Abstract
Objective: By retarding fat digestion, thylakoids, the internal photosynthetic membrane system of green plants, promote the release of satiety hormones. This study examined the effect of consuming a single dose of concentrated extract of thylakoids from spinach on satiety, food intake, lipids, and glucose compared to a placebo.
Design: Sixty overweight and obese individuals enrolled in a double-blind randomized crossover study consumed the spinach extract or placebo in random order at least a week apart. Blood was drawn for assessments of lipids and glucose before a standard breakfast meal, followed 4 hours later by a 5 g dose of the extract and a standard lunch. Visual analog scales were administered before lunch and at intervals until an ad libitum pizza dinner served 4 hours later. Two hours after lunch a second blood draw was conducted. Mixed models were used to analyze response changes.
Results: Compared to placebo, consuming the spinach extract reduced hunger (p < 0.01) and longing for food over 2 hours (p < 0.01) and increased postprandial plasma glucose concentrations (p < 0.01). There were no differences in plasma lipids and energy intake at dinner, but males showed a trend toward decreased energy intake (p = 0.08).
Conclusions: At this dose, the spinach extract containing thylakoids increases satiety over a 2-hour period compared to a placebo. Thylakoid consumption may influence gender-specific food cravings.
Key words: thylakoids, spinach, satiety, food cravings, fat digestion
INTRODUCTION
Appetite reflects a complex interaction among the external environment, the behavioral profile, and subjective states as well as the storage and utilization of energy [1]. Thus, the initiation and termination of ingestive behavior has both metabolic and nonmetabolic components. An eating episode can be sparked by metabolic need, hedonic drive, or an interaction between the two. A neural network sensitive to energy status signals has been identified as the homeostatic control system for the regulation of food intake and energy balance [2]. The system is powerfully designed to protect the lower limits of adiposity by modulating the processing of cognitive and reward functions [2]. However, in the modern world, humans often eat in the absence of any metabolic feedback requiring replenishment of diminished reserves. This nonhomeostatic or hedonic eating involves cognitive, reward, and emotional aspects. Cues that have a reward associated with them, once learned, trigger motivational wanting to secure these rewards [3].
The photosynthetic membrane of chloroplasts consists of a system of paired membranes, the thylakoids. The thylakoid membrane system forms a physically continuous 3-dimensional network that encloses an aqueous space, which is the thylakoid lumen [4]. Approximately 70% of the thylakoid mass consists of the membrane proteins and their bound pigments, such as chlorophyll, carotenes, and xanthophylls. The remaining 30% largely consists of the membrane lipids such as galactolipids, phospholipids, and sulfolipids [5].
Thylakoid membranes are found in green plants such as spinach. A patented [6] extract of spinach containing significant amounts of thylakoids has been shown to have an inhibitory effect on lipase activity [7]. This inhibition is largely mediated by the protein fraction [7], but the membrane galactolipids may also have a role [8]. Delayed fat digestion increases the production of the satiety hormones cholecystokinin [9,10] and glucagon-like peptide-1 (GLP-1) [11], as has been demonstrated in human trials. Additionally, in humans [9] and pigs [12], ingestion of the extract has been shown to suppress the hunger hormone ghrelin. Among the gut hormones involved in appetite regulation, GLP-1 in particular has been associated with the regulation of reward-induced eating behavior [13]. Thylakoid-induced increase in the precursor for enterostatin a peptide involved in appetite suppression and thermogenesis has also been demonstrated [7].
In studies with rats and mice [14,15], significant reductions in body weight and percentage body fat occurred when the diet was supplemented with the spinach extract containing thylakoids. In overweight women, a breakfast meal supplemented with 3.7 or 7.4 g of the spinach extract suppressed subjective hunger compared to a control in a crossover study, with no statistical difference between the 2 doses [10]. Overweight women consuming 5 g of the spinach extract for 3 months demonstrated 43% greater loss of body weight compared to a placebo. The women also exhibited a decreased the urge for sweet and chocolate by 95% and 87%, respectively. The reduced urge for sweets was significant after a single dose and was sustained throughout the study, demonstrating that no tolerance developed during the 3 months of daily usage [11]. Further, unlike pharmaceutical lipase-inhibiting drugs, the thylakoids temporarily delay but do not prevent fat digestion. Thus, the excretion of undigested fat, which is an unpleasant side effect of lipase inhibitor drugs, is avoided.
