Abstract
Muscle- and liver-derived IGF-1 play important roles in muscle anabolism throughout growth and aging. Yet, prolonged food restriction is thought to increase longevity in part by lowering levels of IGF-1, which in turn reduces the risk for developing various cancers. The dietary factors that modulate IGF-1 levels are, however, poorly understood. We tested the hypothesis that the adipokine leptin, which is elevated with food intake and suppressed during fasting, is a key mediator of IGF-1 levels with aging and food restriction. First, leptin levels in peripheral tissues were measured in young mice fed ad libitum, aged mice fed ad libitum, and aged calorie-restricted (CR) mice. A group of aged CR mice were also treated with recombinant leptin for 10 days. Later, aged mice fed ad libitum were treated with saline (VEH) or with a novel leptin receptor antagonist peptide (Allo-aca) and tissue-specific levels of IGF-1 were determined. On one hand, recombinant leptin induced a three-fold increase in liver-derived IGF-1 and a two-fold increase in muscle-derived IGF-1 in aged, CR mice. Leptin also significantly increased serum growth hormone levels in the aged, CR mice. On the other, the leptin receptor antagonist Allo-aca did not alter body weight or muscle mass in treated mice compared to VEH mice. Allo-aca did, however, produce a significant (20%) decline in liver-derived IGF-1 as well as an even more pronounced (>50%) decrease in muscle-derived IGF-1 compared to VEH-treated mice. The reduced IGF-1 levels in Allo-aca treated mice were not accompanied by any significant change in growth hormone levels compared to VEH mice. These findings suggest that leptin receptor antagonists may represent novel therapeutic agents for attenuating IGF-1 signaling associated with aging, and could potentially mimic some of the positive effects of calorie restriction on longevity.
Keywords: aging, calorie restriction, food intake, longevity
1. Introduction
Calorie restriction has been observed to increase longevity in a variety of species including fruit flies, mice, and non-human primates (Heilbronn and Ravussin, 2003). Long-term reductions in food intake are thought to promote longevity at least in part by impacting the growth hormone (GH)-insulin-like growth factor-1 (IGF-1) axis. That is, long-term food restriction leads to relatively low levels of growth hormone and IGF-1, ultimately lowering the risk for developing tumors and hence the risk of mortality due to cancer (Carter et al., 2002; Barzilai and Bartke, 2009). This model is further supported by evidence from mouse models showing that dwarf mice deficient in IGF-1, GH, and the IGF-1 receptor show increased lifespan (Junnilla et al., 2013; Gesing et al., 2014). It is, however, not well understood how reductions in food intake modulate IGF-1 secretion. For example, reductions in overall caloric intake were thought to reduce IGF-1 levels (Barzilai and Bartke, 2009), but recent studies suggest that particular dietary components such as protein may be more important for regulating IGF-1 levels than other components such as carbohydrates or fats (Levine et al., 2014; Solon-Biet et al., 2014).
While specific dietary components such as protein may be involved in modulating IGF-1 levels and thus influencing longevity, there are a number of different hormones that are also responsive to food intake and changes in energy balance. The adipokine leptin, in particular, increases with food intake and is known to modulate satiety and energy balance. Hyperleptinemia is frequently associated with obesity and metabolic syndrome. There is also evidence that leptin may have systemic effects by regulating the GH-IGF1 axis. Leptin-deficient ob/ob mice have significantly lower circulating GH levels than normal, lean mice (Luque et al., 2007), and leptin treatment increases GH levels in ob/ob mice and stimulates growth hormone releasing hormone (GHRH) neurons in the hypothalamus (Carro et al., 1997; Watanobe and Habu, 2002). Other data suggest that leptin may alter IGF-1 and musculoskeletal growth through GH-independent pathways. For example, leptin treatment in fasting rodents increases GH but not IGF-1 levels (Gat-Yablonski et al., 2008). In contrast, recombinant leptin therapy in fasting men and women increased IGF-1 but not GH (Chan et al., 2008), and in pigs exogenous leptin increases tissue-specific IGF-1 with no change in GH (Ajuwon et al., 2003). Thus, leptin may play an important role in linking food intake and caloric restriction with IGF-1 levels, through both GH-dependent and –independent pathways.
Here we tested the hypothesis that leptin can modulate IGF-1 levels in aged animals subjected to caloric restriction. The mice were maintained on long-term caloric restriction, since these mice have been observed to show increased lifespan as well as low levels of leptin and IGF1 (Hamrick et al., 2008). We also used a novel leptin receptor antagonist peptide, Allo-aca (Otvos et al., 2011a, 2011b, 2014), in aged mice fed ad libitum to determine whether or not altered leptin signaling, and interfering thereof, could modulate tissue-specific IGF-1 levels.
