Abstract
Background
A recombinant Mycobacterium bovis BCG (rBCG) vector expressing HIV transgenes is an attractive candidate as a dual vaccine against HIV and TB. However, pre-existing immune responses to mycobacteria may influence immune responses to rBCG. We analyzed data from a rhesus rBCG trial to determine the effect of pre-existing mycobacterial immune responses on the vaccine-induced responses to the vector and expressed transgene.
Methods
Indian-origin rhesus macaques were primed with rBCG expressing simian immunodeficiency virus (SIV) Gag and boosted with attenuated vaccinia NYVAC gag-pol. Mycobacteria responses were measured by M. tuberculosis (Mtb) purified protein derivative (PPD) interferon-γ ELISpot and Mtb whole cell lysate (WCL) ELISA. SIV Gag responses were measured by SIV Gag ELISpot and by p11C tetramer binding.
Results
Baseline Mtb PPD ELISpot responses and Mtb WCL antibody responses in rhesus macaques overlapped those in human populations. Cellular and antibody responses boosted sharply 4 weeks after rBCG vaccination. Mtb WCL antibody titers at 4 weeks correlated with baseline titers. Primates vaccinated with rBCG developed strong SIV Gag ELISpot and p11C tetramer responses after rBCG prime and NYVAC boost. There were no correlations between the pre-existing mycobacterial immune responses and the SIV Gag T cell responses after vaccination.
Conclusions
Rhesus immune responses to SIV Gag expressed by rBCG vectors were independent from pre-existing anti-mycobacterial immunity. Rhesus macaques may serve as a surrogate for investigations of pre-existing anti-mycobacterial immunity in humans.
Keywords: Mycobacterium bovis BCG, HIV/SIV, pre-existing immunity, rhesus macaque
INTRODUCTION
Tuberculosis (TB) and HIV infections are major threats to global public health. Mycobacterium tuberculosis (Mtb) latently infects approximately one third of the global population [1]. Of the 5-20% of individuals who develop active TB disease more than 10% are co-infected with HIV. The majority of TB and HIV co-infected population live in sub-Saharan Africa [1]. BCG is a live-attenuated bacterial vaccine derived from M. bovis that has been used worldwide in several billion people with a well-established distribution network and is currently the only licensed TB vaccine. It is an affordable vaccine with an acceptable safety track record over many decades. BCG's potent adjuvant effects are well-known. BCG, which can be given during infancy, is effective at protecting children from the disseminated forms of TB including TB meningitis and miliary TB [2, 3].
These characteristics make BCG an attractive vaccine vector for the expression of heterologous immunogens with the goal of a combined vaccine with efficacy against both TB and other pathogens [4, 5]. Several research groups have developed vaccine vectors expressing HIV or SIV proteins in recombinant forms of either BCG or attenuated M. tuberculosis [6-14]. However, prior anti-vector immunity has the potential to impact the effectiveness of rBCG vaccine vectors since BCG shares antigens with other mycobacterial species. Adult humans have immune responses to BCG from various sources including Mtb infection, infant BCG vaccination, and exposure to environmental mycobacteria [15]. Multiple lines of evidence suggest that pre-existing anti-mycobacterial immunity reduces the efficacy of BCG against TB, either by interfering with the immune responses generated by BCG or by providing a baseline level of response that is not measurably enhanced by BCG [3, 16, 17]. Pre-existing immunity against inactivated bacterial vectors and viral vectors has also been shown to decrease vaccine efficacy [18-24].
We recently described the effectiveness of rBCG vector-based vaccines as priming immunogens for immune responses to SIV Gag in rhesus macaques [12]. Macaques were vaccinated with rBCG vectors expressing SIV Gag, followed by boosting with NYVAC SIVmac142 gag-pol. rBCG priming led to strong cellular immune responses to SIV Gag. This study provided the opportunity to assess the impact of pre-existing anti-mycobacterial immunity on the immunogenicity of the rBCG vectors. We measured cellular and humoral mycobacterial immune responses at baseline in these macaques, and compared them to the responses in humans from regions with low prevalence (North Carolina, U.S.) or high prevalence (Malawi) of Mtb infection. Analysis of correlations between baseline anti-mycobacterial immunity and rBCG-induced responses to SIV Gag in rhesus monkeys enabled us to investigate whether immune responses to SIV Gag expressed by rBCG vectors were impacted by prior mycobacterial immune responses.
MATERIALS AND METHODS
Animals and immunizations
All animals were maintained in accordance with the Guide for the care and use of laboratory animals [25] and institutional guidelines. The Mamu-A*01+ Indian origin rhesus macaques used were at least two years old and housed at the New England Primate Research Center with animal use protocols approved by the Harvard Medical Area Standing Committee on Animals. They were not derived from specific-pathogen-free breeding colonies. All procedures were conducted under sedation with intramuscular ketamine.
The monkeys were part of an immunogenicity study that aimed to assess the ability of novel rBCG vectors with various SIVmac251gag-expression cassettes as priming vectors to enhance SIV-specific CD8+ cellular responses upon boost immunization, as we recently described [12]; the previous study did not include an evaluation of vector-specific immunity. Prior to study start, the macaques showed a negative TB skin test against purified protein derivative (PPD).
Thirty rhesus macaques were used for the study: Five monkeys per group were immunized with a single intravenous dose of either wild type BCG Danish (WT BCG) strain or one of four variants of rBCG Danish expressing SIV Gag. A temporary peripheral venous catheter was placed, an injection volume of 2 mL was used for the BCG, and the catheter was then flushed with 2 mL buffer. The injection buffer was Dulbecco's phosphate buffered saline (PBS) with 0.05% Tween 20 (Sigma P2287). A positive control group of rhesus monkeys received three injections of 5 mg each of rDNA-SIVgag-pol-nef (Althea Technologies, lot# CP060307B) by the intramuscular route at intervals of four weeks, usingDulbecco's PBS as injection buffer. The total volume of 1.5 mL was split into two injection sites, and administered using a one-inch 23-gauge needle into the left and right shaved thigh.