In this study, subjective satiety ratings and food intake following a single administration of thylakoids from spinach leaves or a placebo were measured. The hedonic and reward responses to food-related stimuli were also evaluated. Plasma glucose and lipid concentrations were measured. It was hypothesized that thylakoid supplementation would produce an increase in satiety that would be accompanied by the appropriate changes in glucose and lipid measures.
SUBJECTS AND METHODS
Participants
Sixty overweight or obese males and females between the ages of 18 and 65 years were recruited from Baton Rouge and the surrounding areas to evaluate the effect of thylakoid supplementation on appetite, satiety, and food intake. Inclusion criteria specified that all subjects should have a body mass index between 25 and 35 kg/m2 and a waist circumference over 35 inches. Subjects were excluded if (1) they had existing medical conditions or were taking medications that could influence their appetite, food absorption, body weight, or mood and (2) were currently or during the previous 2 months on a low-calorie diet.
All subjects participated in an initial screening that involved measurement of body weight, height, waist circumference, and vital signs (blood pressure and pulse rate). At screening, health was assessed through the administration of a medical screening questionnaire and subjects underwent a medical examination to confirm their medical suitability for participation in the study. Sixty subjects passing the screening were randomized to 2 groups of 30 each. This study was approved by the Institutional Review Board of the Pennington Biomedical Research Center, Baton Rouge, where the study was conducted. Participants provided written informed consent. The trial was registered on ClinicaTrials.gov with registration number NCT01919814.
Design
The study followed a double-blind, placebo-controlled randomized crossover design. Each participant was tested on 2 days. On one occasion the test product consisted of a concentrated extract of thylakoids from spinach (Appethyl™, Green Leaf Medical, Stockholm, Sweden) and on the other occasion the test product consisted of the placebo served in random, balanced order. At the first test visit, subjects arrived at the center after a 12-hour overnight fast and having avoided strenuous exercise for 24 hours prior to arrival. Body fat was measured using bioelectrical impedance analysis (Tanita Corporation of America Inc., Arlington Heights, IL) and subjects were allowed to rest for a few minutes prior to having a blood sample drawn for assessment of serum triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), free fatty acids (FFA), high-sensitivity C-reactive protein (hs-CRP), and glucose. After eating a standardized 300 kcal breakfast meal, they were advised to remain in the dining area of the metabolic kitchen and refrain from any food or snack consumption until lunch. Before eating lunch, subjects were required to rate their satiety using electronic visual analog scales (VAS) [16,17]. They then consumed the spinach extract or placebo prior to being served a standardized 750 kcal lunch meal.
Breakfast and lunch were supervised to ensure that the entire meal was eaten. Visual analog scales assessing satiety were administered at 30, 60, 120, and 240 minutes after the start of the lunch meal. Two hours after lunch, a second blood draw was conducted to obtain postprandial measures of TG, total cholesterol, HDL-C, LDL-C, FFA, hsCRP, and glucose. Liking and wanting [18] were assessed 4 hours after lunch followed by an ad libitum dinner meal consisting of pizza. The pizza meals were preweighed and participants were presented with a meal that was in excess of what they could possibly eat. They were told to eat to satisfaction over 20 minutes, after which the remains of the meal were weighed. Subjects were required to remain at the center between lunch and dinner. The food intake at dinner was determined by subtracting the weight of the uneaten food from its original weight. The energy and macronutrient intakes were calculated using product information.
Study Products and Meals
Five grams of the spinach extract were well mixed with 2 fl. oz. of diet Blueberry Pomegranate Juice (Ocean Spray) and one teaspoon of corn oil. For the placebo, 2.5 g of cornstarch, 2.5 g of all-purpose flour, 5 g of glycerin, 1 teaspoon of corn oil, and food coloring were well mixed with 2 fl. oz. of diet Blueberry Pomegranate Juice. The test products were prepared and served immediately. A description of the standardized breakfast and lunch meals is provided in Table 1. Pepperoni pizza (Tombstone™ Original, Glendale, CA) was served at the ad libitum diner meal.
Table 1.
