Abstract
Receptor protein tyrosine phosphatases (RPTPs) are extensively expressed in the central nervous system (CNS), and have distinct spatial and temporal patterns in different cell types during development. Previous studies have demonstrated possible roles for RPTPs in axon outgrowth, guidance, and synaptogenesis. In the present study, our results revealed that protein tyrosine phosphatase, receptor type D (PTPRD) was initially expressed in mature neurons in embryonic CNS, and later in oligodendroglial cells at postnatal stages when oligodendrocyte undergo active axonal myelination process. In PTPRD mutants, oligodendrocyte differentiation was normal and a transient myelination delay occurred at early postnatal stages, indicating the contribution of PTPRD to the initiation of axonal myelination. Our results also showed that the remyelination process was not affected in the absence of PTPRD function after a cuprizone-induced demyelination in adult animals.
Keywords: axonal myelination, spinal cord, cuprizone, corpus callosum
INTRODUCTION
As an important cellular component of the brain and spinal cord, the oligodendrocyte is mainly responsible for synthesizing myelin and wrapping around axons to provide insulation and facilitate the rapid and efficient conduction of electrical impulses (Bunge, 1968). In the developing central nervous system (CNS), oligodendrocyte progenitor cells (OPCs) are initially generated from the ventricular zone and rapidly dispersed into the surrounding white matter (Miller et al., 1999, Richardson et al., 2000). While a small number of them remain as progenitor cells, the majority of them undergo terminal differentiation to become mature myelinating oligodendrocytes (Richardson et al., 1997). Multiple and sequential steps are involved in myelination process, including the migration of oligodendrocytes to target axons, the recognition and adhesion of oligodendrocyte process to axons, the surrounding of oligodendrocyte process around axons and the final compacting of myelin sheath (Baumann and Pham-Dinh, 2001). However, little is known about the molecular mechanisms and signals that control this complex process.
Development of the myelination process requires dramatic changes in the pattern of gene expression (Wegner, 2000). It is clear that exposure of cells to extracellular signals leads to changes in gene expression which assure appropriate physiological and biological responses during development. The most common regulatory mechanism involves protein phosphorylation and dephosphorylation of various transcriptional factors (Hunter and Karin, 1992, Hill and Treisman, 1995). Within these signal transducing pathways, the interaction between extracellular signaling molecules and their specific membrane receptors is the first step. Previous studies showed that in vertebrates, the Type IIa receptor tyrosine phosphatases (RPTPs) which consist of Leukocyte antigen-related (LAR), protein tyrosine phosphatase, receptor type S (PTPRS) and type D (PTPRD) are highly expressed in the developing nervous system (Sommer et al., 1997, Schaapveld et al., 1998, Tian et al., 1999, Johnson and Van Vactor, 2003, Thompson et al., 2003). The extracellular regions of Type IIa RPTPs resemble cell adhesion molecules as they all contain multiple extracellular Ig-like domains and two to nine type-III fibronectin repeats. Both the Ig-like domains and fibronectin repeats are known to participate in cell-cell and cell-matrix interactions (O’Grady et al., 1998, Haj et al., 1999). This suggests a role of these RPTPs in cell surface recognition or cell adhesion besides their dephosphorylation function. Consistently, Type IIa RPTPs are known to be concentrated in axons and growth cones (Stoker, 1994, Gershon et al., 1998, Zhang et al., 1998), raising the possibility that these adhesion surface molecules may participate in axon-glia interaction and axonal myelination.
To investigate the possible involvement of protein phosphorylation and dephosphorylation in oligodendrocyte differentiation and axonal myelination, we recently performed a large-scale screening of RPTP expression by RNA in situ hybridization in perinatal CNS tissues. In this study, we reported that PTPRD, a member of Type IIa RPTPs, was transiently expressed in mature myelinating oligodendrocytes at postnatal stages. Analysis of PTPRD knock-out mice revealed a developmental delay during early myelination, suggesting a potential function of PTPRD in CNS myelination process. However, the differentiation of OPCs was not significantly affected during development and after the cuprizone-induced demyelination.