The four rBCG SIVgag variants (BCG-pSL10, AF25-pSL10, J13-pSL10, J13-pSL7) were generated on a BCG Danish backbone. Each expressed a codon-optimized transgene SIVmac239 gag on a multicopy episomal pMV261 plasmid [12], with a 60-kDa heat shock protein promoter, a human influenza hemagglutinin tag for detection, and a hygromycin resistance marker. Whereas the pSL10-SIVgag expression cassette used an antigen 85 secretion signal, the pSL7-SIVgag employed a 19kDa signal sequence. While BCG-pSL10 was constructed on the unmutated BCG backbone, the AF25 and J13 variants had deletions of operon BCG_2587-2590 (uncharacterized function) or cmaA2 gene (required for mycolic acid cyclopropanation), respectively, as recently described [12].
For NHP experiments, inocula of WT BCG Danish or rBCG-SIVgag variant strains were taken out of fresh culture grown rocking at 37°C in Middlebrook 7H9 broth (Difco, Becton Dickinson) supplemented with 10% albumin-dextrose-saline (ADS: 8.1g NaCl, 50g BSA, 20g D-dextrose per liter water), 0.5% glycerol (Sigma-Aldrich), and either 0.05% Tween 80 (Fisher) or 0.05% tyloxapol nonionic surfactant (Sigma-Aldrich) to an optical density at 600nm (OD600) around 1 corresponding to approximately 1.5×108 cfu/ml, pelleted by centrifugation, and resuspended in phosphate-buffered saline (PBS) with 0.05% Tween 20 (Sigma-Aldrich) for injection.
All BCG strains were injected by intravenous route at 3×108 cfu. In a previous NHP study, an earlier generation rBCG Pasteur vector expressing SIVgag-pol and SIVenv did not elicit detectable SIV-specific immune responses after the priming rBCG immunizations (at 1×106–1×109 cfu by intravenous, intradermal, or intramuscular route), but only after a recombinant adenovirus 5 (Ad5) boost immunization [14]. The intravenous route and high dose was chosen in the present study to attempt to induce measurable SIV Gag cellular responses after priming rBCG immunization.
All macaques were boosted with 1×107 pfu NYVAC SIVmac142 gag-pol (provided by Sanofi Pasteur, lot #17Dec2010) by intramuscular route ten months after prime immunization. The injection buffer was Dulbecco's PBS. The total volume of 1.5 mL, split into two injection sites, was administered into the left and right shaved thigh using a one-inch 23-gauge needle.
Human specimens
Human plasma and matching peripheral blood mononuclear cell (PBMC) samples were provided by the Center for HIV-AIDS Vaccine Immunology (CHAVI) Repository of Duke University. De-identified specimens were obtained from HIV-negative volunteers in North Carolina or HIV-negative patients in a sexually-transmitted-disease (STD) clinic in Malawi. Informed written consent was obtained from participants, and the study was approved by the Duke University Medical Center Institutional Review Board.
Humoral immunity assay
Plasma antibody titers against Mtb whole cell lysate (WCL) (Colorado State University, CO) were assessed by standard enzyme-linked immunosorbent assay (ELISA). M. tuberculosis H37Rv whole cell lysate was obtained from Colorado State University through BEI Resources. The antigenic preparation was diluted to 2 μg/mL in CBC Buffer (7.5 mM sodium carbonate, 17.4 mM sodium bicarbonate, pH 9.0), plated in 96-well high binding plates (Costar) at a concentration of 200ng/well, and incubated overnight at 4°C. Plates were washed with PBS, pH 7.4 containing 0.5% Tween-20 (PBST), prior to blocking with 5% dry milk in CBC buffer for one hour at room temperature. After an additional PBST wash, plates were incubated with serial dilutions of immune sera in 5% dry milk in PBST overnight at 4°C. A 1:128 dilution of serum from humans with Mtb disease or BCG-vaccinated primates was used as a positive control, and 5% dry milk in PBST was used as a negative control. Plates were then washed in PBST and incubated for one hour at room temperature in 5% dry milk in PBST with a 1:3,000 dilution of alkaline phosphatase coupled goat-anti-human or goat-anti-monkey secondary antibody. After a final wash, plates were incubated in 1 mg/ml para-nitrophenyl phosphate (PNP) solution in CBC buffer containing 1.1 mM magnesium chloride and read when the positive control wells reached an OD of 2.0, as measured by 405 nm spectrophotometry. ELISA titers were defined as the reciprocal dilution equal to 5% of the positive control after subtraction of the negative control.
Cellular immunity assays
Enzyme-linked immunosorbent spot (ELISpot) assays were used to determine interferon-gamma (IFN-γ) production of PBMC, as previously described [14]. Briefly, 2×105 PBMC/well were plated on Multiscreen Immobilon-P microtiter plates (96-well; Millipore, Bedford, MA) and stimulated with pooled overlapping 15-mer peptides spanning SIVmac239 Gag (obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH; 1 μg/ml final concentration), mammalian PPD (human isolates, Tuberculin-OT, Synbiotics; 12.5 μg/ml final concentration), or the mitogen phytohemagglutinin M (PHAM, Sigma; 5 μg/ml final concentration) as positive control; addition of medium (i.e. unstimulated) served as negative control. Developed plates were read on an ImmunoSpot analyzer using CTL ImmunoSpot image-processing software (Cellular Technology, Cleveland, OH). The numbers of IFN-γ spot forming cells (SFC)/106 PBMC were calculated from the median SFC counts per triplicate with the background of the negative control subtracted. Median SFC values exceeding twice the background were considered positive. PPD ELISpot data from humans was generated using frozen PBMCs, and this data was compared to data derived from frozen rhesus PBMCs. All other analyses were carried out using fresh rhesus PBMCs.