Energy and Macronutrient Content of Standard Lunch and Breakfast Meals
| Food | Energy (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) |
|---|---|---|---|---|
| Breakfast | ||||
| Whole wheat bread | 133.0 | 4.9 | 22.6 | 1.6 |
| Butter, salted | 22.0 | 0.0 | 0.0 | 2.4 |
| Cheese, cheddar | 52.0 | 3.2 | 0.2 | 4.3 |
| Extra-lean ham | 43.0 | 5.7 | 0.7 | 1.4 |
| Egg, scrambled | 50.0 | 3.3 | 0.7 | 3.7 |
| Water | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 300.0 | 17.1 | 24.2 | 13.4 |
| Lunch | ||||
| Oven-roasted turkey | 75.0 | 15.0 | 1.5 | 0.8 |
| Cheese, Swiss | 120.0 | 8.5 | 1.7 | 8.8 |
| Whole wheat bread | 165.0 | 6.0 | 28.0 | 2.0 |
| Lettuce | 4.0 | 0.3 | 0.8 | 0.1 |
| Tomatoes | 5.0 | 0.3 | 1.2 | 0.1 |
| Mayonnaise | 115.0 | 0.2 | 0.6 | 12.5 |
| Mustard, yellow | 7.0 | 0.4 | 0.5 | 0.4 |
| Fritolay Sunchips | 209.0 | 3.4 | 28.6 | 9.0 |
| Fruit salad | 50.0 | 0.0 | 15.0 | 0.0 |
| Water | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 750.0 | 34.1 | 77.9 | 33.7 |
Subjective Satiety, Liking, and Wanting Assessment
Hunger, fullness, desire to eat, prospective intake, satisfaction, thirst, and appetite for sweet, salty, and savory foods were assessed using VAS. Liking refers to the affective reaction reflecting the hedonic response to a food and is the result of a central process that integrates the sensory properties as well as the individual's physiological state and associative history. Using the method developed by Finlayson et al. [18], liking was measured through VAS ratings associated with food image stimuli varying across the dimensions of fat (high or low) and taste (sweet or savory). Thus, using 16 pictures, the foods could be arranged into combined categories where 4 were high-fat sweet, 4 were high-fat savory, 4 were low-fat sweet, and 4 were low-fat savory foods. These foods could also be arranged into generic groups with 8 foods in each of the categories high-fat, low-fat, sweet, and savory. The images were relatively similar in size and color. The order in which images were presented to subjects was randomized by time point using a random number generator. Each image was paired with the following questions administered using VAS: (1) How pleasant would it be to experience a mouthful of this food now? (2) How often do you eat this food? (3) How pleasant do you find this food? (4) How much do you want some of this food now?
Wanting refers to an underlying implicit and objective drive process that mediates an intent or desire to consume a food [18]. Called incentive salience, it reflects a motivating desire that the brain attributes to reward-predicting cues [19]. Wanting was measured by presenting an image of a food stimulus paired with another image of a different food stimulus and asking subjects to select the food they “most want to eat now.” The images used in the wanting procedure were the same images used for the liking procedure and each image in the set was uniquely paired with every possible image outside of its food category. For instance, a high-fat sweet food was paired with every low-fat sweet, high-fat savory, and low-fat savory food. Thus, using 16 images, 96 pairs of food images were presented and participants had to choose one of the 2 foods presented, imagining that they could eat as much or as little of that food as they wanted.
Statistical Analyses
To analyze the differences in the VAS ratings of satiety between the spinach extract and placebo conditions, a linear mixed model was used to estimate how the ratings for each of the questions changed over time. The covariates in the model were time and crossover order effect, and an unstructured covariance matrix was used to model the relation between time points for each subject. The responses defined as change from baseline (the measure just prior to lunch) included measures at 30, 60, 120, and 240 minutes following the lunch meal. t Tests based on the least squares means from the model were used to determine differences between the 2 conditions.
The hedonic impact of each food category was assessed by calculating the mean liking scores and the differences in scores between the test and the control conditions for each of the questions asked. The differences in wanting were assessed by calculating the mean frequency scores for food selection in each food category. Food intake was evaluated as the difference in mean scores between the spinach extract and placebo conditions using a linear model that adjusted for a crossover effect. A subanalysis based on gender was also performed. Metabolic parameters were assessed as change from baseline using a linear model adjusted for a crossover effect. t Tests were used to determine differences between the 2 conditions. All values are expressed as least squares means ± standard error. Statistical analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC).
RESULTS
Sixty participants, 30 males and 30 females, were enrolled in the study. One female participant had a severe headache, which the participant related to the temperature in the testing room. It was treated as a serious adverse event and the available data relating to this participant were not included in the analysis. Additionally, 2 other participants were dropped from the study due to one being diagnosed with diabetes and the other having a schedule conflict. Descriptive characteristics of participants included in the analyses are presented in Table 2.
