Abstract
Benzodiazepines are commonly used medications that alter sleep spindles during non-rapid eye movement (NREM) sleep, however the topographic changes to these functionally significant waveforms have yet to be fully elucidated. This study utilized high-density electroencephalography (hdEEG) to investigate topographic changes in sleep spindles and spindle-range activity caused by temazepam during NREM sleep in 18 healthy adults. After an accommodation night, sleep for all participants was recorded on two separate nights after taking either placebo or oral temazepam 15mg. Sleep was monitored using 256-channel hdEEG. Spectral analysis and spindle waveform detection of sleep EEG data were performed for each participant night. Global and topographic data were subsequently compared between temazepam and placebo conditions. Temazepam was associated with significant increases in spectral power from 10.33–13.83Hz. Within this frequency band, temazepam broadly increased sleep spindle duration, and topographically increased spindle amplitude and density in frontal and central-posterior regions, respectively. Higher frequency sleep spindles demonstrated increased spindle amplitude and a paradoxical decrease in spindle density in frontal and centroparietal regions. Further analysis demonstrated temazepam both slowed the average frequency of spindle waveforms and increased the relative proportion of spindles at peak frequencies in frontal and centroparietal regions. These findings suggest that benzodiazepines have diverse effects on sleep spindles that vary by frequency and cortical topography. Further research that explores the relationships between topographic and frequency-dependent changes in pharmacologically-induced sleep spindles and the functional effects of these waveforms is indicated.
Keywords: sleep spindle, temazepam, benzodiazepine, high density EEG
Introduction
Sleep spindles are characteristic electroencephalographic waveforms that occur during non-rapid eye movement (NREM) sleep. These waxing-waning oscillations play functionally significant roles in cortical development, sleep-dependent memory consolidation, and sleep maintenance (Fogel and Smith, 2011; Khazipov et al., 2004; Steriade, 2003). In addition, multiple investigations have demonstrated that reductions in sleep spindles may be a biomarker in schizophrenia and reflect thalamic reticular and/or thalamocortical deficits in the disorder (Ferrarelli et al., 2007; Ferrarelli et al., 2010; Genzel et al., 2015; Manoach et al., 2010; Phillips et al., 2012; Suh et al., 2013; Wamsley et al., 2012). Thus, understanding the effects pharmacologic agents have on sleep spindles is important for several spheres of research, including those related to sleep, plasticity, cognitive function, and neuropsychiatric disease.
Benzodiazepines are common psychotropic agents utilized clinically for their anxiolytic, anticonvulsant, muscle relaxant, and/or sedative-hypnotic properties, for which a sizeable prior literature details their effects on the sleep electroencephalogram (EEG) (Lancel, 1999). Multiple investigations have demonstrated that benzodiazepines increase spectral power in the spindle range (~10–16Hz) (Aeschbach et al., 1994a; Aeschbach et al., 1994b; Borbély et al., 1985; Dijk et al., 1989; Feige et al., 1999; Trachsel et al., 1990). Moreover, myriad investigations that have utilized methods to quantify sleep spindles have demonstrated that benzodiazepines increase the occurrence of these waveforms (Azumi and Shirakawa, 1982; Hirshkowitz et al., 1982; Johnson and Spinweber, 1981; Johnson et al., 1983; Monti and Altier, 1973; Suetsugi et al., 2001). This increase in sleep spindles has been hypothesized to result from positive allosteric modulation of the GABA-A receptor, which plays a key role in the generation of sleep spindles in the thalamic reticular nucleus (De Gennaro and Ferrara, 2003).
Despite several studies that have previously examined the effects of benzodiazepines on sleep spindles, prior investigations have typically utilized a limited number of EEG derivations (usually 1–2 central channels) in their analyses. This is an important limitation of prior research because sleep spindles have a well-described topographic distribution, in which the frequency of the oscillation increases in a rostral to caudal gradient, with the occurrence of sleep spindles more prominent in midline derivations, particularly for fast (centroparietal) spindles (Andrillon et al., 2011; Peter-Derex et al., 2012; Schabus et al., 2007). Because a limited number of central channels may fail to detect pertinent alterations in sleep spindles (Ferrarelli et al., 2007), this study sought to utilize high-density EEG (hdEEG) to evaluate the topographic effects of the non-selective benzodiazepine, temazepam, on these functionally significant waveforms. Based on their previously described topography, we hypothesized that temazepam would increase slow and fast sleep spindles, most prominently in frontal and centroparietal derivations, respectively.
Experimental Procedures
Participants
All participants and sleep EEG data utilized in this study were drawn post hoc from a larger study that examined the effects of medications on sleep restriction. The University of Wisconsin-Madison Health Sciences Institutional Review Board approved this study and subsequent analyses. Participants were healthy volunteers recruited from the greater Madison, WI area and provided written-informed consent. Inclusion criteria included age 18–35 years, right-handedness, and body mass index (BMI) ≥19 and ≤32kg/m2. Baseline evaluation included the Structured Clinical Interview for DSM-IV-TR Axis I disorders (First et al., 2002), urine drug screen, and urine pregnancy test (for female participants). Exclusion criteria included current or past psychiatric disorder (based on SCID interview), current or recent major medical or neurologic illness, use of centrally acting pharmacologic agents, reported caffeine intake greater than 300mg/day, average alcohol intake greater than 3 drinks/day or 8 drinks/week, daily use of nicotine within 6 months prior to enrollment, use of illicit drugs, night/evening shift work, or travel across ≥3 time zones in the month preceding enrollment. Female participants were excluded if they were pregnant, planning to become pregnant during the study, nursing, or lactating. At baseline participants had to endorse a typical bedtime between 2100 and 0100 hours and a usual nightly sleep duration between 6.5 and 8.5 hours. Participants were free of clinically significant sleep-related breathing or movement disorders, verified by clinical history and screening polysomnogram, with participants excluded if they demonstrated an apnea-hypopnea index >10/hr or a periodic limb movement arousal index >10/hr. In addition, participants were excluded if they were excessively sleepy, based on either score ≥10 on the Epworth Sleepiness Scale (Johns, 1991) or mean sleep latency <8 minutes on baseline multiple sleep latency testing (Sullivan and Kushida, 2008)
The sleep data utilized in these analyses occurred on two separate nights of sleep (within-subjects design) that each occurred prior to a supervised sleep restriction and recovery protocol. The placebo night was drawn from the placebo-arm of a randomized study of an investigational drug; the temazepam night was drawn from an open-label extension arm of this parent study. Prior to both (placebo and temazepam) nights, all participants had spent at least two nights in the sleep laboratory during eligibility screening (which included an accommodation night with hdEEG monitoring), and at least three weeks had elapsed since undergoing any prior sleep restriction. Sleep-wake patterns were monitored between in-laboratory testing sessions using actigraphy (Actiwatch, Mini-Mitter, Bend, OR). Participants took either oral temazepam 15mg (open-label) or placebo (single tablet, not designed to be identical to temazepam) prior to bedtime on study nights.