The frequency of CD8+ T lymphocytes specific for the SIVmac251 Gag peptide p11C, an immunodominant Mamu-A*01-restricted cytotoxic T lymphocyte epitope, was quantified by staining fresh peripheral blood samples with the tetrameric Mamu-A*01/p11C, C-M (CTPYDINQM) complex [26] conjugated to phycoerythrin-labeled streptavidin, allophycocyanin-labeled anti-rhesus CD3 (SP34; custom conjugate, Becton Dickinson) and fluorescein isothiocyanate-labeled anti-human CD8α (SK1; BD Biosciences, San Jose, CA), as previously described [14]. Flow cytometric analysis was performed on a FACSCalibur utilizing CellQuest software (Becton Dickinson). Assessment of p11C-specific CD8+ tetramer binding was performed on lymphocytes gated on the CD3+CD8+ lymphocyte population using FlowJo Software (TreeStar, Ashland, OR).
Statistical analysis
Prism Software (GraphPad, San Diego, CA) was used for statistical analyses. Correlation between datasets was computed using Pearson correlation with two-tailed P values. Linear regression lines were plotted by least squares analysis. Pairwise comparisons were carried out using the nonparametric Mann-Whitney test. Comparisons among multiple groups were performed using the nonparametric Kruskal-Wallis test with Dunn's correction for multiple comparisons. P values < 0.05 were considered significant.
RESULTS
Pre-existing immune responses in rhesus macaques and humans
Plasma and PBMCs were collected from rhesus macaques (n=30) prior to immunization. Baseline cellular and humoral responses to mycobacteria were determined by ELISpot and ELISA assays, respectively, and compared to two distinct human populations: healthy HIV-negative, PPD skin-test-negative volunteers from North Carolina, U.S. (n=15) and HIV-negative patients recruited from a Malawian STD-clinic (n=19). BCG vaccine is routinely provided at birth in Malawi, and PPD skin-test reactivity was not tested in this group.
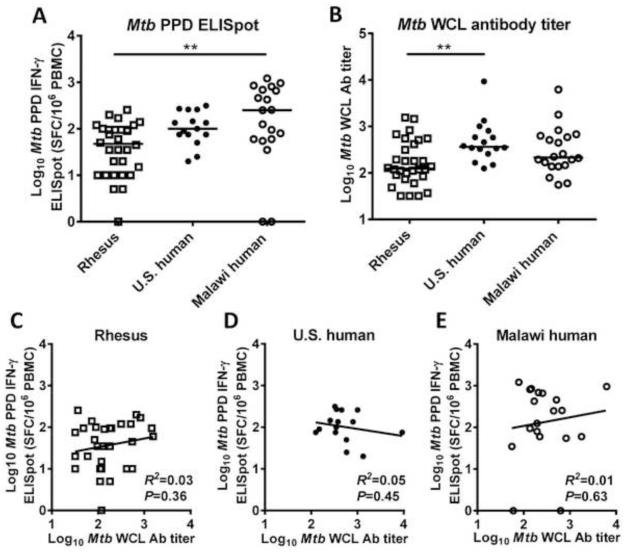
Cellular immune responses to mycobacteria were measured by Mtb PPD IFN-γ ELISpot assay on frozen PBMCs (Fig. 1 A). PPD-specific ELISpot responses differed significantly among the groups; baseline PPD-specific IFN-γ ELISpot responses in the rhesus macaques were lower than those in Malawi humans and similar to those in U.S. humans (Kruskal-Wallis test: P=0.003; Dunn's multiple comparisons test: P=0.005 or P>0.05 respectively). Baseline cellular immune responses in the rhesus macaques showed substantial overlap with both human populations.
Fig. 1. Baseline M. tuberculosis (Mtb) cellular and humoral immune responses in rhesus macaques and humans.
(A) Cellular responses to Mtb purified protein derivative (PPD) in PBMCs from PPD-negative rhesus macaques (open squares, n=30), PPD-negative humans residing in the U.S. (closed circles, n=16), and humans residing in Malawi (open circles, n=20) expressed as log10 IFNγ-ELISpot spot-forming cells/million PBMCs. (B) Humoral responses to Mtb whole cell lysate (WCL) from the same groups based on log10 total Ig ELISA titer. (C-E) Mtb cellular responses plotted against Mtb humoral response. No significant correlation was identified in any of the three groups between cellular and humoral immune responses to Mtb. Bars in panels A and B represent medians. Zero values for ELISpot are plotted on the axis. Comparisons in panels A and B based on Kruskal-Wallis test with Dunn's correction for multiple comparisons. **P<0.01.
Humoral responses to mycobacteria at baseline were measured in rhesus macaques and humans by ELISA to Mtb WCL (Fig. 1 B). Mtb WCL antibody differed significantly among the groups; baseline antibody titers in rhesus macaques were lower than those in U.S. humans and similar to those in Malawi humans (Kruskal-Wallis test: P=0.007; Dunn's multiple comparisons test: P=0.007 or P>0.05 respectively). Although specific positive control sera and species-specific secondary antibodies were used, titers were defined by the same criteria in both species. Baseline antibody titers in the rhesus macaques overlapped with those in both human populations.