Table 2.
Characteristics at Baseline of 59 Participants Who Were Included in the Mixed Model Analysis
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 35.3 | 12.4 | 18–64 |
| Height (cm) | 171.4 | 9.6 | 153.2–194.5 |
| Weight (kg) | 88.0 | 10.9 | 67.3–114.0 |
| BMI (kg/m2) | 29.9 | 2.6 | 25.2–34.5 |
| Percentage body fat | 35.1 | 8.3 | 20.4–47.8 |
| Waist circumference (cm) | 97.8 | 6.4 | 88.9–114.3 |
BMI = body mass index.
Visual Analog Scales
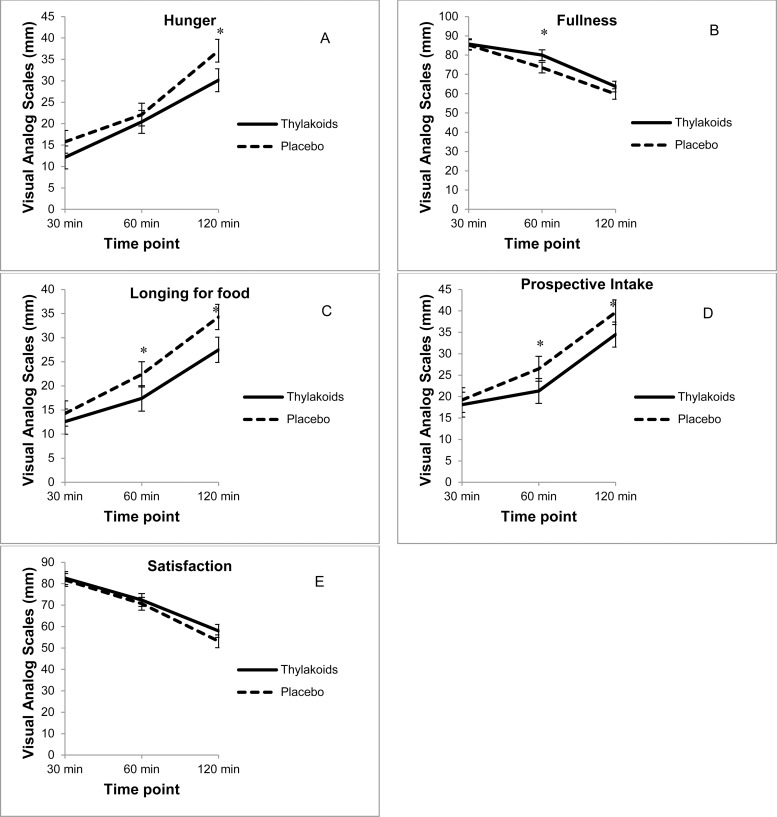
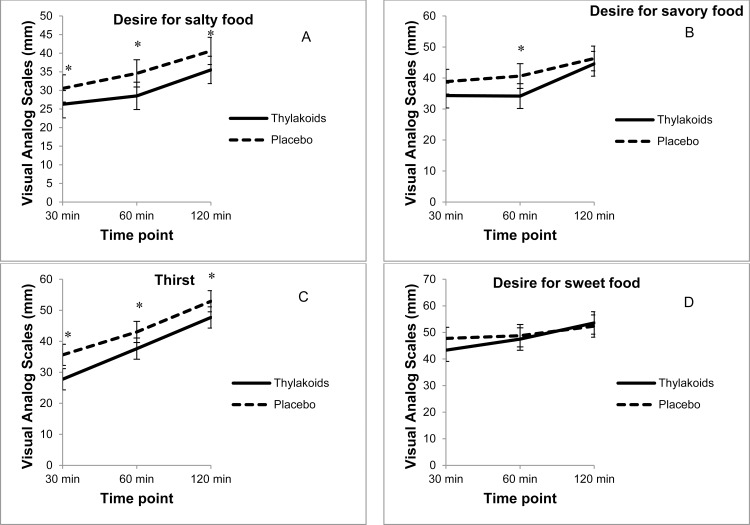
Analysis of individual satiety ratings revealed that compared to the placebo, consumption of the spinach extract significantly increased fullness (p = 0.04) and reduced hunger (p < 0.01), longing for food (p < 0.01), and prospective intake (p = 0.01) over the 2-hour period following lunch (Figs. 1A–1D), whereas satisfaction was not significantly different (Fig. 1E). In addition, consumption of the spinach extract reduced the desire for something salty (p < 0.01), desire for something savory (p < 0.01), and thirst (p < 0.01) over the 2-hour period following lunch, compared to the placebo (Figs. 2A–2C); however, the desire for something sweet was not significantly different (Fig. 2D). At 4 hours, thirst was still suppressed after consuming the spinach extract (p = 0.02), whereas there were no significant differences between the 2 conditions for the other ratings.