Sleep EEG
HdEEG signals were collected with a vertex reference and 500 Hz sampling rate, first-order high-pass (0.1Hz) filtered and band-pass (0.3–50Hz) filtered in NetStation (Electrical Geodesics, Eugene, OR). HdEEG signals were then downsampled to 128 Hz, high-pass filtered (2-way least-squares FIR, 1Hz) and re-referenced to the average scalp voltage computed in all channels in MATLAB (MathWorks, Natick, MA). Semi-automatic artifact rejection was conducted to remove channels with interrupted contact with the scalp or high-frequency artifact. Specifically, thresholds were calculated for low (1–4 Hz) and high (20–30 Hz) frequency ranges at the 99.8th and 99.5th percentile, respectively, for each channel. Spectral power in these ranges across all 6-second NREM epochs for each channel were plotted for visual inspection. In cases where epochs with substantially greater low or high frequency power did not exceed the automatic threshold, the threshold was manually lowered and all epochs exceeding the threshold were removed. Also, channels with artifact affecting a majority of the recording were removed.
Spectral analysis of NREM sleep (all N2 and N3 epochs) was performed for each channel in consecutive 6-second epochs (Welch’s averaged modified periodogram with a Hamming window; frequency resolution 0.17Hz), which maintained congruence between spectral analysis and traditional sleep staging based on 30-second epochs (Goldstein et al., 2012; Plante et al., 2012). Sleep staging was performed by a registered technologist according to standard criteria (Iber et al., 2007) using Alice® Sleepware (Philips Respironics, Murrysville, PA) based on 6 EEG channels at approximate 10–20 locations (F3, F4, C3, C4, O1, and O2) re-referenced to the mastoids, sub-mental electromyogram and electrooculogram.
Spindle Detection and Characterization
Spindle detection was performed with a custom spindle detection algorithm in MATLAB with similar specifications as prior research from our laboratory (Ferrarelli et al., 2007; Ferrarelli et al., 2010; Plante et al., 2013). NREM epochs were filtered within frequency bands in the spindle range (10–16Hz), and rectified filtered signals were used as the time series for each channel. In order to increase the likelihood of identifying pertinent between group differences, our a priori analysis of spindle parameters (detailed below) was designed to focus on spindles detected within frequency bands in the spindle range that demonstrated significant between-condition differences using global spectral power. In addition, because pertinent topographic differences in sleep spindles could be missed solely using this approach, additional exploratory analyses examined spindles in residual frequency bands, as well as the entire spindle range (10–16Hz). Also, because prior literature has suggested functional and topographic differences between slow (~10 to ~13–14Hz) and fast (~13–14 to ~16Hz) sleep spindles (Astori et al., 2013), with variable frequency ranges used to define this dichotomous classification among investigators, spindles categorized using standard frequency cut-offs between slow (10 to 13–14Hz) and fast (13–14 to 16Hz) spindles were also examined for comparison purposes and to clarify results.
Details of the spindle detection algorithm have been described elsewhere (Ferrarelli et al., 2007). Briefly, a spindle was detected when the mean signal amplitude exceeded an upper threshold (6 times the mean amplitude). The peak amplitude for each spindle was the local maximum above the upper threshold. The beginning and end of a spindle occurred when the amplitude of the time series fell below a lower threshold (2 times the mean amplitude), occurring ≥0.25 seconds from the peak. We examined the following spindle parameters: density (number of spindles divided by artifact-free NREM sleep time), maximal amplitude, duration, and integrated spindle activity (ISA; integrated absolute amplitude values of each spindle divided by artifact-free NREM sleep time) (Ferrarelli et al., 2010).
Statistics
Sleep staging variables, as well as spectral power and spindle morphology data were compared between conditions (placebo vs. temazepam) using paired t-tests. To increase the signal-to-noise ratio, all hdEEG analyses were restricted to channels falling within a plotting radius of 0.57 specified in the topoplot function of the EEGLAB plug-in for MATLAB (Delorme and Makeig, 2004), resulting in 173 channels overlaying the scalp. For analyses, global spectral power was defined as the average of these 173 channels. Topographic comparisons of spectral and spindle data were performed using channel-by-channel paired t-tests. Statistical non-parametric mapping (SnPM) with suprathreshold cluster testing was utilized to correct for multiple comparisons of topographic data using a t-value threshold corresponding to alpha=0.05 for the uncorrected comparisons (Nichols and Holmes, 2002), with missing data for a given channel interpolated using the average of surrounding channels in order to maintain maximal degrees of freedom without altering the mean signal of the group. All statistical analyses were performed using MATLAB.
Results
Sleep staging, spectral activity and spindle morphology
Participants (11 female; 7 male) were aged 23.5±3.6 years (range 18–29). Sleep staging demonstrated no significant differences between placebo (PLC) and temazepam (TMZ) nights on standard variables (Table 1). Sixteen of the 18 participants had intact actigraphy for analysis (two had device failure) that confirmed similar bedtimes (mean 00:03 hours for PLC and 00:08 hours for TMZ) and sleep duration (7.68 hours for PLC and 7.48 hours for TMZ, p=0.25) prior to polysomnography. There were no significant between condition differences in percent of retained N2/N3 epochs (PLC: 94.3±2.6% vs. TMZ: 93.2±5.8%; p=0.40) or retained channels (PLC: 96.0±4.7% vs. TMZ: 96.0±4.8%; p=0.97) after artifact rejection.
Table 1.