2. Materials & Methods
2.1 Ethics Statement
All animal procedures were approved by the Institutional Animal Care and Use Committee of Georgia Regents University (formerly Georgia Health Sciences University).
2.2 Animals & Assays
All experiments described were approved by the Institutional Animal Care & Use Committee (IACUC) at Georgia Regents University (formerly Georgia Health Sciences University). In the first experiment, mice aged 12 months (n=6) and 20 months fed ad-libitum (AL) (n=6) and mice 20 months on caloric restriction (CR) (n=12) were obtained from the aged rodent colony at the National Institute on Aging (NIA; Bethesda, MD, USA). CR follows the NIA protocol and is initiated at 14 weeks of age at 10% restriction, increased to 25% restriction at 15 weeks, and to 40% restriction at 16 weeks where it is maintained throughout the life of the animal. CR and AL diets follow those described in Hamrick et al. (2008). Half of the mice 20 months of age on CR were treated with recombinant mouse leptin (intraperitoneal injection; R&D Systems, Minneapolis, MN, USA cat # 498-OB-05M) for 10 days at 10 mg/kg body weight, and the other half of each group treated with vehicle control (saline; VEH). In the second experiment 20 mice aged 22 months were obtained from the National Institute on Aging and randomly assigned to two groups. One group of ten mice received saline i.p. injections (VEH) daily for ten days, whereas the other group of ten mice received daily i.p. injections (0.2 ml volume, 1.0 mg/kg) of Allo-aca for ten days. Peptide Allo-aca was synthesized by solid-phase methods and was purified by reversed-phase high performance liquid chromatography (RP-HPLC). The final product was characterized by RP-HPLC and matrix-assisted laser ionization/desorption mass spectrometry (MALDI-MS) and was >95% pure. The peptide was lyophilized twice from 2% acetic acid before testing in biological systems. ELISA assays were performed for GH on mouse serum samples (Millipore EZRMGH-45K) and for IGF-1 (R&D Systems MG100) in liver and skeletal muscle (quadriceps or tibialis anterior) following the manufacturer's instructions. Leptin levels in peripheral tissues and in serum were measured using ELISA (R&D Systems MOB00) following the manufacturer's protocol.
2.3 Tissue Collection
Animals were weighed and then euthanized by CO2 overdose and were subjected to thoracotomy following procedures approved by the Institutional Animal Care and Use Committee of Georgia Regents University. Blood was collected via cardiac puncture, and blood was allowed to clot at room temperature before spinning down for serum at 4500 rpm (2250 × g) for 15 min. Serum was removed and stored at -20°C until assayed. Hindlimb muscles (quadriceps femoris and tibialis anterior) were dissected out and weighed before being snap frozen in liquid nitrogen. Both femora were disarticulated and snap frozen. Inguinal fat pads and a 10 mm2 piece of the liver were removed and also snap frozen in liquid nitrogen. All tissue was rinsed with PBS before being homogenized using an ultrasonicator in 500μl of PBS. Homogenates went through two freeze thaw cycles to disrupt the plasma membrane then briefly centrifuged. Supernatant was removed and stored at -80C until assayed. Mice were not perfused prior to tissue collection, and though tissues were washed thoroughly it is still possible that some circulating peptides remained in the tissue homogenates.
2.4 Statistical Analysis
ANOVA was used to detect between-group differences and Fisher's LSD test used for post-hoc pairwise comparisons. Student's t-tests were used to compare mean values between treatments for a particular tissue.
3. Results
Aged, food-restricted (CR) mice weighed less than their ad-libitum counterparts, and CR mice treated with leptin weighed significantly less than CR mice receiving saline (Fig. 1A). Hindlimb muscle mass did not differ among the aged mice, and all aged mice had lower muscle mass than the 12 month mice (Fig. 1B); however, relative muscle mass normalized for body weight was increased with leptin treatment in the CR mice, such that young AL mice did not differ significantly in terms of relative muscle mass from aged CR mice treated with leptin (Fig. 1C). Leptin levels in peripheral tissues were similar between aged and young (12 month) mice fed ad libitum (data not shown). Calorie restriction was found to significantly decrease leptin levels in both fat and in serum of aged mice (Fig. 2A), indicating that although leptin is abundant in peripheral tissues such as liver and muscle, leptin levels in these tissues are not significantly altered by changes in food intake.
Figure 1.