Rhesus macaques demonstrated substantial variability in their cellular and humoral immune responses to mycobacteria prior to vaccination. This baseline variability was important for the goals of this analysis as it means that baseline responses to the vector could be correlated with the responses to both vector and transgene after vaccination.
Baseline Mtb PPD IFN-γ ELISpot and Mtb WCL antibody responses were not significantly correlated in any of the three populations studied (Fig. 1 C-E). As the R2 value calculated was low in each case (rhesus macaques R2=0.03, N.C. humans R2=0.05, Malawi humans R2=0.01), the mycobacterial cellular and humoral immune responses were largely independent.
Boosting of IFN-γ PPD-specific ELISpot responses and Mtb WCL antibody titers after rBCG vaccination
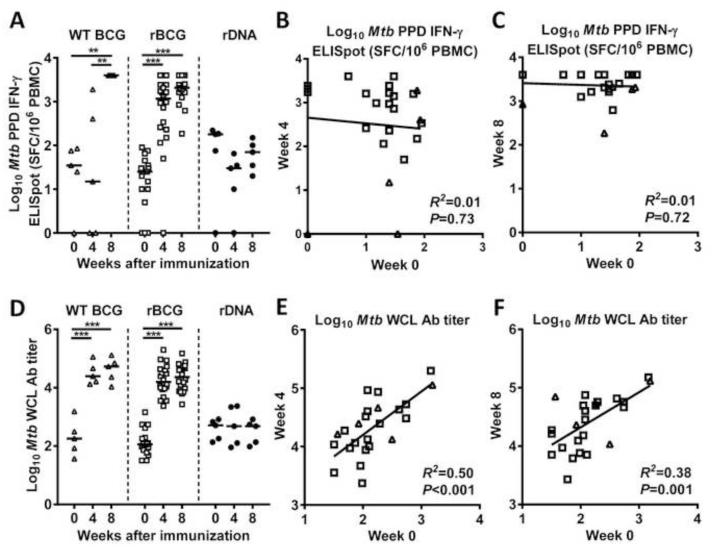
As expected, PPD-specific IFN-γ ELISpot responses increased in both WT BCG- and rBCG-treated macaques in the weeks following immunization (Fig. 2 A). NHPs immunized with WT BCG showed significantly elevated titers by eight weeks post vaccination with a 2.1 log10 increase compared to baseline (P= 0.008), while NHPs immunized with rBCG showed significantly elevated titers at four and eight weeks after vaccination (P<0.001 for both comparisons). As expected, rDNA immunization did not cause an increase in PPD IFN-γ ELISpot titers in the rDNA control primates. There was no significant correlation between ELISpot titers at baseline and four or eight weeks post infection among the primates receiving either WT BCG or rBCG (Fig. 2 B-C). Note that the baseline PPD ELISpot data in Figure 2A differs slightly from that shown in Figure 1A. All data in Figure 2 was derived from fresh rhesus PBMCs. The data in Figure 1A was derived from frozen rhesus PBMCs so it could be compared directly to data from frozen human PBMCs. PPD ELISpot responses measured independently on frozen and fresh rhesus cells were correlated (R2=0.59, p<0.0001; based on log-transformed values).
Fig. 2. Changes in Mtb cellular and humoral immune responses in rhesus macaques after immunization with wild-type BCG, rBCG, or rDNA.
Rhesus macaques were immunized with either wild type (WT) BCG (open triangles, n=5), rBCG expressing SIV Gag (open squares, n=20), or rDNA expressing SIV Gag, Pol, and Nef (closed circles, n=5). (A) Cellular responses to Mtb PPD at baseline, and at 4 and 8 weeks after immunization. (B-C) Correlation between Mtb cellular responses at baseline and 4 and 8 weeks post immunization for the rhesus macaques given either WT BCG or rBCG (n=25). (D) Humoral responses to Mtb WCL in the same groups. (E-F) Correlation between Mtb humoral responses at baseline and 4 and 8 weeks post immunization for the same groups. Bars in panels A and D represent medians. Zero values for ELISpot are plotted on the axis. Comparisons between two groups by Mann Whitney Rank Sum Test. **P<0.01, ***P<0.001.
All macaques receiving WT BCG or rBCG demonstrated a sharp increase in the Mtb WCL titers (Fig. 2 D). Titers became elevated by four weeks post rBCG vaccination (WT BCG Δ=2.1 log10, P<0.001; rBCG Δ=2.1 log10, P<0.001) and did not increase further at eight weeks post vaccination (WT BCG Δ=0.3 log10, P>0.05; rBCG Δ=0.2 log10, P>0.05). As expected, rDNA immunization did not cause a change of the Mtb WCL titer in the control NHP group. A significant correlation was observed between baseline Mtb WCL antibody titer and the titer four and eight weeks after vaccination when the 25 macaques that received either WT or rBCG were combined (Fig. 2 E-F), or when the 20 macaques that received rBCG were considered separately. There was no correlation for the 5 macaques that received WT BCG, but this analysis is limited by the small group size.