Sleep staging data.
| PLC | TMZ | p-value | |
|---|---|---|---|
| TST (min) | 424.7 (47.4) | 436.6 (41.1) | 0.32 |
| WASO (min) | 35.8 (32.1) | 35.4 (43.7) | 0.97 |
| SE (%) | 90.1 (8.5) | 91.1 (9.1) | 0.54 |
| SOL (min) | 12.2 (14.3) | 7.4 (6.8) | 0.16 |
| N1 (min) | 51.7 (34.6) | 36.3 (25.0) | 0.15 |
| N1 (%) | 12.6 (9.3) | 8.3 (5.6) | 0.10 |
| N2 (min) | 260.7 (50.0) | 280.5 (52.6) | 0.19 |
| N2 (%) | 61.2 (8.0) | 64.3 (10.1) | 0.29 |
| N3 (min) | 44.5 (30.5) | 51.4 (25.5) | 0.46 |
| N3 (%) | 10.2 (6.7) | 11.8 (5.8) | 0.44 |
| REM (min) | 68.0 (32.2) | 68.4 (33.8) | 0.95 |
| REM (%) | 16.0 (7.6) | 15.6 (7.5) | 0.82 |
| REM Latency (min) | 144.5 (92.3) | 141.8 (92.4) | 0.93 |
PLC, placebo; TMZ, temazepam; TST, total sleep time; WASO, wake after sleep onset; SE, sleep efficiency (TST/time in bed); SOL, sleep onset latency; N1/2/3, NREM stage 1/2/3 (minutes and % of TST); REM, stage REM (minutes and % of TST); REML, REM latency (time from sleep onset to first REM sleep epoch).
Values are displayed as mean (standard deviation).
p-value derived using 2-tailed, paired t-tests.
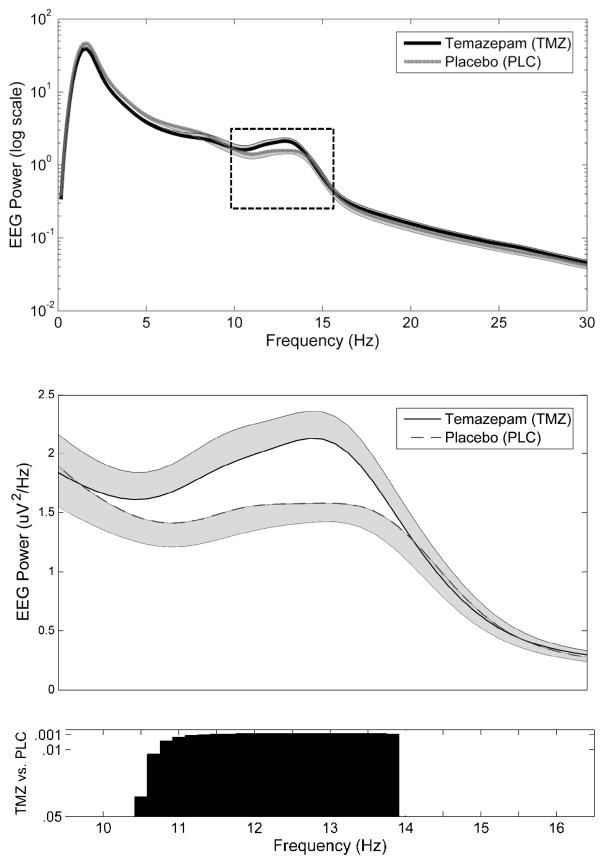
Analyses of global (average of 173 channels) spectral power in the spindle range demonstrated TMZ was associated with significant increases from 10.33–13.83Hz (Figure 1). Topographic analyses of spectral activity within this frequency band demonstrated increases that were most prominent in frontal and parieto-occipital channels in midline and bilateral derivations in TMZ relative to PLC (Figure 2).
Figure 1.
Differences in global spectral power in the spindle range between TMZ and PLC nights during NREM sleep. Global (average 173 channels overlying the scalp) EEG spectral power ± SEM for TMZ and PLC plotted for each 0.17Hz frequency bin from 1–30Hz (top panel) and 10–16Hz (middle panel). Corresponding p-values for comparison between TMZ vs. PLC from 10–16Hz (lower panel). Significant differences in global power were observed between conditions for bins 10.33–13.83Hz.
Figure 2.
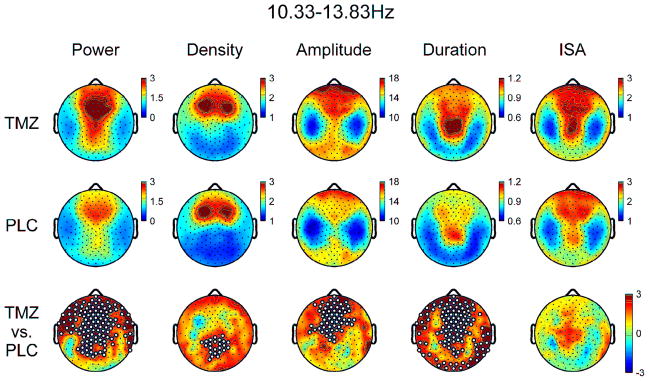
Topographic EEG power and slow spindle parameters for TMZ and PLC nights from 10.33–13.83Hz. Bottom row denotes t-values for channel-by-channel paired t-tests between TMZ and PLC. White dots denote significant channels after statistical non-parametric mapping with suprathreshold cluster test.
Using global spindle detection data within 10.33–13.83Hz, spindle duration and amplitude were significantly increased for TMZ relative to PLC, without significant changes in density or ISA (Table 2). Topographic analysis demonstrated the scalp distribution of density of these slower sleep spindles was more prominent in bilateral frontocentral areas for both PLC and TMZ conditions, consistent with prior literature (Figure 2) (Andrillon et al., 2011; Peter-Derex et al., 2012; Schabus et al., 2007). However, topographic increases in spindle density were observed in central-posterior derivations (e.g. outside of frontocentral regions) for TMZ relative to PLC (Figure 2). In addition, spindle amplitude significantly increased in frontocentral regions (Figure 2). Topographic analysis also demonstrated spindle duration increased in the vast majority of channels, except for a few centrolateral derivations (Figure 2). There were no significant topographic changes for ISA observed. Similar global and topographic patterns were observed for spindles detected with frequencies 10–14Hz and 10–13Hz (data not shown).
Table 2.