Fig. 1.
Visual analog scale ratings for satiety (n = 59) over 2 hours after consuming the spinach extract or a placebo. (A) Hunger: overall differences, 4.0720 mm ± 1.52 (p < 0.01), *120 minutes (p < 0.01). (B) Fullness: overall differences, 3.62 mm (p = 0.04), *60 minutes (p = 0.03). (C) Longing for food: overall differences, 4.50 mm ± 1.32 (p < 0.01), *60 minutes (p < 0.03) and *120 minutes (p < 0.01). (D) Prospective intake: overall differences, 3.83 mm ± 1.35 (p < 0.01), *60 minutes (p = 0.03) and *120 minutes (p = 0.03). (E) Satisfaction: overall differences were not significant. Values are mean ± standard error.
Fig. 2.
Visual analog scale ratings for satiety (n = 59) over 2 hours after consuming the spinach extract or a placebo. Values are mean ± standard error. (A) Desire for salty food: overall differences, 5.14 mm ± 1.09 (p < 0.01), *30 minutes (p = 0.02), *60 minutes (p < 0.01), and *120 minutes (p < 0.01). (B) Desire for savory food: overall differences, 4.22 mm ± 1.4 (p < 0.01) *60 minutes (p < 0.01). (C) Desire for sweet food: overall differences were not significant. (D) Thirst: overall differences, 6.16 mm ± 1.46 (p < 0.01) *30 minutes (p < 0.01), *60 minutes (p = 0.03), and *120 minutes (p = 0.04).
Intake at Dinner Meal
There were no significant differences in food intake at the dinner meal served 4 hours after lunch, between the 2 conditions. The total weight of food consumed and energy intake were also not significantly different. Males exhibited a trend toward decreased consumption, reducing their energy intake by 49.3 g (125.7 kcal, p = 0.08), whereas females increased their intake by 3.2 g (8.1 kcal, p = 0.85).
Liking and Wanting
There were no significant differences in the mean scores for all the questions asked in each food category in the test to assess differences in liking between the spinach extract and placebo conditions. No significant differences were found in the mean scores for frequency of choosing foods in each food category in the test to assess the differences in wanting between the 2 conditions.
Metabolic Parameters
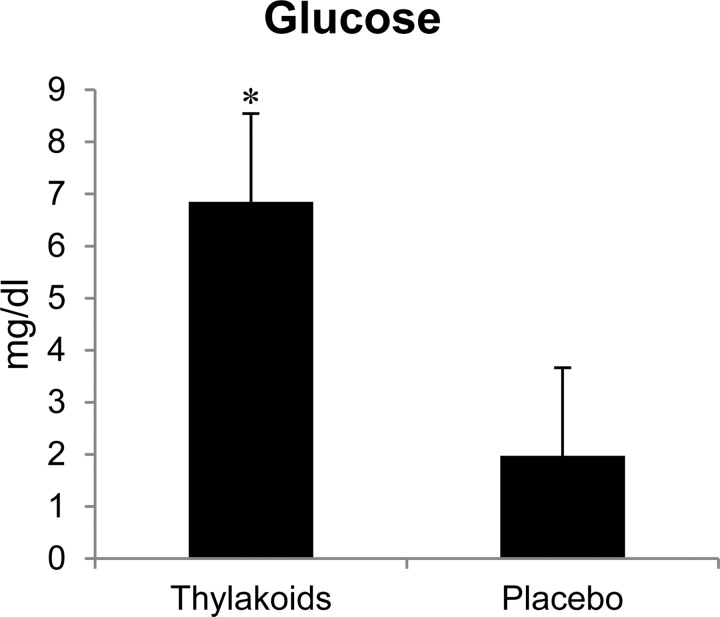
There were no differences in the serum concentrations of TG, total cholesterol, HDL-C, LDL-C, FFA, and hs-CRP. However, the difference in fasting and post-lunch serum glucose concentrations was greater (p < 0.01) in the spinach extract condition compared to the placebo (Fig. 3).