Impact of baseline PPD IFN-γ ELISpot and WCL antibody titers on SIV Gag responses after immunization
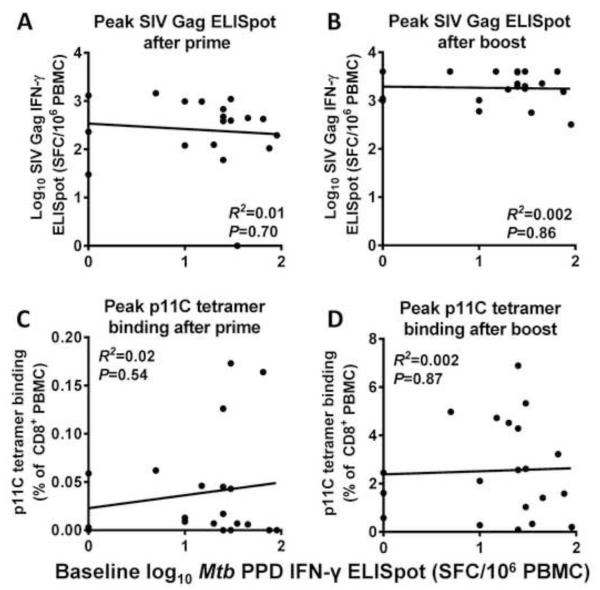
To evaluate the potential impact of prior anti-vector-specific immunity on immune responses directed against the transgene of the rBCG vector, we assessed the correlation between the baseline mycobacterial immune responses (PPD IFN-γ ELISpot or Mtb WCL titer) and two measures of SIV Gag responses chosen in advance as co-primary endpoints: SIV Gag pooled peptide-stimulated IFN-γ ELISpot responses and p11C-specific CD8+ tetramer binding. Both responses were measured after prime immunization with the mycobacterial vector and after boost with a NYVAC-SIVgag-pol vaccine. There was no correlation between baseline PPD IFN-γ ELISpot and the peak SIV Gag-specific SFC/106 PBMCs either after prime (R2=0.01, P=0.70) or after boost (R2=0.002, P=0.86) (Fig. 3 A-B). Similarly, no correlation was noted between baseline PPD IFN-γ ELISpot titer and peak percentage of p11C+ CD8+ T cells (prime: R2=0.02, P=0.54, boost: R2=0.002, P=0.87) (Fig. 3 C-D). The lack of correlation between baseline PPD IFN-γ ELISpot response and vaccine-induced SIV Gag response was confirmed when each of the four rBCG-vaccinated NHP groups was considered separately (data not shown).
Fig. 3. Lack of correlation between cellular responses to SIV Gag after vaccination and baseline Mtb cellular responses.
Rhesus macaques (n=20) were immunized with rBCG expressing SIV Gag, then boosted 10 months later with recombinant NYVAC expressing SIV Gag and SIV Pol. Cellular responses to SIV Gag were measured two weeks after the initial rBCG prime and two weeks after the rNYVAC boost. Each panel shows cellular responses to SIV Gag on the y-axis, and baseline Mtb cellular responses on the x-axis. Shown are IFN-γ ELISpot responses to an SIV Gag peptide pool after prime (A) and boost (B), and tetramer binding to the SIV Gag p11C epitope after prime (C) and boost (D). No significant correlation was seen between cellular responses to SIV Gag and baseline Mtb cellular responses.
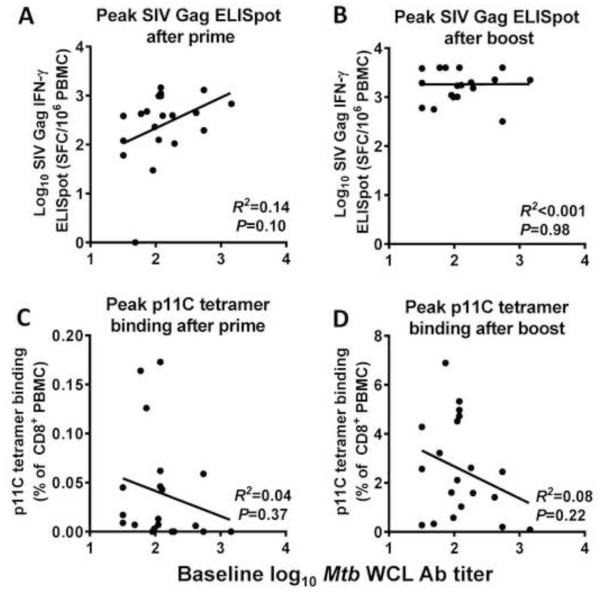
The same lack of correlation was observed when baseline WCL antibody titers were compared to peak SIV Gag-specific SFC/106 PBMCs or peak percentage of p11C+ CD8+ T cells (Fig. 4). Pre-existing antibody responses did not correlate with peak SIV Gag-specific SFC/106 PBMCs after prime (R2=0.14, P=0.10) or boost (R2<0.001, P=0.98), nor did they correlate with peak percentage of p11C+ CD8+ T cells (prime: R2=0.04, P=0.37; boost: R2=0.08, P=0.22). The lack of correlation between baseline Mtb WCL antibody titer and vaccine-induced SIV Gag response was confirmed when each of the four rBCG-vaccinated NHP groups was considered separately (data not shown).
Fig. 4. Lack of correlation between cellular responses to SIV Gag after vaccination and baseline Mtb antibody responses.
Rhesus macaques (n=20) were immunized with rBCG expressing SIV Gag, then boosted 10 months later with recombinant NYVAC expressing SIV Gag and SIV Pol. Cellular responses to SIV Gag were measured two weeks after the initial rBCG prime and two weeks after the rNYVAC boost. Each panel shows cellular responses to SIV Gag on the y-axis, and baseline Mtb humoral responses on the x-axis. Shown are peak IFN-γ ELISpot responses to an SIV Gag peptide pool after prime (A) and boost (B), and tetramer binding to the SIV Gag p11C epitope after prime (C) and boost (D). No significant correlation was seen between cellular responses to SIV Gag and baseline Mtb antibody responses.