Global spindle characteristics.
| PLC | TMZ | p-value | |
|---|---|---|---|
| Spindle Frequency: 10.33–13.83Hz | |||
| Density (count/min) | 2.01 (0.68) | 2.13 (0.68) | 0.17 |
| Amplitude (μV) | 14.13 (3.80) | 15.10 (3.67) | 0.01 |
| Duration (sec) | 0.89 (0.13) | 0.98 (0.19) | 0.0008 |
| ISA (μV2/min) | 2.22 (0.78) | 2.37 (0.91) | 0.37 |
| Spindle Frequency: 13.83–16Hz | |||
| Density (count/min) | 0.84 (0.49) | 0.67 (0.40) | 0.01 |
| Amplitude (μV) | 13.59 (3.62) | 14.64 (3.49) | 0.002 |
| Duration (sec) | 0.61 (0.10) | 0.59 (0.11) | 0.11 |
| ISA (μV2/min) | 1.46 (0.55) | 1.45 (0.47) | 0.88 |
| Spindle Frequency: 10–16Hz | |||
| Density (count/min) | 2.80 (0.58) | 2.85 (0.63) | 0.36 |
| Amplitude (μV) | 13.99 (3.76) | 14.99 (3.67) | 0.006 |
| Duration (sec) | 0.82 (0.13) | 0.91 (0.19) | 0.0007 |
| ISA (μV2/min) | 2.07 (0.76) | 2.22 (0.91) | 0.33 |
Global values derived from the average of 173 channels overlying the scalp. PLC, placebo; TMZ, temazepam. Values are displayed as mean (standard deviation).
p-value derived using 2-tailed, paired t-tests uncorrected for multiple comparisons.
Significant between condition values marked in bold.
Spindle detection within a residual fast spindle range (13.83–16Hz) demonstrated significant increases in global spindle amplitude and decreases in spindle density, without significant differences in duration or ISA (Table 2). Topographic analysis of spectral power in this band demonstrated no significant differences between conditions (Figure 3). Topographic data demonstrated these faster spindles were more common in central midline derivations for both conditions, consistent with prior literature (Figure 3) (Andrillon et al., 2011; Peter-Derex et al., 2012; Schabus et al., 2007). Notably, topographic analysis also demonstrated a decrease in spindle density and a concurrent increase in spindle amplitude in frontal and central midline derivations in TMZ relative to PLC (Figure 3). Additionally, a minor right posterolateral increase in spindle duration was observed with TMZ, without topographic changes for ISA (Figure 3). Similar global and topographic patterns were observed for spindles detected between 14–16Hz and 13–16Hz (data not shown).
Figure 3.
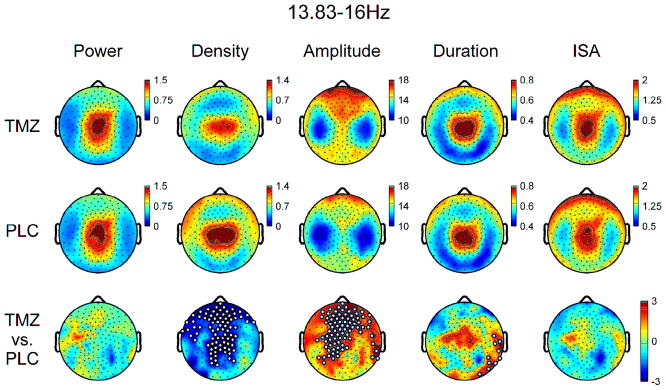
Topographic EEG power and fast spindle parameters for TMZ and PLC nights from 13.83–16Hz. Bottom row denotes t-values for channel-by-channel paired t-tests between TMZ and PLC. White dots denote significant channels after statistical non-parametric mapping with suprathreshold cluster test.
For comparison purposes, data using the full spindle range (10–16Hz) were additionally analyzed. Global data demonstrated a similar pattern to slower (i.e. 10.33–13.83Hz) spindles with significant increases in spindle duration and amplitude in TMZ relative to PLC, without significant differences in density or ISA (Table 2). Topographically, as observed in both slower and faster (i.e. 13.83–16Hz) spindles, amplitude was increased by TMZ in frontocentral regions (Figure 4). In addition, TMZ broadly increased spindle duration in frontal, central, and lateroposterior areas, with no significant topographic differences in spindle density or ISA between conditions.
Figure 4.
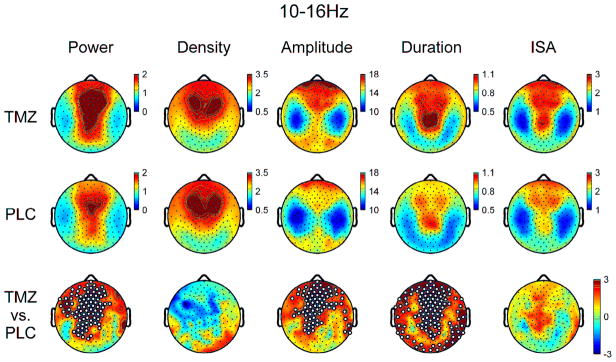
Topographic EEG power and spindle parameters for TMZ and PLC nights for all detected spindles 10–16Hz. Bottom row denotes t-values for channel-by-channel paired t-tests between TMZ and PLC. White dots denote significant channels after statistical non-parametric mapping with suprathreshold cluster test.