EXPERIMENTAL PROCEDURES
Mice
Mice were housed under standard laboratory conditions at the animal facility of the University of Louisville. All experimental procedures conformed to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Louisville.
The Olig1 and PTPRD homozygous null pups were obtained by the interbreeding of heterozygous animals. CNP+/cre;Nkx2.2fl/fl mice were obtained through intercross between Nkx2.2 floxed mouse lines and CNPcre transgenic mice. Genomic DNA extracted from tails was used for genotyping by Southern analysis or by PCR. Genotyping of PTPRD, Olig1, Nkx2.2, CNPCre and Nkx2.2 floxed mutants were described earlier (Lu et al., 2000, Uetani et al., 2000, Qi et al., 2001, Lu et al., 2002, Lappe-Siefke et al., 2003, Uetani et al., 2006, Mastracci et al., 2013).
Immunofluorescence and immunohistochemistry
Postnatal mice were fixed by cardiac perfusion with 4% paraformaldehyde (PFA). Brain and spinal cord tissues were dissected out and fixed in 4% PFA at 4 °C overnight. Following fixation, tissues were transferred to 20% sucrose in PBS overnight, embedded in OCT media and then sectioned (20 μm thickness) on a cryostat. After rinsing with PBS, sections were permeabilized in 0.1% Triton X-100 in PBS, incubated in blocking solution (5% normal serum in PBS plus 1% BSA) at room temperature for 1 hour, and incubated in diluted primary antibody in blocking solution at 4°C overnight. The next day, sections were washed in PBS and incubated in the secondary antibody in blocking solution at room temperature for 1 hour. After incubation, sections were washed in PBS and mounted in Mowiol mounting medium on glass slides. The dilution ratio of primary antibodies is as follows: anti-mouse Olig2 (1:3,000, gift from Drs. Stiles and Alberta), anti-mouse APC/CC1 (1:50, Oncogene), anti-mouse MBP (1:5000, Chemicon). The Alexa-488 or Alexa-594 conjugated secondary antibodies were obtained from Molecular Probes (Eugene, OR).
In situ RNA hybridization
In situ RNA hybridization or the combined immunohistochemistry and in situ hybridization (ISH) was performed as described in Schaeren-Wiemers and Gerfin-Moser (Schaeren-Wiemers and Gerfin-Moser, 1993) with minor modifications.
Ultrastructural analyses of myelin structures
Wild-type and PTPRD mutant littermates from various age groups (P4, P7, P14) were cardially perfused with 3% glutaraldehyde in 0.1 M cacodylate buffer, pH7.2., spinal cord (at T6 level) were removed and postfixed for overnight. Tissues were then washed several times with cacodylate buffer, postfix in 1% osmium tetroxide for 1 h, washed again with the buffer before dehydration through a series of graded alcohol. Fixed tissues were subsequently embedded in epony plastic and sectioned at 800–1000 Å on a diamond knife and mount on 200 mesh copper grids. Ultra thin sections were stained with uranium acetate and lead citrate, and examined under a Philips CM10 EM.
For statistical analyses of axonal myelination, the number of axons that were wrapped by compact myelin sheaths was calculated from each micrograph for each genotype (n √3). Statistical significance (p value) was assessed by Student’s t test. The areas of myelinated fibers and inner axons were calculated by Photoshop CS6. Statistical significance for g-ratio analysis was analyzed with SigmaStat using the Mann-Whitney (rank sum) test. Statistical significance was set at P<0.05.
Western blotting
Spinal cord tissues were removed from the anesthetized mice and lysed in tissue lysis buffer (Sigma) with protease inhibitor cocktail (Sigma). A 20 or 40 g protein from control and transgenic mouse tissues were loaded for SDS-PAGE electrophoresis and subsequently detected with rabbit anti-MBP (Millipore) according to the standard protocol. β-actin was used as an internal control.