DISCUSSION
Pre-existing immunity limits the immunogenicity of an array of viral [18-23] or bacterial vaccine vectors [24]. Failure of revaccination with BCG to boost protection against pulmonary TB [27] has led to the suggestion that prior anti-BCG immunity reduces the immunogenicity of a BCG vaccine delivered later in life. Differential exposure to nontuberculous mycobacteria in differing geographic areas may also influence the efficacy of BCG vaccination [3] or revaccination [28]. Yet, several observations from the present study suggest that pre-existing anti-mycobacterial immune responses may not present a substantial limitation for the immune responses to foreign proteins expressed by rBCG.
In the present study, rhesus monkeys vaccinated with rBCG-SIVgag immunogens had preexisting immune responses to mycobacteria. Prior anti-mycobacterial immune responses in the naïve rhesus macaques were likely due to environmental exposure to nontuberculous mycobacteria (e.g., M. avium complex) [29, 30]. There was no history of prior BCG vaccination in the macaques, and a negative PPD skin test showed no indication of Mtb infection. Baseline immune responses to mycobacteria in rhesus macaques overlapped those of human populations from North Carolina and Malawi, and showed similar large variations, making the rhesus macaque model a suitable surrogate for investigations of anti-mycobacterial immunity in humans.
The two human comparator populations chosen represent a spectrum in TB prevalence and use of BCG vaccine: U.S. populations have a low TB prevalence and do not vaccinate with BCG at birth, while Malawi has a high TB incidence and incorporates infant BCG vaccination [1], though some Malawian adults lack BCG scars [31]. According to the World Health Organization, Malawi is one of 41 high TB/HIV burden countries in the world with an estimated one or two TB cases in 1,000 Malawians. In 2012 approximately 59% of tested TB patients in Malawi were HIV-positive, whereas only 7 % of TB patients in the U.S. were HIV-positive [1]. Since Malawians are a high-risk population for HIV infection, they are a candidate population for an HIV-1 vaccine trial. The significantly higher PPD-specific IFN-γ ELISpot responses in the Malawian humans than in the NHP cohort could be due to infant BCG vaccination, latent or active TB infections, and cross-reactive cellular immunity to environmental mycobacteria [29, 32]. We did not screen the Malawian population for latent tuberculosis infection. Humans in Malawi had a higher median response than those in North Carolina (0.4 logs or 2.5 fold). This difference was not statistically significant but the sample sizes were small. Comparisons between these two populations are limited based on the multiple differences between the two groups. The extent of exposure to environmental mycobacteria is multifactorial including differences by geographical region, climate (including humidity, aerosol, and dust exposure), personal hygiene, housing, living, and human working conditions.
Baseline mycobacterial cellular and humoral responses were independent in both humans and NHP. We previously noted this independence among humans with active TB [15]. Similarly, baseline cellular and humoral responses to adenovirus serotype 5 (Ad5) are also independent in human populations [33]. Noteworthy, mycobacterial antibody and cellular responses in NHP were significantly boosted after rBCG vaccination. Moreover, post-vaccination anti-mycobacterial antibody responses in the macaques were correlated to pre-vaccination responses. Thus, baseline anti-Mtb antibody titers predicted peak anti-Mtb antibody titers following rBCG immunization, indicating that rBCG boosted these titers. This is in contrast to the experience with viral vectors in which pre-existing antibody responses interfere with response to the vector. Antibodies to viral vectors may prevent cell entry or lead to more rapid clearance. In contrast, pre-existing antibodies may have a more limited effect on BCG replication.
Post-vaccination anti-mycobacterial T cell responses did not correlate with pre-vaccination responses, indicating an absence of a boosting effect based on prior responses. The interplay of pre-existing immunity and the immune response to BCG in human populations is complex [34, 35]. Pre-existing cellular immune responses may blunt or even block the immune response to BCG [34, 36]. In general, cellular responses to BCG have been more robust in human population with low levels of pre-existing immunity [31]. In one study, Gambians who responded immunologically to BCG vaccination had lower pre-existing response, while those who did not respond had higher pre-existing responses [36].
Our results with mycobacteria contrast to the experience with Ad5-based vaccines. Pre-existing Ad5 immune responses blunt vaccine immunogenicity by recombinant Ad5 vaccines [18]. Additionally, the Step Study clinical trial of a recombinant Ad5 HIV-1 gag/pol/nef vaccine showed a higher rate of HIV infections in men with pre-existing Ad5 immunity [37], suggesting that baseline responses to Ad5 vaccine vectors can be a safety concern.
Rhesus macaques in the present study generated robust SIV Gag-specific immune responses following immunization with rBCG-SIVgag vaccines. There was no correlation between prior cellular or humoral mycobacterial responses and immune responses elicited to the SIV Gag-transgene expressed by rBCG vectors. This indicates that the immune responses against the SIV Gag immunogen were not altered by prior anti-vector immunity. Moreover, studies in mice using attenuated Listeria monocytogenes, another intracellular bacteria, as a vaccine delivery vector showed that pre-existing immunity has a minimal impact on subsequent vaccination [38]. Taken together, these findings suggest that baseline immunity to intracellular bacteria may not have the same ramifications for immunogenicity as pre-existing immunity to viral vectors.
Our study is subject to limitations that may be addressed in future NHP studies. First, we administered rBCG vaccine vectors by the intravenous route at a high dose (3×108 cfu). We chose this route and dose with the goal of generating measurable immune responses after prime and boost. This dose and route was tolerated well in the macaques in this study, but is not likely to be replicated in future human trials. Pre-existing immune responses may have a greater impact on rBCG vaccine vectors delivered at lower doses or by other routes such as intradermal or subcutaneous injection. Recent murine studies have demonstrated that rBCG vector dose can influence the character of the immune response against both the vector and the insert protein [39].