Additional Exploratory Analyses
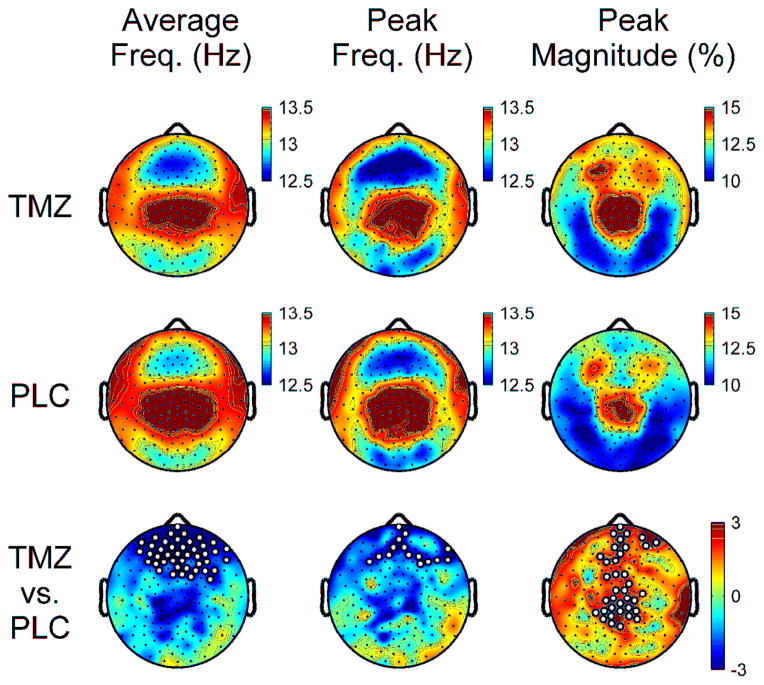
Since the effects of TMZ on sleep spindles varied depending on the frequency range in which detected spindles were evaluated, the effects of TMZ on the distribution of detected spindles across the broad spindle frequency range were analyzed. The average frequency for detected spindles at each channel was compared between conditions (Figure 5). In addition, the distribution of detected spindles was apportioned into adjacent 0.25Hz bins, and the peak frequency and relative magnitude (% of total detected spindles) of the peak of the distribution were compared between TMZ and PLC (Figure 5). TMZ reduced the average and peak frequencies of detected spindles, most prominently in frontal derivations, while increasing the peak magnitude of the distribution in frontal and midline channels. For visualization purposes, these effects are displayed in twelve midline derivations in Supplemental Figure 1, illustrating the leftward shift (i.e. reduction) of the frequency distribution of detected spindles and an increased percentage of spindles at the peak frequency in TMZ relative to PLC. Thus the decreased density of fast sleep spindles observed occurred in the context of generalized slowing of all detected spindles, that was most pronounced in frontocentral derivations.
Figure 5.
Topography of spindle frequency and peak magnitude of the distribution of detected spindles for TMZ and PLC nights. Average frequency analyzed as a continuous variable and represents the mean spindle frequency for all detected spindles at each channel. Peak frequency derived by apportioning detected spindles at each channel into adjacent 0.25Hz bins from 10–16Hz and determining the bin of maximal proportion of oscillations relative to the total number of detected spindles at each channel (peak magnitude). Bottom row denotes t-values for channel-by-channel paired t-tests between TMZ and PLC. White dots denote significant channels after statistical non-parametric mapping with suprathreshold cluster test.
Also, because the maximal amplitude of a sleep spindle is a function of waxing and waning amplitudes, which are hypothesized to result from different mechanisms (Urakami et al., 2012), we examined whether TMZ differentially affected the latency to the maximal amplitude for detected spindles. We found no significant topographic differences between conditions for latency to the maximal amplitude (both absolute and normalized to spindle duration), nor the waxing or waning slope of the oscillation (derived from spindle onset to maximal amplitude and maximal amplitude to termination, respectively) (Supplemental Figure 2).
Although we chose to examine sleep spindles in all N2/N3 sleep to minimize the chance that differences in sleep architecture/staging or intra-individual differences in drug metabolism during the course of the night might influence results, we additionally explored differences in sleep spindles between PLC and TMZ in separate sleep cycles [i.e. separate NREM episodes using previously defined criteria (Kurth et al., 2010)]. Similar topographic findings were observed for spindle amplitude and duration for both slow and fast spindles for the first three NREM episodes. Spindle density also showed similar topographic patterns for slow and fast spindles, except the increased density of slow spindles due to TMZ expanded to include frontocentral channels in the first NREM sleep cycle (data not shown).
To examine whether BMI might have affected findings given a fixed dose of temazepam within a heterogeneous participant group (mean BMI 23.6, range 19.3–31.8kg/m2), we explored correlations between BMI and change in spindle density, amplitude, and duration. We found no significant correlations between BMI and these parameters for either slow or fast spindles using global measures. Additionally, we performed exploratory correlations between global slow wave activity (EEG power 1–4.5Hz for all epochs of N2/N3 sleep) and spindle parameters (density, duration, and amplitude) for fast and slow spindles, finding no significant correlations among these variables for either PLC or TMZ conditions.
Discussion
Consistent with prior investigations, our findings demonstrate that the oral administration of the non-selective benzodiazepine temazepam results in alterations in spectral activity in the spindle range, as well as spindle density and morphology during non-rapid eye movement sleep. In regards to global spectral power, our results are consistent with several prior reports demonstrating that the increase in spindle range activity during NREM sleep associated with benzodiazepines is typically below ~14Hz (Aeschbach et al., 1994a; Aeschbach et al., 1994b; Borbély et al., 1985; Dijk et al., 1989; Trachsel et al., 1990). Our study and the use of high-density EEG to evaluate sleep spindles additionally further extends the previous literature in several ways. First, we found that the density, amplitude, and duration of slower (~10–14Hz) sleep spindles were differentially increased by temazepam, with varying cortical topographies for these measures. Second, temazepam resulted in an unexpected topographic decrease in spindle density of faster (~14–16Hz) sleep spindles, despite a concurrent increase in amplitude. Third, the differential effects of temazepam on slow and fast spindles observed in this study were mediated, at least in part, by a shift in the distribution of spindles to lower frequencies, without an overall net change in spindle density from 10–16Hz.
Although this study is not designed to evaluate the cellular and molecular mechanisms through which temazepam alters sleep spindle morphology, it is most likely that the drug enacts its effects through agonism of the GABA-A receptor at thalamic reticular neurons. The spindle rhythm is generated by rhythmic interactions between inhibitory neurons of the reticular nucleus and excitatory thalamocortical neurons (Urakami et al., 2012). Recent work has suggested that the duration of a sleep spindle is dependent on the inhibitory activity of reticular neurons, such that termination of the oscillation occurs once inhibitory input decreases below a minimal value required for evoking rebound bursts in thalamocortical cells (Barthó et al., 2014). Similarly, the degree and/or duration of inhibitory post-synaptic potentials imposed by reticular neurons on thalamocortical cells is the primary determinant of intra-spindle frequency (Urakami et al., 2012). Of note, the duration of slow spindles in both placebo and temazepam conditions in this investigation was greater than that of fast spindles, a finding that has also been observed in other investigations most prominently in frontal electrodes (Ujma et al., 2015). Therefore the changes in spindle duration and frequency caused by temazepam observed in this study would most likely result from potentiation of the GABA-A receptor in thalamic reticular neurons, and although speculative, suggests that potentiation of the GABA-A receptor may have more substantial effects on the duration of slow relative to fast spindles.