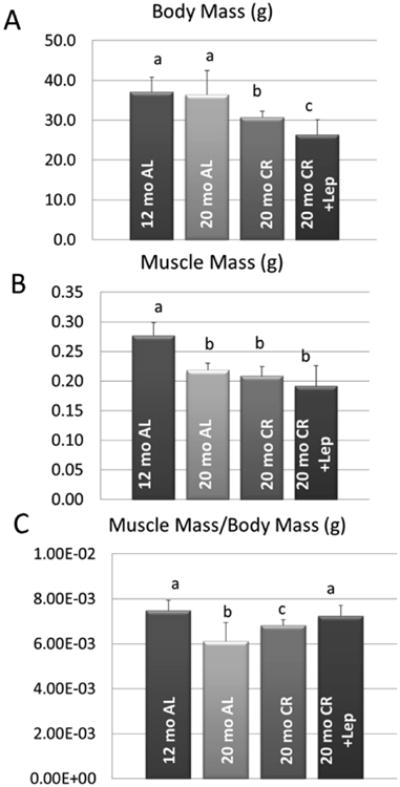
Body weight and composition parameters for mice fed ad libitum (AL) and on calorie restriction (CR). Data are provided for body mass (A), quadriceps muscle mass (B), and quadriceps muscle mass normalized for body weight (C). Solid bars represent group means and error bars represent one standard deviation. Means with different superscripts differ significantly (P<.05) from one another in pairwise comparisons.
Figure 2.
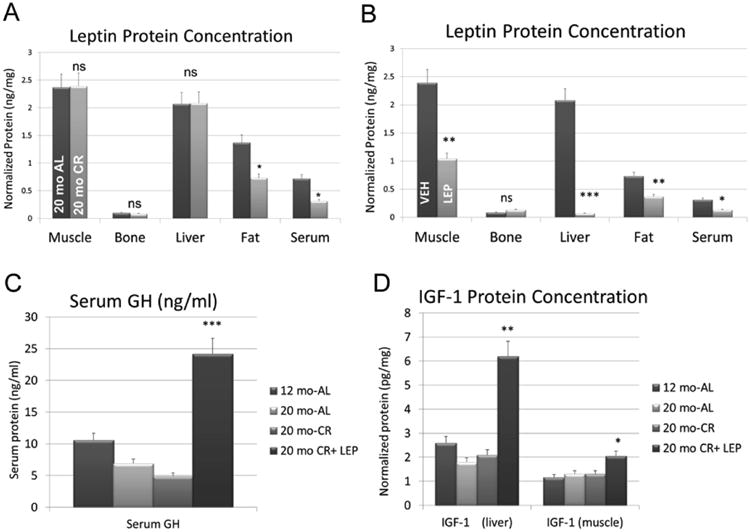
ELISA data for leptin, growth hormone (GH) and IGF-1. Data are shown for A. leptin levels in peripheral tissues and serum of aged mice fed ad libitum (AL) and on calorie restriction (CR), B. leptin levels in peripheral tissues and serum in aged, CR mice receiving saline (VEH) and receiving leptin (LEP), C. serum levels of growth hormone (GH) in mice fed ad libitum (AL), on caloric restriction (CR), and calorie-restricted treated with leptin (CR + Lep), and D. IGF-1 in tissues of mice fed ad libitum (AL), on caloric restriction (CR), and calorie-restricted treated with leptin (CR + Lep). Vertical bars represent group means and error bars are the standard error. *P<.05,** P<.01,***P<.001, ns=non-significant.
A negative feedback loop was observed in skeletal muscle, liver, and fat of aged CR mice, where exogenous leptin suppressed leptin expression in these tissues (Fig. 2B). A dramatic five-fold increase in circulating GH was seen with leptin treatment in aged, CR mice (Fig. 2C). This increase was associated with a marked three-fold increase in liver-derived IGF-1 (Fig. 2D), and a two-fold increase in muscle-derived IGF-1 (Fig. 2D). Aged mice fed ad-libitum treated with the leptin antagonist Allo-aca did not differ significantly in body weight from vehicle-treated mice either before or after the ten-day treatment period (Fig. 3A). Hindlimb muscle mass also did not differ significantly between the control and treated mice, either in absolute terms or when normalized by body weight (Fig. 3B, C). Allo-aca did, however, induce a significant decrease in liver-derived IGF-1 (Fig. 3D) and an even more pronounced decrease in muscle-derived IGF-1 (Fig. 3E). The decline in liver- and muscle-specific IGF-1 with Allo-aca treatment was not accompanied by any change in circulating GH (Fig. 3F).
Figure 3.