Second, the pre-existing mycobacteria immune responses in these primates were generated by natural exposure to environmental mycobacteria rather than by prior BCG vaccination or prior Mtb infection. The latter sources of immune response are present in human populations that are candidates for future HIV vaccines. However, humans with Mtb disease exhibit immune responses that are cross-reactive against antigenic preparations from environmental mycobacteria [15]. To assess the effect of infant BCG vaccination on the immunogenicity of rBCG vectors, prospective NHP trials may model standard BCG newborn vaccination prior to rBCG-SIV/HIV immunization in adults.
In conclusion, we demonstrated that the rBCG vaccine vectors used in the present study were suitable priming immunogens for anti-SIV Gag immune responses. The immunogenicity of the rBCG vaccine constructs in rhesus macaques did not appear to be blunted by prior mycobacterial immune responses. Future studies will be required to demonstrate the efficacy of rBCG vectors against SIV or SHIV challenges in primates and demonstration of immunogenicity in humans. Assessment of pre-existing immune responses to the rBCG vector will be a relevant component of future NHP and human studies. These investigations will have implications for the use of rBCG vectors as combined vaccines against TB and HIV.
highlights.
We generated recombinant vectors using the Mycobacterium bovis BCG vaccine strain.
We vaccinated primates with recombinant BCG vectors expressing SIV Gag.
Primates had baseline humoral and cellular immune responses to mycobacteria.
Vaccinated primates generated strong CD8 T cell responses to SIV Gag.
Responses to SIV Gag were independent from baseline mycobacterial immunity.
ACKNOWLEDGMENTS
We are grateful to Harikrishnan Balachandran, Linh Mach, Leila Eslamizar, and Michelle A. Lifton for technical support. Funding for this study was provided by Collaboration for AIDS Vaccine Discovery (CAVD) program grants OPP38614 and OPP1033104 from the Bill & Melinda Gates Foundation. Additional funding support was provided by the NIH grants AI060354 (Harvard University Center for AIDS Research), OD011103 (New England Primate Research Center), AI067854 (Center for HIV/AIDS Vaccine Immunology), AI100645 (Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery), AI057157 (Southeast Regional Center of Excellence for Emerging Infections and Biodefense), and AI058607 (Regional Biocontainment Laboratory at Duke), and National Science Foundation Graduate Research Fellowship 2011083676 (C.C.P.). We acknowledge the CHAVI Repository for providing human plasma and PBMC samples. M. tuberculosis whole cell lysate was produced at Colorado State University under NIH Contract HHSN266200400091C (Tuberculosis Vaccine Testing and Research Materials). The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Gag (15-mer) Peptides - Complete Set.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflicts of interest exist.
REFERENCES
- [1].World Health Organization Global tuberculosis report 2013. 2013:306. [Google Scholar]
- [2].Centers for Disease Control The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 1996;45:1–18. [PubMed] [Google Scholar]
- [3].Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–80. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- [4].Dennehy M, Williamson AL. Factors influencing the immune response to foreign antigen expressed in recombinant BCG vaccines. Vaccine. 2005;23:1209–24. doi: 10.1016/j.vaccine.2004.08.039. [DOI] [PubMed] [Google Scholar]
- [5].Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–60. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- [6].Fuerst TR, Stover CK, de la Cruz VF. Development of BCG as a live recombinant vector system: potential use as an HIV vaccine. Biotechnology therapeutics. 1991;2:159. [PubMed] [Google Scholar]
- [7].Jensen K, Ranganathan UD, Van Rompay KK, Canfield DR, Khan I, Ravindran R, et al. A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virus-infected infant macaques. Clinical and vaccine immunology : CVI. 2012;19:1170–81. doi: 10.1128/CVI.00184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jensen K, Pena MG, Wilson RL, Ranganathan UD, Jacobs WR, Jr., Fennelly G, et al. A neonatal oral -SIV prime / intramuscular MVA-SIV boost combination vaccine induces both SIV and -specific immune responses in i 449 nfant macaques. Trials in vaccinology. 2013;2:53–63. doi: 10.1016/j.trivac.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saubi N, Mbewe-Mvula A, Gea-Mallorqui E, Rosario M, Gatell JM, Hanke T, et al. Pre-clinical development of BCG.HIVA(CAT), an antibiotic-free selection strain, for HIV-TB pediatric vaccine vectored by lysine auxotroph of BCG. PloS one. 2012;7:e42559. doi: 10.1371/journal.pone.0042559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hopkins R, Bridgeman A, Joseph J, Gilbert SC, McShane H, Hanke T. Dual neonate vaccine platform against HIV-1 and M. tuberculosis. PloS one. 2011;6:e20067. doi: 10.1371/journal.pone.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosario M, Fulkerson J, Soneji S, Parker J, Im EJ, Borthwick N, et al. Safety and immunogenicity of novel recombinant BCG and modified vaccinia virus Ankara vaccines in neonate rhesus macaques. Journal of virology. 2010;84:7815–21. doi: 10.1128/JVI.00726-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sixsmith JD, Panas MW, Lee S, Gillard GO, White K, Lifton MA, et al. Recombinant Mycobacterium bovis bacillus Calmette-Guerin vectors prime for strong cellular responses to simian immunodeficiency virus gag in rhesus macaques. Clinical and vaccine immunology : CVI. 2014;21:1385–95. doi: 10.1128/CVI.