, such as heightened visual preference for repetitive geometric images, are limited in their ability to capture and parse all of the heterogeneity and complexity of a multifactorial disorder such as ASD. Interestingly, while percent fixation levels towards geometric images was highest in the ASD group, it was not significantly different between those toddlers with a final ASD diagnosis, and those that only showed ASD Features, further underscoring the dimensionality of the disorder. Consider that while it is generally believed that autism has a strong genetic component (60, 61) studies also suggest that non-genetic, currently unknown environmental factors may account for up to 50% of the total variance (61). It may be the case that combining multiple measures with low sensitivity but extremely high accuracy and specificity, such as the GeoPref Test, will be an effective method for detecting a larger portion of the highly heterogeneous ASD population in the future.
Interestingly, findings showed that all toddlers, ASD, TD and contrast cases alike, visually fixated on geometric images more strongly with increasing age. This is likely due to the fact that during very early development typically developing infants and toddlers are strongly drawn to the human face (28) while other factors such as an increased response to novelty emerge during early childhood that may impact the ability of the GeoPref Test to discriminate ASD from other disorders at older ages.
In terms of the predictive ability of the GeoPref Test to correctly identify ASD children, we found high values in classifying ASD children within our sample (i.e., 90% PPV when using 69% fixation cutoff). However, our sample, as with all research samples, does not reflect the base rate of ASD in the general population. Instead, in the current study, the majority of participants were referred for eye tracking after they failed a broadband developmental screen (i.e., the CSBS) at their pediatrician’s office or after a delay was suspected by a parent or healthcare provider (21). As such, eye-tracking as applied to our sample is highly reflective of a 2nd tier screening approach. When considering eye-tracking as a possible 1st tier screening approach, PPV and NPV should be calculated using ASD population base rates. In this case, PPV decreases to 17%, as only ~1.4 out of 100 children will develop ASD (27). Changes to the PPV as reflected in a 2nd or 1st tier screening approach highlight the importance of the population and setting in which a classification test will be used (see (62) for a discussion). Moreover, our study was limited in the sense that the number of normal control participants was somewhat arbitrary in count, which could also impact estimates of PPV and NPV.
At this stage of the science, the presence of an ASD biomarker in a toddler, such as heightened visual preference for dynamic geometric images, should not replace clinical diagnosis. Rather, the presence of this trait has considerable scientific and clinical value as an early biomarker of ASD that could hasten the pace of early identification and treatment, and provide valuable prognostic information. Toddlers that show a preference for geometric images have worse symptoms than other toddlers with ASD and as such may have distinctive underlying neurobiological attributes. It is conceivable, although not yet empirically tested, that the subtype of toddlers with ASD who have the geometric responder profile may require specific treatments tailored to their unique biology.
While it is unclear what the future will hold, it is possible that diagnosis may move away from a purely clinical judgment DSM approach, to a more objective research domain approach that instead focuses on the measurement of reproducible biological traits (63, 64). As such, large adequately powered research studies, such as the current study, are essential to enable definition of clinically-relevant biomarkers and comparison of biomarker levels across different diagnostic groups.