The probable mechanism through which temazepam increased spindle amplitude in our study is less clear. The maximal amplitude of the spindle oscillation is a function of waxing and waning amplitudes, the former correlated to the amplitude of neuronal excitatory post-synaptic potentials and the latter likely resulting from cortical feedback to the thalamus as well as deterioration of synchronization of thalamocortical neurons, with resultant asynchrony of reticular neurons (Urakami et al., 2012). Since temazepam should increase cortical inhibitory post-synaptic potentials, increases in spindle amplitude via effects on the waning rather than waxing amplitude seem more likely. However, because there were no observable differences between placebo and temazepam conditions in our study for either latency to maximal amplitude or the waxing/waning slopes of the oscillation, our data do not point to differential effects of temazepam on the waxing versus waning portions of sleep spindles (i.e. the symmetry of the waveform), and thus mechanisms though which temazepam increases spindle amplitude are highly speculative. However, regardless of the mechanism through which temazepam increases spindle amplitude, the results of this study are consistent with the findings of prior investigations (Hirshkowitz et al., 1982).
Our results are further consistent with prior research that has demonstrated that slow (~10 to ~13–14Hz) and fast (~13–14 to ~16Hz) spindles have different cortical topographies, with slow spindles more prominent in frontal regions, and fast spindles more pronounced in centroparietal areas (Andrillon et al., 2011; Peter-Derex et al., 2012; Schabus et al., 2007). It has been suggested that slow and fast spindles have varying functional roles, particularly in the context of sleep-dependent memory consolidation (Astori et al., 2013). It is noteworthy that our results suggest temazepam decreases the density of fast sleep spindles, as prior studies that have not utilized hdEEG have reported increases in sleep spindles associated with benzodiazepine administration. In this context, our results may elucidate the paradox that benzodiazepines have been associated with increases in sleep spindles using limited EEG montages, but decrements in some forms of sleep-related memory consolidation.
The motor sequence task (MST) is a widely used paradigm to used investigate the effects of sleep and EEG waveforms on motor memory (Albouy et al., 2013; Barakat et al., 2011; Nishida and Walker, 2007; Wamsley et al., 2013; Wamsley et al., 2012). Improvement on the MST has been positively associated with spindle density, particularly with fast spindles in centroparietal regions (Barakat et al., 2011; Nishida and Walker, 2007). Notably, the non-selective benzodiazepine triazolam paradoxically worsens overnight performance on the MST, despite reports that this medication increases the density of sleep spindles (Johnson and Spinweber, 1981; Morgan et al., 2010). Our results suggest that impairment on the MST caused by benzodiazepines may be due to topographic decreases in fast sleep spindles, that may not have been previously observed in other studies due to either inadequate topographic resolution of sleep EEG and/or failure to distinguish between slow and fast spindles. However, it is certainly also plausible that other factors such as slowed response time or functionally different properties of pharmacologically-induced sleep spindles compared to those produced endogenously, may account for the paradoxical worsening on the MST associated with benzodiazepines.
To extend our results utilizing temazepam to other non-selective benzodiazepines, and thus assume that the alterations in sleep spindles observed in this study are a class effect, must be done with caution. Although benzodiazepines have a shared mechanism of action, they have a diversity of pharmacokinetic profiles that could theoretically result in variable effects on sleep spindles. Moreover, the results of this study cannot be extended to other GABAergic sedative hypnotics, such as non-benzodiazepine benzodiazepine receptor agonists (BZRAs). Both zolpidem and eszopiclone have been shown to increase spindle density without changes in frequency, amplitude, or duration of spindles (Mednick et al., 2013; Wamsley et al., 2013). Notably, the reticular nucleus is devoid of GABA-A α1-receptors, and synaptic inhibition is mediated by α3-receptors; while α1-receptors mediate inhibition in thalamocotrical relay neurons (Huntsman et al., 1999; Jia et al., 2005; Pirker et al., 2000). Correspondingly, in vitro studies have demonstrated that zolpidem exerts larger effects on thalamocortical neurons, while eszopiclone has larger effects on reticular neurons (Jia et al., 2009). Because these GABAergic agents have also demonstrated divergent effects on the MST (Mednick et al., 2013; Morgan et al., 2010; Wamsley et al., 2013), future research that examines the relationship between GABA-A subtype receptor affinity, sleep spindle density/morphology, and pharmacological enhancement of sleep-dependent memory consolidation, may yield further insights into the relationships between these factors.
There are limitations of this study that merit discussion. First, this was a fixed-dose study, and thus observed differences between temazepam and placebo conditions may have differed if a higher dose were utilized, particularly since dose-response effects of benzodiazepines on sleep spindle incidence have been previously reported (Hirshkowitz et al., 1982). Second, the temazepam night was part of an open-label extension, and thus our unblinded design may have affected results. However, prior studies utilizing temazepam have demonstrated that even in double-blind protocols, study participants are usually able to discern whether they are on active drug or placebo (Morin et al., 1995), which reduces the likelihood that our results would have differed using a blinded protocol. Third, there may have been an order effect as TMZ nights occurred after PLC nights. However, the risks of order effects are minimized by the relative stability of spindle activity from night to night (Curcio et al., 2004; Le Bon et al., 2001). Fourth, caffeine intake was based on self-report and not verified by objective assay, which may have affected results since caffeine can increase power in the sigma range (Drapeau et al., 2006; Landolt et al., 2004). Fifth, our spindle detection algorithm was selected due to its previous application in hdEEG studies (Ferrarelli et al., 2007; Ferrarelli et al., 2010; Plante et al., 2013) and to maximize precision over recall (Warby et al., 2014), to minimize the likelihood that false positive detections would lead to spurious results. However, our results may have differed had we utilized a different spindle detection algorithm, particularly one that utilizes intra-individual thresholds for slow and fast spindles, such as proposed by Bódizs and colleagues (Bódizs et al., 2009). Finally, the phase of the menstrual cycle at the time of hdEEG recordings was not ascertained for female participants, which may have affected results, as spindle range activity has been reported to increase in the luteal relative to the follicular phase (Baker et al., 2007; Baker et al., 2012). Because prior research has also demonstrated a sex and menstrual phase effect on sleep-related memory consolidation and sleep spindle activity after learning (Genzel et al., 2012), future research that examines the effects of exogenous benzodiazepines on sleep EEG across the menstrual cycle, and their interactive effects on learning, may prove a fruitful avenue of investigation.