Electrophysiology
All transcranial magnetic motor-evoked potential (tcMMEP) recordings were obtained from awake, non-anesthetized, restrained mice. To perform the tcMMEPs in wild-type and PTPRD mutant mice (n = 4), the edge of the 5 cm diameter stimulating coil was placed at the inion with the coil tilted at a 45° angle. A single maximal MS (100%) elicited descending motor potentials. Hind limbs were exposed through the stockinet with electrodes placed into both gastrocnemius muscles. Bipolar platinum needle electrodes were used, with the active electrode inserted into the muscle belly, and the reference electrode placed into the muscle tendon. The ground electrode was positioned into the tail. Interelectrode impedances were maintained below 3.5–5 KΩ. The amplifier gain ranged between 1 and 2 K, and the bandpass filter was 10–3000 Hz. Evoked responses were displayed on the Cadwell Excel monitor (Cadwell Laboratories, Kennewick, WA).
Beam walking assay
Mice were evaluated using a graded series of metal beams (24.13 cm in length) of various widths: 2, 1.6, 1.2, 0.8, and 0.4 cm. The beams were suspended (15 cm high) across a plastic tank filled with 5–6 cm of soft bedding. The narrowest beam each mouse could traverse was recorded, along with the number of errors across four trials. Hindpaw and whole-body falls were both counted as errors. Data for this test were obtained by taking the average of four trials per beam per animal.
Cuprizone treatment
Wild-type and PTPRD mutant littermates at age of 8–10 weeks were fed a diet of ground mouse chow containing 0.2% cuprizone (w/w) (biscyclohexane oxaldihydrazone; Sigma-Aldrich) ad libitum for 6 weeks to induce demyelination. Remyelination was assayed by returning mice to normal chow for 1 week following 6 weeks of cuprizone treatment. Control mice were maintained on a normal diet for the duration of the experiment.
RESULTS
PTPRD is selectively expressed in mature oligodendrocytes
To investigate the role of PTPRD in axonal myelination, we first examined its expression in the developing spinal cord by in situ RNA hybridization (ISH). Throughout embryonic development, PTPRD was mainly expressed in motor neurons in the ventral gray matter of the spinal cord (Fig. 1A, A′). However, starting at around postnatal day 1 (P1), PTPRD was detected in the white matter of the spinal cord (data not shown). Its expression was progressively increased between P3 and P15 (Fig. 1B–D) and then gradually down-regulated afterwards. By P30, PTPRD expression is restricted only to the gray matter region (Fig 1E, E′). The marked up-regulation of PTPRD in white matter between P7 and P15 when oligodendrocytes undergo active myelination suggested that this receptor molecule is likely expressed in mature myelinating oligodendrocytes. This possibility was examined by PTPRD ISH followed by anti-APC immunohistochemical staining (Fig 2A–C). In the CNS, APC is specifically expressed in differentiated oligodendrocytes (Bhat et al., 1996, Southwood et al., 2004). Double labeling experiments revealed that many PTPRD+ cells co-expressed APC in P7 spinal cord tissue (Fig 2 A–B), especially in the white matter region. In addition, PTPRD+/APC+ double positive cell are also found in the white matter (corpus callosum) of the forebrain (Fig 2C). Thus, PTPRD is selectively expressed in differentiated oligodendrocytes in the developing CNS.
Figure 1.
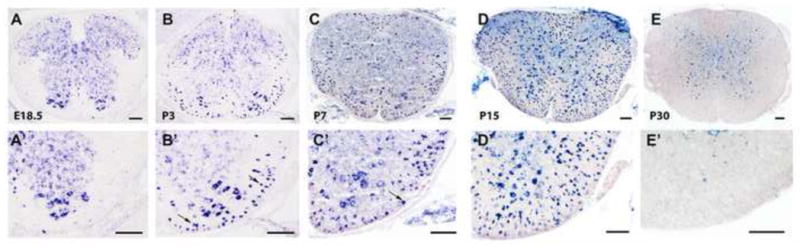
Spatiotemporal expression of PTPRD in the developing spinal cord. Spinal cord sections from E18.5 (A,A′), P3 (B,B′), P7 (C,C′), P15 (D,D′) and P30 (E,E′) wild-type animals were subject to in situ RNA hybridization (ISH) with PTPRD riboprobes. Arrows show the PTPRD+ cells in the white matter. A′-E′, higher magnification of the ventrolateral region of A–E. Scale bars: 100 μm.