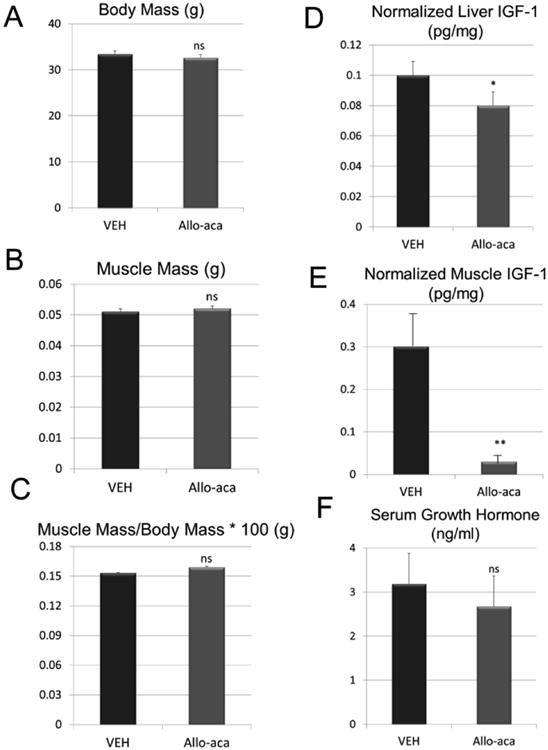
Body composition and ELISA data for aged mice fed ad libitum treated with saline (VEH) or the leptin antagonist Allo-aca. Data for liver- and muscle (tibialis anterior)-derived IGF-1 are normalized to total protein but data for serum growth hormone were not normalized. Vertical bars represent group means and error bars are the standard error. *P<.05,** P<.01, ns=non-significant.
4. Discussion
IGF-1 has significant anabolic effects on skeletal muscle through its activation of Akt and mTor (Luo et al., 2013; Sandri et al., 2013). We have previously observed low levels of leptin, IGF-1, and decreased muscle mass with caloric restriction in mice (Hamrick et al., 2008), and these findings are consistent with our previous work showing that leptin deficiency and the absence of functional leptin receptors are associated with decreased muscle and bone mass (Bartell et al., 2011; Arounleut et al., 2013). Thus, leptin deficiency is frequently associated with IGF-1 deficiency, and the two are likely to lead to reduced muscle mass. This association is further corroborated by our observations here showing that leptin treatment normalizes relative muscle mass, such that muscle mass relative to body weight is similar between young mice and aged, CR mice treated with leptin (Fig. 1A). These data suggest that leptin levels may have a positive, direct relationship with muscle mass, and that this relationship may change with age (Hamrick et al. 2006); however, though leptin treatment seems to increase both muscle fiber size and relative muscle mass in aged mice (Hamrick et al., 2010), we detected no change in absolute muscle mass or relative muscle mass with leptin antagonist treatment. This may be related to the duration of treatment (10 days), and a longer period of exposure to the antagonist could potentially lead to muscle loss. Our results are, however, encouraging in that the leptin antagonist could potentially lower IGF-1 levels in various tissues but not have a significant effect on muscle mass.
As noted above, the molecular mechanisms by which leptin may modulate muscle mass are thought to involve IGF-1 signaling (Arounleut et al., 2013). For example, leptin receptors are abundant in skeletal muscle, and their expression increases with exercise (Guerra et al., 2007). Our earlier studies have shown that caloric restriction decreases muscle mass as well as circulating leptin and IGF-1 in rodents (Hamrick et al., 2008), whereas leptin treatment in ob/ob mice increases muscle mass and serum IGF-1 (Bartell et al., 2008); however, it was not known if these effects were mediated by GH, and if these leptin-mediated effects could also be observed in aged animals. Our findings presented in this report indicate that leptin can stimulate the GH-IGF1 axis in aged, food-restricted rodents. Our findings also reveal a potential new role for leptin antagonists in modulating tissue-specific IGF-1 levels with aging. These molecules have recently been studied for their effects on cancer, muscle wasting (cachexia), and metabolic syndrome (Gertler and Elinav, 2014). Our data indicate that the leptin receptor antagonist Allo-aca can suppress IGF-1 levels in both liver and skeletal muscle independent of GH alterations. These data suggest that antagonism of leptin signaling with high-affinity leptin receptor antagonists could perhaps attenuate the potentially negative impact of leptin-IGF1 actions on longevity, and thus could mimic some of the positive effects of calorie restriction on aging and lifespan.
Highlights.
Calorie restriction is thought to increase longevity in part by lowering levels of IGF-1, which in turn reduces the risk for developing various cancers.