In conclusion, we have demonstrated temazepam results in global and topographic changes in spectral power and spindle density/morphology during the sleep EEG. These findings suggest that benzodiazepines have myriad effects on spectral activity and sleep spindles that vary depending on cortical topography and frequency of the waveform. Further studies that explore the relationship between topographic and morphological changes in sleep spindles induced by benzodiazepines and other related compounds, and their effects on sleep-dependent memory consolidation, development, and neuropsychiatric disorders, are indicated to clarify the functional significance of these findings.
Supplementary Material
Representative analysis of the frequency distribution of detected spindles for TMZ and PLC nights using twelve midline derivations. A) Schematic of locations of derivations used. B) Distribution of detected spindles by frequency for selected frontal (blue) and central (red) channels. Distribution defined by 0.25Hz frequency bins 10–16Hz. Dashed lines correspond to PLC night; solid lines correspond to TMZ night. C) Plots illustrating the relative proportion of spindles across the frequency distribution of detected spindles for TMZ and PLC nights for all 12 midline channels, as an extension of B), and comparisons between the two. White dots indicate significant difference between conditions (uncorrected). Note the leftward shift of the distribution and increase in peak amplitudes for TMZ relative to PLC.
Topography of the waxing and waning oscillation of detected spindles for TMZ and PLC nights. Absolute latency derived as the time (seconds) from start of spindle envelope to maximal amplitude. Relative latency (percent) is the time from start of spindle envelope to maximal amplitude normalized to the duration of each detected spindle. Wax Slope (μV/sec) is the slope from the start of the spindle to the maximal amplitude; Wane slope (μV/sec) is the time from the maximal amplitude to the termination of the spindle. Duration of detected spindles is re-plotted for comparison purposes. Bottom row denotes t-values for channel-by-channel paired t-tests between TMZ and PLC. White dots denote significant channels after statistical non-parametric mapping with suprathreshold cluster test.
Acknowledgments
This research was funded by an investigator-initiated grant by Sanofi to Dr. Peterson. Dr. Plante is supported by grants from NIMH (K23MH099234), the American Sleep Medicine Foundation, and The Brain and Behavior Research Foundation.
ROLE OF FUNDING SOURCE
Sanofi had no further role in the design of this study, collection of data, analysis and interpretation of data, or writing of the manuscript. Sanofi was provided courtesy review of the manuscript prior to submission, but did not play a role in the decision to submit the report for publication.
Footnotes
CONTRIBUTORS
Dr. Plante designed the study, managed literature searches and analyses, and wrote the first draft of the manuscript. Mr. Goldstein and Mr. Cook performed statistical analyses, hdEEG processing and analysis, design of figures/tables, and contributed to study design and writing the manuscript. Mr. Smith, Dr. Rumble, Ms. Jelenchick, and Ms. Roth contributed to the evaluation of participants, collection of hdEEG data, and writing the article. Dr. Riedner contributed to analysis and interpretation of the data and writing the manuscript. Dr. Tononi and Dr. Benca contributed to the study design, data analysis and interpretation, and to writing the article. Dr. Peterson contributed by being the principal investigator of the parent study, contributing to the study design, participating in data analysis and interpretation, and writing the article. All authors contributed to and have approved the final manuscript.
CONFLICTS OF INTEREST
Dr. Plante has received royalties from Cambridge University Press. Dr. Riedner is financially supported in part by a grant from Philips Respironics and is involved in several patent applications resulting from research supported by Philips Respironics. Dr. Tononi has consulted for Sanofi-Aventis and Takeda, and he is currently the David P. White Chair in Sleep Medicine at the University of Wisconsin–Madison, endowed by Phillips Respironics. Dr. Tononi has also received unrelated research support from Phillips Respironics. Dr. Benca is a consultant for Merck and Jazz Pharmaceuticals and receives grant support from Merck. Dr. Peterson is a member of the medical advisory panel for Otsuka Pharmaceuticals. All other authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeschbach D, Cajochen C, Tobler I, Dijk DJ, Borbély AA. Sleep in a sitting position: effect of triazolam on sleep stages and EEG power spectra. Psychopharmacology (Berl) 1994a;114:209–214. doi: 10.1007/BF02244838. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbély AA. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology. 1994b;11:237–244. doi: 10.1038/sj.npp.1380110. [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8:e52805. doi: 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, Fried I. Sleep spindles in humans: insights from intracranial EEG and unit recordings. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:17821–17834. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Lüthi A. Manipulating sleep spindles - expanding views on sleep, memory, and disease. Trends Neurosci. 2013;36:738–748. doi: 10.1016/j.tins.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Azumi K, Shirakawa S. Characteristics of spindle activity and their use in evaluation of hypnotics. Sleep. 1982;5:95–105. doi: 10.1093/sleep/5.1.95. [DOI] [PubMed] [Google Scholar]
- Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, Colrain IM. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21:535–545. doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behavioural brain research. 2011;217:117–121. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Barthó P, Slézia A, Mátyás F, Faradzs-Zade L, Ulbert I, Harris KD, Acsády L. Ongoing Network State Controls the Length of Sleep Spindles via Inhibitory Activity. Neuron. 2014;82:1367–1379. doi: 10.1016/j.neuron.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4:189–194. [PubMed] [Google Scholar]
- Bódizs R, Körmendi J, Rigó P, Lázár AS. The individual adjustment method of sleep spindle analysis: methodological improvements and roots in the fingerprint paradigm. J Neurosci Methods. 2009;178:205–213. doi: 10.1016/j.jneumeth.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115:1178–1188. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Daan S, van den Hoofdakker RH. Effects of seganserin, a 5-HT2 antagonist, and temazepam on human sleep stages and EEG power spectra. Eur J Pharmacol. 1989;171:207–218. doi: 10.1016/0014-2999(89)90109-x. [DOI] [PubMed] [Google Scholar]