Supplementary Material
Acknowledgements
All phases of this study were supported by NIH grants R01-MH080134 awarded to Karen Pierce and P50-MH081755 awarded to Eric Courchesne and a National Foundation for Autism Research grant awarded to Karen Pierce. Thank you to Eric Courchesne for his helpful comments on final drafts of this manuscript, to Clelia Ahrens-Barbeau for administrative and overall support, James Proudfoot for statistical consultation and to Dr. Richard Stoner, Dr. Tiziano Pramparo, David Conant, and Adrienne Moore for assistance with some of the figures. A very special thank you to pediatricians in San Diego for the hundreds of children they have referred to our research program. And most importantly, thank you to the children with and without ASD in San Diego and their parents, without whom this work would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
An invention disclosure form was filed by K. Pierce with the University of California, San Diego, on March 5th, 2010. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon EC, Dufek S, Schreibman L, Stahmer AC, Pierce K, Courchesne E. Measuring outcome in an early intervention program for toddlers with autism spectrum disorder: use of a curriculum-based assessment. Autism research and treatment. 2014;2014:964704. doi: 10.1155/2014/964704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozonoff S, Iosif AM, Young GS, Hepburn S, Thompson M, Colombi C, et al. Onset patterns in autism: correspondence between home video and parent report. J Am Acad Child Adolesc Psychiatry. 2011;50:796–806. e791. doi: 10.1016/j.jaac.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strimbu K, Tavel JA. What are biomarkers? Current opinion in HIV and AIDS. 2010;5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce K, Glatt S, Liptak GS LM. The Power and Promise of Identifying Autism Early: Insights From the Search for Clinical and Biological Markers. Annals of Clinical Psychiatry. 2009;21:132–147. [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 8.Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naber F, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Dietz C, van Daalen E, Swinkels SH, et al. Joint attention development in toddlers with autism. Eur Child Adolesc Psychiatry. 2008;17:143–152. doi: 10.1007/s00787-007-0648-6. [DOI] [PubMed] [Google Scholar]
- 11.Sacrey LA, Bryson SE, Zwaigenbaum L. Prospective Examination of Visual Attention during Play in Infants at High-Risk for Autism Spectrum Disorder: A Longitudinal Study from 6 to 36 Months of Age. Behav Brain Res. 2013;256:441–450. doi: 10.1016/j.bbr.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives General Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 14.Jones W, Klin A. Attention to eyes is present but in decline in 2–6 month old infatns later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shic F, Bradshaw J, Klin A, Scassellati B, Chawarska K. Limited activity monitoring in toddlers with autism spectrum disorder. Brain Res. 2011;1380:246–254. doi: 10.1016/j.brainres.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, et al. Precursors to Social and Communication Difficulties in Infants At-Risk for Autism: Gaze Following and Attentional Engagement. J Autism Dev Disord. 2012;42:2208–2218. doi: 10.1007/s10803-012-1450-y. [DOI] [PubMed] [Google Scholar]
- 17.Chawarska K, Macari S, Shic F. Context modulates attention to social scenes in toddlers with autism. J Child Psychol Psychiatry. 2012;53:903–913. doi: 10.1111/j.1469-7610.2012.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillon Q, Hadjikhani N, Baduel S, Roge B. Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neurosci Biobehav Rev. 2014;42C:279–297. doi: 10.1016/j.neubiorev.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Dev Sci. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce K, Conant D, Hazin R, Stoner R, Desmond J. Preference for geometric patterns early in life as a risk factor for autism. Archives of general psychiatry. 2011;68:101–109. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce K, Carter C, Weinfeld M, Desmond J, Hazin R, Bjork R, et al. Detecting, Studying, and Treating Autism Early: The One-Year Well-Baby Check-Up Approach. Journal of Pediatrics. 2011;159:458–465. doi: 10.1016/j.jpeds.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wetherby A, Prizant B. Communication and symbolic behavior scales developmental profile - first normed edition. Baltimore, MD: Paul H. Brookes; 2002. [Google Scholar]
- 23.Wetherby AM, Brosnan-Maddox S, Peace V, Newton L. Validation of the Infant- Toddler Checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism. 2008;12:487–511. doi: 10.1177/1362361308094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 2001. [Google Scholar]
- 25.Mullen EM. Mullen Scales of Early Learning. AGS ed. MN: American Guidance Service Inc; 1995. [Google Scholar]