Fig. 3.
Difference in fasting and 2-hour post-lunch plasma glucose concentrations between the spinach extract and placebo conditions. *Significantly different at p < 0.01. Values are mean ± standard error.
DISCUSSION
Compared to a placebo, a single supplementation with 5 g of thylakoids increased satiety measured subjectively over 2 hours. This was accompanied by a greater increase in the postprandial plasma glucose response. Satisfaction, which introduces a hedonic component into the measurement of satiety [20], was included to determine whether satiety measures were judged from a comparable baseline during the repeated testing. As expected, it was not significantly different between the conditions. However, over a 4-hour period the differences in satiety measures were no longer significant. Differences in energy intake at the ad libitum meal and in the liking and wanting components of food reward measured at 4 hours after consuming thylakoids were also not significant between the 2 conditions.
The spinach extract (Appethyl™) contained concentrated thylakoids extracted from the choloroplasts of spinach leaves. By interacting with lipids and retarding fat digestion, thylakoid membranes promote the release of satiety hormones such as cholecystokinin and reduce the hunger stimulating hormone ghrelin [9]. In vitro, thylakoid membrane proteins bind to lipid droplets at the oil–water interface as well as to the lipase–colipase complex [7]. Thus, inhibition of lipolysis could occur through blocking the access of the lipase–colipase complex to the fat droplet or blocking of the active site of the lipase–colipase complex and preventing the enzyme complex from coming in contact with the fat droplet [7]. It has also been suggested that the thylakoid membrane lipid digalactosyldiacylglycerol, with its large polar head groups, may shield colipase and sterically hinder the formation of the lipase–colipase complex necessary for lipolysis [8].
Thylakoid membranes effectively suppressed lipolytic activity in a dose-dependent manner during in vitro hydrolysis of an emulsion of TG dispersed in bile salts [7]. Suppression of lipase–colipase activity stimulates a compensatory increase in endogenous secretion and production of lipase and colipase. This compensatory release has been suggested as a mechanism for increasing the appetite suppressing peptide enterostatin in response to thylakoid-supplemented high-fat meals [7].
In the present study, there was no difference in the serum lipid concentrations between the thylakoid and placebo conditions at 2 hours. Following a fat-containing meal, TG reach maximal concentrations 3–4 hours after consuming a single meal and return to baseline within 6–8 hours [21–24]. Following sequential meals, the plasma appearance of TG peaks at 60 minutes and thereafter falls up to 240 minutes [23]. Free or nonesterified fatty acid concentrations fall in response to a single meal and rise thereafter, peaking between 4 and 5 hours postprandially [21–24]. Following 2 sequential meals, FFA increase within 30 to 60 minutes and thereafter fall [23]. Based on the kinetics of fat absorption, it is difficult to determine whether there was a delay in lipid absorption without repeated testing before and after the 2-hour period.
The prolonged presence of nutrients in the gastrointestinal tract as well as cholecystokinin release prompts the release of GLP-1 from the distal regions of the intestine [11]. Supplementation with 5 g of the spinach extract significantly increased the difference between the pre- and postprandial GLP-1 levels after a single instance as well as following 12 weeks of supplementation compared to a placebo-treated group [11]. GLP-1 acts on the reward system [13]; hence, it may provide an explanation for the decrease among males in energy intake at the dinner meal served 4 hours after consuming the spinach extract. Males exhibit a yearning for savory foods [25–27], whereas females prefer high-fat sweet foods such as chocolate [25,27,28]. Although not significant, which may perhaps be because the study was not powered to detect differences in food intake by gender, males reduced their intake of savory food (pizza) by 126 kcal after consuming the spinach extract.
It has been demonstrated that thylakoids consumption reduces the urge for chocolate among women [11]. However, in the present study, the option of sweet foods was not presented when food intake was evaluated at the ad libitum dinner meal. This resulted in an overall nonsignificant change in energy intake between the spinach extract and control conditions. The absence of a sweet food at the dinner meal may also explain why desire to eat something savory decreased with supplementation of the spinach extract, but desire to eat something sweet was not different between the conditions. Nevertheless, it is estimated that weight gain in 90% of the adult population in the United States is due to a positive energy balance of 100 kcal/day or less [29]. Thus, a reduction in energy intake of 126 kcal may have clinical relevance.