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu JS, Peacock JW, Vanleeuwen S, Hsu T, Jacobs WR, Jr., Cayabyab MJ, et al. Generation of mucosal anti-human immunodeficiency virus type 1 T-cell responses by recombinant Mycobacterium smegmatis. Clinical and vaccine immunology : CVI. 2006;13:1204–11. doi: 10.1128/CVI.00195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cayabyab MJ, Korioth-Schmitz B, Sun Y, Carville A, Balachandran H, Miura A, et al. Recombinant Mycobacterium bovis BCG prime-recombinant adenovirus boost vaccination in rhesus monkeys elicits robust polyfunctional simian immunodeficiency virus-specific T469 cell responses. Journal of virology. 2009;83:5505–13. doi: 10.1128/JVI.02544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Perley CC, Frahm M, Click EM, Dobos KM, Ferrari G, Stout JE, et al. The human antibody response to the surface of Mycobacterium tuberculosis. PloS one. 2014;9:e98938. doi: 10.1371/journal.pone.0098938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Flaherty DK, Vesosky B, Beamer G472L, Stromberg P, Turner J. Exposure to Mycobacterium avium can modulate established immunity against Mycobacterium tuberculosis infection generated by Mycobacterium bovis BCG vaccination. Journal of leukocyte biology. 2006;80:1262–71. doi: 10.1189/jlb.0606407. [DOI] [PubMed] [Google Scholar]
- [17].Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, et al. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infection and immunity. 2002;70:672–8. doi: 10.1128/iai.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. Journal of immunology. 2004;172:6290–7. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- [19].Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Current opinion in immunology. 2011;23:377–82. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [20].Lin J, Calcedo R, Vandenberghe LH, Figueredo JM, Wilson JM. Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Human gene therapy. 2008;19:663–9. doi: 10.1089/hum.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cafaro A, Macchia I, Maggiorella MT, Titti F, Ensoli B. Innovative approaches to develop prophylactic and therapeutic vaccines against HIV/AIDS. Advances in experimental medicine and biology. 2009;655:189–242. doi: 10.1007/978-1-4419-1132-2_14. [DOI] [PubMed] [Google Scholar]
- [22].Basner-Tschakarjan E, Bijjiga E, Martino AT. Pre-Clinical Assessment of Immune Responses to Adeno-Associated Virus (AAV) Vectors. Frontiers in immunology. 2014;5:28. doi: 10.3389/fimmu.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kannanganat S, Nigam P, Velu V, Earl PL, Lai L, Chennareddi L, et al. Preexisting vaccinia virus immunity decreases SIV-specific cellular immunity but does not diminish humoral immunity and efficacy 495 of a DNA/MVA vaccine. Journal of immunology. 2010;185:7262–73. doi: 10.4049/jimmunol.1000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sevil Domenech VE, Panthel K, Meinel KM, Winter SE, Russmann H. Pre-existing anti-Salmonella vector immunity prevents the development of protective antigen-specific CD8 T-cell frequencies against murine listeriosis. Microbes and infection / Institut Pasteur. 2007;9:1447–53. doi: 10.1016/j.micinf.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [25].National Research Council . Guide for the care and use of laboratory animals. 8th National Academies Press; Washington, D.C.: 2011. [Google Scholar]
- [26].Kuroda MJ, Schmitz JE, Barouch DH, Craiu A, Allen TM, Sette A, et al. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. The Journal of experimental medicine. 1998;187:1373–81. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366:1290–5. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]
- [28].Barreto ML, Pereira SM, Pilger D, Cruz AA, Cunha SS, Sant'Anna C, et al. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine. 2011;29:4875–7. doi: 10.1016/j.vaccine.2011.05.023. [DOI] [PubMed] [Google Scholar]
- [29].Demangel C, Garnier T, Rosenkrands I, Cole ST. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infection and immunity. 2005;73:2190–6. doi: 10.1128/IAI.73.4.2190-2196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lozes E, Denis O, Drowart A, Jurion F, Palfliet K, Vanonckelen A, et al. Cross-reactive immune responses against Mycobacterium bovis BCG in mice infected with non-tuberculous mycobacteria 518 belonging to the MAIS-Group. Scandinavian journal of immunology. 1997;46:16–26. doi: 10.1046/j.1365-3083.1997.d01-99.x. [DOI] [PubMed] [Google Scholar]
- [31].Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359:1393–401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- [32].Mantilla-Beniers NB, Gomes MG. Mycobacterial ecology as a modulator of tuberculosis vaccine success. Theoretical population biology. 2009;75:142–52. doi: 10.1016/j.tpb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [33].O'Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nature medicine. 2009;15:873–5. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature reviews Microbiology. 2005;3:656–62. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- [35].Parkash O. Vaccine against tuberculosis: a view. J Med Microbiol. 2014;63:777–9. doi: 10.1099/jmm.0.070425-0. [DOI] [PubMed] [Google Scholar]
- [36].Ota MO, Brookes RH, Hill PC, Owiafe PK, Ibanga HB, Donkor S, et al. The effect of tuberculin skin test and BCG vaccination on the expansion of PPD-specific IFN-gamma producing cells ex vivo. Vaccine. 2007;25:8861–7. doi: 10.1016/j.vaccine.2007.10.025. [DOI] [PubMed] [Google Scholar]
- [37].McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leong ML, Hampl J, Liu W, Mathur S, Bahjat KS, Luckett W, et al. Impact of preexisting vector-specific immunity on vaccine potency: characterization of listeria monocytogenes-specific humoral and cellular 540 immunity in humans and modeling studies using recombinant vaccines in mice. Infection and immunity. 2009;77:3958–68. doi: 10.1128/IAI.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Power C, Marfleet TW, Qualtiere L, Xiao W, Bretscher P. Development of Th1 imprints to rBCG expressing a foreign protein: implications for vaccination against HIV-1 and diverse influenza strains. Journal of biomedicine & biotechnology. 2010;2010:591348. doi: 10.1155/2010/591348. [DOI] [PMC free article] [PubMed] [Google Scholar]