- 26.Sparrow S, Cicchetti D, Balla D. Vineland-II scales of adaptive behavior: survey form manual. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- 27.Network A. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 28.Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- 29.Sheinkopf SJ, Iverson JM, Rinaldi ML, Lester BM. Atypical cry acoustics in 6- month-old infants at risk for autism spectrum disorder. Autism Res. 2012;5:331–339. doi: 10.1002/aur.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, Johnson MH. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol Psychiatry. 2013;74:189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. J Child Psychol Psychiatry. 2013;54:763–771. doi: 10.1111/jcpp.12032. [DOI] [PubMed] [Google Scholar]
- 32.Matsunami N, Hadley D, Hensel CH, Christensen GB, Kim C, Frackelton E, et al. Identification of rare recurrent copy number variants in high-risk autism families and their prevalence in a large ASD population. PLoS One. 2013;8:e52239. doi: 10.1371/journal.pone.0052239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatt SJ, Tsuang MT, Winn M, Chandler SD, Collins M, Lopez L, et al. Blood-based gene expression signatures of infants and toddlers with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:934–944. e932. doi: 10.1016/j.jaac.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skafidas E, Testa R, Zantomio D, Chana G, Everall IP, Pantelis C. Predicting the diagnosis of autism spectrum disorder using gene pathway analysis. Mol Psychiatry. 2012;19:504–510. doi: 10.1038/mp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013;136:2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135:949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Courchesne E, Karns C, Davis HR, Ziccardi R, Carper R, Tigue Z, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 38.Townsend J, Courchesne E, Egaas B. Slowed orienting of covert visual-spatial attention in autism: Specific deficits associated with cerebellar and parietal abnormality. Development and Psychopathology. 1996;8:563–584. [Google Scholar]
- 39.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 40.Shi F, Wang L, Peng Z, Wee CY, Shen D. Altered modular organization of structural cortical networks in children with autism. PLoS One. 2013;8:e63131. doi: 10.1371/journal.pone.0063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 42.Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, et al. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- 43.Hobson RP, Lee A. Hello and goodbye: a study of social engagement in autism. Journal of Autism and Developmental Disorders. 1998;28:117–127. doi: 10.1023/a:1026088531558. [DOI] [PubMed] [Google Scholar]
- 44.Phillips W, Baron-Cohen S, Rutter M. The role of eye contact in goal detection: Evidence from normal infants and children with autism or mental handicap. Development & Psychopathology. 1992;4:375–383. [Google Scholar]
- 45.Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- 46.Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20:115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- 47.Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: an investigation of empathy, pretend play, joint attention, and imitation. Developmental psychology. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- 48.Gillespie-Lynch K, Elias R, Escudero P, Hutman T, Johnson SP. Atypical Gaze Following in Autism: A Comparison of Three Potential Mechanisms. J Autism Dev Disord. 2013;43:2779–2792. doi: 10.1007/s10803-013-1818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 50.Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 51.Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Dev Sci. 2011;14:980–988. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweatt JD. Experience-dependent Epigenetic Modifications in the CNS. Biological Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry. 2004;45:212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 55.Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, et al. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. J Autism Dev Disord. 2007;37:98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- 56.Eigsti IM, Fein DA. More Is Less: Pitch Discrimination and Language Delays in Children with Optimal Outcomes from Autism. Autism Res. 2013;6:605–613. doi: 10.1002/aur.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fein D, Barton M, Eigsti IM, Kelley E, Naigles L, Schultz RT, et al. Optimal outcome in individuals with a history of autism. J Child Psychol Psychiatry. 2013;54:195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert M, et al. Can children with autism recover? If so, how? Neuropsychol Rev. 2008;18:339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- 59.Leboyer M, Bellivier F, Nosten-Bertrand M, Jouvent R, Pauls D, Mallet J. Psychiatric genetics: search for phenotypes. Trends Neurosci. 1998;21:102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- 60.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Ercument Cicek A, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffin R, Westbury C. Infant EEG activity as a biomarker for autism: a promising approach or a false promise? BMC medicine. 2011;9:61. doi: 10.1186/1741-7015-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- 64.Cuthbert BN. Translating intermediate phenotypes to psychopathology: The NIMH Research Domain Criteria. Psychophysiology. 2014;51:1205–1206. doi: 10.1111/psyp.12342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.