The increase in plasma glucose concentrations at the 2-hour time point may be explained by a decrease in insulin secretion. In humans, a single thylakoid-enriched high-fat meal increased the satiety hormones cholecystokinin and leptin while reducing levels of insulin and the hunger-stimulating hormone ghrelin [9]. A reduction in insulin secretion by supplementation with thylakoids has also been demonstrated in a porcine study [12]. In a previous study [10], a high-carbohydrate meal (71% of energy) supplemented with the spinach extract resulted in a tendency toward higher plasma glucose from 90 minutes following the meal compared to a control meal. The greatest difference occurred at 120 minutes, although over 4 hours the differences were not significant. In that study, the trend toward increased blood glucose was accompanied by suppressed hunger from 180 minutes.
In the present study, significantly higher plasma glucose concentrations coincided with greater satiety. Increasing extracellular glucose concentration inhibits the orexigenic agouti-related peptide/neuropeptide Y-expressing neurons and stimulates the anorexigenic proopiomelanocortin and cocaine-and amphetamine-related transcript expressing neurons [30]. Thus, the greater increase in plasma glucose concentration 2 hours post-lunch may in part explain the increase in satiety following supplementation with the spinach extract compared to the placebo. Longer term (90 days, 5 g/day) consumption of thylakoids decreased body weight but there were no differences in blood glucose or insulin concentrations between control and treated groups [11]. Thus, the decrease in insulin secretion and rise in 2-hour postprandial glucose concentrations do not seem to adversely affect glycemic or insulinemic control over time. Moreover, thylakoids administered with a low-carbohydrate breakfast had no effect on serum glucose concentration at 2 hours [9]. Therefore, the effect of thylakoids on glucose homeostasis may depend on the carbohydrate content of the meal. The glycemic and insulinemic effect may also dissipate before 90 days; however, more studies are needed to establish these relationships.
The main limitation of this study is that metabolic parameters were assessed at a single time point. Frequent and more prolonged testing would have provided a better determination of the physiological mechanisms influencing satiety. Moreover, insulin and gut hormones were not measured. Further, males and females differ in their food cravings and the ad libitum meal did not cater to both gender preferences. A larger sample of males and females than the study provided would have helped to clarify potential mechanisms relating to the effects of thylakoids on the reward system.
CONCLUSIONS
Consistent with previous research, a single meal supplemented with 5 g of a concentrated extract of thylakoids from spinach increases satiety over the 2-hour period following consumption. Thylakoids supplementation may influence food cravings by acting on the reward system, thereby offering a novel way to address a positive energy balance in a manner that is minimally burdensome on the consumer. Gender-based studies of the effect of thylakoids consumption on appetite regulation, employing appropriate sample sizes, are needed.
ACKNOWLEDGMENT
We would like to thank Jim Roufs for his valuable contribution.
FUNDING
This study was funded by Greenleaf Medical, Stockholm, Sweden. This study is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center.
REFERENCES
- Blundell JE, Lawton CL, Cotton JR, Macdiarmid JI. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr. 1996;16:285–319. doi: 10.1146/annurev.nu.16.070196.001441. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: “liking,” “wanting,” and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouril R, Dekker JP, Boekema EJ. Supramolecular organization of photosystem II in green plants. Biochim Biophys Acta. 2012;1817:2–12. doi: 10.1016/j.bbabio.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Rayner M, Ljusberg H, Emek SC, Sellman E, Erlanson-Albertsson C, Albertsson PA. Chloroplast thylakoid membrane-stabilised emulsions. J Sci Food Agric. 2011;91:315–321. doi: 10.1002/jsfa.4187. [DOI] [PubMed] [Google Scholar]
- Albertsson, PA, Albertsson-Erlanson CA: Use of plant cell membrane for the treatment of obesity. US patent 8,642,098 B2. 2014 http://www.uspto.gov/web/patents/patog/week05/OG/html/1399-1/US08642098-20140204.html September 30. [Google Scholar]