- Drapeau C, Hamel-Hébert I, Robillard R, Selmaoui B, Filipini D, Carrier J. Challenging sleep in aging: the effects of 200 mg of caffeine during the evening in young and middle-aged moderate caffeine consumers. J Sleep Res. 2006;15:133–141. doi: 10.1111/j.1365-2869.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- Feige B, Voderholzer U, Riemann D, Hohagen F, Berger M. Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol. 1999;110:1965–1974. doi: 10.1016/s1388-2457(99)00147-9. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Cornu M, Jäger E, Konrad B, Adamczyk M, Friess E, Steiger A, Czisch M, Goya-Maldonado R. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77:177–186. doi: 10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Genzel L, Kiefer T, Renner L, Wehrle R, Kluge M, Grözinger M, Steiger A, Dresler M. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology. 2012;37:987–998. doi: 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Goldstein MR, Plante DT, Hulse BK, Sarasso S, Landsness EC, Tononi G, Benca RM. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr Scand. 2012;125:468–477. doi: 10.1111/j.1600-0447.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Thornby JI, Karacan I. Sleep spindles: pharmacological effects in humans. Sleep. 1982;5:85–94. doi: 10.1093/sleep/5.1.85. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SFftAAoSM. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. American Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009;328:1000–1006. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Spinweber CL. Effect of a short-acting benzodiazepine on brain electrical activity during sleep. Electroencephalogr Clin Neurophysiol. 1981;52:89–97. doi: 10.1016/0013-4694(81)90193-0. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Spinweber CL, Seidel WF, Dement WC. Sleep spindle and delta changes during chronic use of a short-acting and a long-acting benzodiazepine hypnotic. Electroencephalogr Clin Neurophysiol. 1983;55:662–667. doi: 10.1016/0013-4694(83)90276-6. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Retey JV, Tonz K, Gottselig JM, Khatami R, Buckelmuller I, Achermann P. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1933–1939. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Staner L, Hoffmann G, Dramaix M, San Sebastian I, Murphy JR, Kentos M, Pelc I, Linkowski P. The first-night effect may last more than one night. J Psychiatr Res. 2001;35:165–172. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Altier H. Flunitrazepam (Ro 5–4200) and sleep cycle in normal subjects. Psychopharmacologia. 1973;32:343–349. doi: 10.1007/BF00429470. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Kehne JH, Sprenger KJ, Malison RT. Retrograde effects of triazolam and zolpidem on sleep-dependent motor learning in humans. J Sleep Res. 2010;19:157–164. doi: 10.1111/j.1365-2869.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Brink D, Astruc M, Mercer J, Remsberg S. How “blind” are double-blind placebo-controlled trials of benzodiazepine hypnotics? Sleep. 1995;18:240–245. doi: 10.1093/sleep/18.4.240. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human brain mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PloS one. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Derex L, Comte JC, Mauguière F, Salin PA. Density and frequency caudo-rostral gradients of sleep spindles recorded in the human cortex. Sleep. 2012;35:69–79. doi: 10.5665/sleep.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, Jones MW. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012;76:526–533. doi: 10.1016/j.neuron.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Plante DT, Goldstein MR, Landsness EC, Peterson MJ, Riedner BA, Ferrarelli F, Wanger T, Guokas JJ, Tononi G, Benca RM. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146:120–125. doi: 10.1016/j.jad.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, Goldstein MR, Riedner BA, Wanger T, Guokas JJ, Tononi G, Benca RM. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density EEG investigation. BMC psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, Maquet P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Neuronal Substrates of Sleep and Epilepsy. Cambridge University Press; Cambridge, United Kingdom: 2003. [Google Scholar]
- Suetsugi M, Mizuki Y, Ushijima I, Kobayashi T, Watanabe Y. The effects of diazepam on sleep spindles: a qualitative and quantitative analysis. Neuropsychobiology. 2001;43:49–53. doi: 10.1159/000054865. [DOI] [PubMed] [Google Scholar]
- Suh J, Foster DJ, Davoudi H, Wilson MA, Tonegawa S. Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia. Neuron. 2013;80:484–493. doi: 10.1016/j.neuron.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SS, Kushida CA. Multiple sleep latency test and maintenance of wakefulness test. Chest. 2008;134:854–861. doi: 10.1378/chest.08-0822. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Dijk DJ, Brunner DP, Klene C, Borbély AA. Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology. 1990;3:11–18. [PubMed] [Google Scholar]
- Ujma PP, Gombos F, Genzel L, Konrad BN, Simor P, Steiger A, Dresler M, Bodizs R. A comparison of two sleep spindle detection methods based on all night averages: adjusted vs. fixed frequencies. Frontiers in Human Neuroscience. 2015;9:1–11. doi: 10.3389/fnhum.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakami Y, Ioannides AA, Kostopoulos GK. Sleep Spindles - As a Biomarker of Brain Function and Plasticity. Advances in Clinical Neurophysiology. 2012:73–108. [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36:1369–1376. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warby SC, Wendt SL, Welinder P, Munk EG, Carrillo O, Sorensen HB, Jennum P, Peppard PE, Perona P, Mignot E. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014;11:385–392. doi: 10.1038/nmeth.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative analysis of the frequency distribution of detected spindles for TMZ and PLC nights using twelve midline derivations. A) Schematic of locations of derivations used. B) Distribution of detected spindles by frequency for selected frontal (blue) and central (red) channels. Distribution defined by 0.25Hz frequency bins 10–16Hz. Dashed lines correspond to PLC night; solid lines correspond to TMZ night. C) Plots illustrating the relative proportion of spindles across the frequency distribution of detected spindles for TMZ and PLC nights for all 12 midline channels, as an extension of B), and comparisons between the two. White dots indicate significant difference between conditions (uncorrected). Note the leftward shift of the distribution and increase in peak amplitudes for TMZ relative to PLC.
Topography of the waxing and waning oscillation of detected spindles for TMZ and PLC nights. Absolute latency derived as the time (seconds) from start of spindle envelope to maximal amplitude. Relative latency (percent) is the time from start of spindle envelope to maximal amplitude normalized to the duration of each detected spindle. Wax Slope (μV/sec) is the slope from the start of the spindle to the maximal amplitude; Wane slope (μV/sec) is the time from the maximal amplitude to the termination of the spindle. Duration of detected spindles is re-plotted for comparison purposes. Bottom row denotes t-values for channel-by-channel paired t-tests between TMZ and PLC. White dots denote significant channels after statistical non-parametric mapping with suprathreshold cluster test.