The adipokine leptin, which is elevated with food intake and suppressed during fasting, induced a three-fold increase in liver-derived IGF-1 and a two-fold increase in muscle-derived IGF-1 in aged, calorie-restricted mice.
A leptin receptor antagonist significantly reduced both liver- and muscle-derived IGF-1 in aged mice, suggesting that leptin receptor antagonists could potentially mimic some of the positive effects of calorie restriction on longevity.
Acknowledgments
This work was supported by the National Institutes of Health (NIA-AG036675-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: The authors declare that there are no conflicts of interest regarding the publication of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajuwon K, Kuske J, Ragland D, et al. The regulation of IGF-1 by leptin in the pig is tissue specific and independent of changes in growth hormone. J Nutr Biochem. 2003;14:522–530. doi: 10.1016/s0955-2863(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Arounleut P, Bowser M, Upadhyay S, Shi XM, Fulzele S, Johnson MH, Stranahan AM, Hill WD, Isales CM, Hamrick MW. Absence of functional leptin receptor isoforms in the POUND (Lepr(db/lb)) mouse is associated with muscle atrophy and altered myoblast proliferation and differentiation. PLoS One. 2013;1:e72330. doi: 10.1371/journal.pone.0072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Rayalam S, Ambati S, Gaddam DR, Hartzell DL, Hamrick M, She JX, Della-Fera MA, Baile CA. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–20. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Bartke A. Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci. 2009;64:187–91. doi: 10.1093/gerona/gln061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Senaris R, Considine R, Casanueva F, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology. 1997;138:2203–2206. doi: 10.1210/endo.138.5.5238. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Chan J, Williams C, Raciti P, et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-1 in leptin-deficient states. J Clin Endocrinol Metab. 2008;93:2819–27. doi: 10.1210/jc.2008-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat-Yablonski G, Ben-Ari T, Shtaif B, Potievsky O, Moran O, Eshet R, Maor G, Segev Y, Phillip M. Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology. 2004;145:343–50. doi: 10.1210/en.2003-0910. [DOI] [PubMed] [Google Scholar]
- Gertler A, Elinav E. Novel superactive leptin antagonists and their potential therapeutic applications. Curr Pharm Des. 2014;20:659–65. doi: 10.2174/13816128113199990014. [DOI] [PubMed] [Google Scholar]
- Gesing A, Al-Regaiey KA, Bartke A, Masternak MM. Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice. Exp Gerontol. 2014;58:219–29. doi: 10.1016/j.exger.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra B, Santana A, Fuentes T, Delgado-Guerra S, Cabrera-Socorro A, et al. Leptin receptors in human skeletal muscle. J Appl Physiol. 2007;102:1786–1792. doi: 10.1152/japplphysiol.01313.2006. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu Y, Howard B, Immel D, McNeil P, Borlongan C, Bollag W, Curl W, Yu JC, Isales CM. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39:845–853. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–879. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, Zhou L, Isales CM, Mi QS. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. 2010;400:379–83. doi: 10.1016/j.bbrc.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn L, Ravussin R. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–69. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9:366–76. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–17. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu J, Li X, Qin ZH. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol. 2013;48:427–36. doi: 10.1016/j.exger.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Luque R, Huang Z, Shah B, Mazzone T, Kineman R. Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am J Phys Endocrinol Metab. 2007;292:E891–E899. doi: 10.1152/ajpendo.00258.2006. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD, Surmacz E. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011a;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Shao WH, Vanniasinghe AS, Amon MA, Holub MC, Kovalszky I, Wade JD, Doll M, Cohen PL, Manolios N, Surmacz E. Toward understanding the role of leptin and leptin receptor antagonism in preclinical models of rheumatoid arthritis. Peptides. 2011b;32:1567–1574. doi: 10.1016/j.peptides.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Vetter SW, Koladia M, Knappe D, Schmidt R, Ostorhazi E, Kovalszky I, Bionda N, Cudic P, Surmacz E, Wade JD, Hoffmann R. The designer leptin antagonist peptide Allo-aca compensates for short serum half-life with very tight binding to the receptor. Amino Acids. 2014;46:873–82. doi: 10.1007/s00726-013-1650-6. [DOI] [PubMed] [Google Scholar]
- Sandri M, Barberi L, Bijlsma AY, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303–23. doi: 10.1007/s10522-013-9432-9. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–30. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanobe H, Habu S. Leptin regulates growth hormone-releasing factor, somatostatin, and alpha-melanocyte-stimulating hormone but not neuropeptide Y release in rat hypothalamus in vivo: relation with growth hormone secretion. J Neurosci. 2002;22:6265–71. doi: 10.1523/JNEUROSCI.22-14-06265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