- Albertsson PA, Kohnke R, Emek SC, Mei J, Rehfeld JF, Akerlund HE, Erlanson-Albertsson C. Chloroplast membranes retard fat digestion and induce satiety: effect of biological membranes on pancreatic lipase/co-lipase. Biochem J. 2007;401:727–733. doi: 10.1042/BJ20061463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu BS, Rich GT, Ridout MJ, Faulks RM, Wickham MS, Wilde PJ. Modulating pancreatic lipase activity with galactolipids: effects of emulsion interfacial composition. Langmuir. 2009;25:9352–9360. doi: 10.1021/la9008174. [DOI] [PubMed] [Google Scholar]
- Kohnke R, Lindbo A, Larsson T, Lindqvist A, Rayner M, Emek SC, Albertsson PA, Rehfeld JF, Landin-Olsson M, Erlanson-Albertsson C. Thylakoids promote release of the satiety hormone cholecystokinin while reducing insulin in healthy humans. Scand J Gastroenterol. 2009;44:712–719. doi: 10.1080/00365520902803499. [DOI] [PubMed] [Google Scholar]
- Stenblom EL, Montelius C, Ostbring K, Hakansson M, Nilsson S, Rehfeld JF, Erlanson-Albertsson C. Supplementation by thylakoids to a high carbohydrate meal decreases feelings of hunger, elevates CCK levels and prevents postprandial hypoglycaemia in overweight women. Appetite. 2013;68:118–123. doi: 10.1016/j.appet.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Montelius C, Erlandsson D, Vitija E, Stenblom EL, Egecioglu E, Erlanson-Albertsson C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite. 2014;81:295–304. doi: 10.1016/j.appet.2014.06.101. [DOI] [PubMed] [Google Scholar]
- Montelius C, Szwiec K, Kardas M, Lozinska L, Erlanson-Albertsson C, Pierzynowski S, Rehfeld JF, Westrom B. Dietary thylakoids suppress blood glucose and modulate appetite-regulating hormones in pigs exposed to oral glucose tolerance test. doi: 10.1016/j.clnu.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emek SC, Szilagyi A, Akerlund HE, Albertsson PA, Kohnke R, Holm A, Erlanson-Albertsson C. A large scale method for preparation of plant thylakoids for use in body weight regulation. Prep Biochem Biotechnol. 2010;40:13–27. doi: 10.1080/10826060903413057. [DOI] [PubMed] [Google Scholar]
- Kohnke R, Lindqvist A, Goransson N, Emek SC, Albertsson PA, Rehfeld JF, Hultgardh-Nilsson A, Erlanson-Albertsson C. Thylakoids suppress appetite by increasing cholecystokinin resulting in lower food intake and body weight in high-fat fed mice. Phytother Res. 2009;23:1778–1783. doi: 10.1002/ptr.2855. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE. Is it possible to dissociate “liking” and “wanting” for foods in humans? A novel experimental procedure. Physiol Behav. 2007;90:36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Weltens N, Zhao D, Van Oudenhove L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol Motil. 2014;26:303–315. doi: 10.1111/nmo.12309. [DOI] [PubMed] [Google Scholar]
- Cardello AV, Schutz HG, Lesher LL, Merrill E. Development and testing of a labeled magnitude scale of perceived satiety. Appetite. 2005;44:1–13. doi: 10.1016/j.appet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kagan ML, West AL, Zante C, Calder PC. Acute appearance of fatty acids in human plasma—a comparative study between polar-lipid rich oil from the microalgae Nannochloropsis oculata and krill oil in healthy young males. Lipids Health Dis. 2013;12:102. doi: 10.1186/1476-511X-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J Lipid Res. 2003;44:2065–2072. doi: 10.1194/jlr.M300167-JLR200. [DOI] [PubMed] [Google Scholar]
- Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am J Physiol Endocrinol Metab. 2007;292:E732–E739. doi: 10.1152/ajpendo.00409.2006. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Powell J, Calder PC. Lack of effect of meal fatty acid composition on postprandial lipid, glucose and insulin responses in men and women aged 50–65 years consuming their habitual diets. Br J Nutr. 2006;96:489–500. [PubMed] [Google Scholar]
- Pelchat ML. Food cravings in young and elderly adults. Appetite. 1997;28:103–113. doi: 10.1006/appe.1996.0063. [DOI] [PubMed] [Google Scholar]
- Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991;17:167–175. doi: 10.1016/0195-6663(91)90019-o. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Garriga-Trillo A, Rohm E, Centeno S, Parker S. Food liking and craving: a cross-cultural approach. Appetite. 1999;33:61–70. doi: 10.1006/appe.1999.0234. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Metabolic determinants of binge eating. Addict Behav. 1995;20:733–745. doi: 10.1016/0306-4603(95)00105-0. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Jordan SD, Konner AC, Bruning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci. 2010;67:3255–3273. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]