Figure 2.
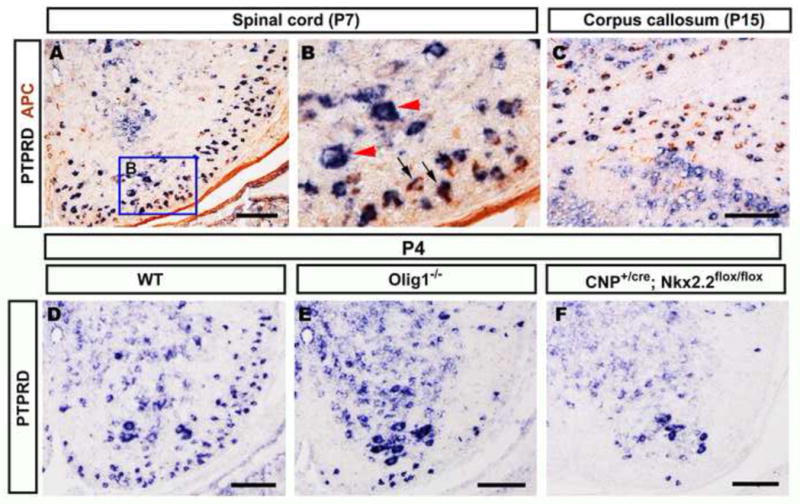
Selective expression of PTPRD in differentiated oligodendrocyte lineage. A–C, P7 wild-type mouse spinal cord and corpus callosum sections were subjected to PTPRD ISH followed by anti-APC immunohistochemical staining. Double positive cells are represented by blue arrows; PTPRD positive only motor neurons are indicated by red arrowheads (B). D–F, P4 spinal cords from wild-type (D), Olig1−/− (E) and CNP+/cre;Nkx2.2fl/fl (F) were hybridized with PTPRD riboprobe. PTPRD expression in the white matter region was dramatically reduced in Olig1−/− and CNP+/cre;Nkx2.2fl/fl mutants. Scale bar: 100 μm.
To further confirm the selective expression of PTPRD in mature oligodendrocytes, we also examined the PTPRD expression in the Olig1 and Nkx2.2 mutant spinal cords, in which the differentiation, but not the generation, of OPCs is inhibited (Qi et al., 2001, Lu et al., 2002, Zhu et al., 2014). Consistent with the idea that PTPRD was expressed in mature oligodendrocytes, expression of this receptor was significantly diminished in the white matter of P4 spinal cords of both Olig1−/− and CNP+/cre;Nkx2.2flox/flox mutants (Fig. 2 D–F). Expression of PTPRD in motor neurons in the gray matter was not affected.
Oligodendrocytes differentiate normally in PTPRD mutant mice
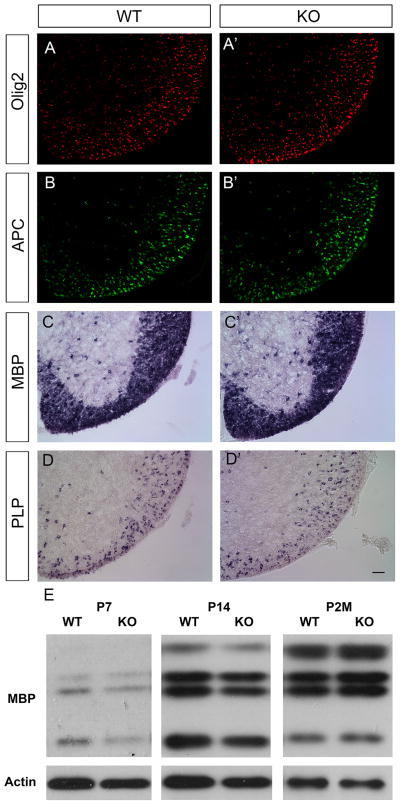
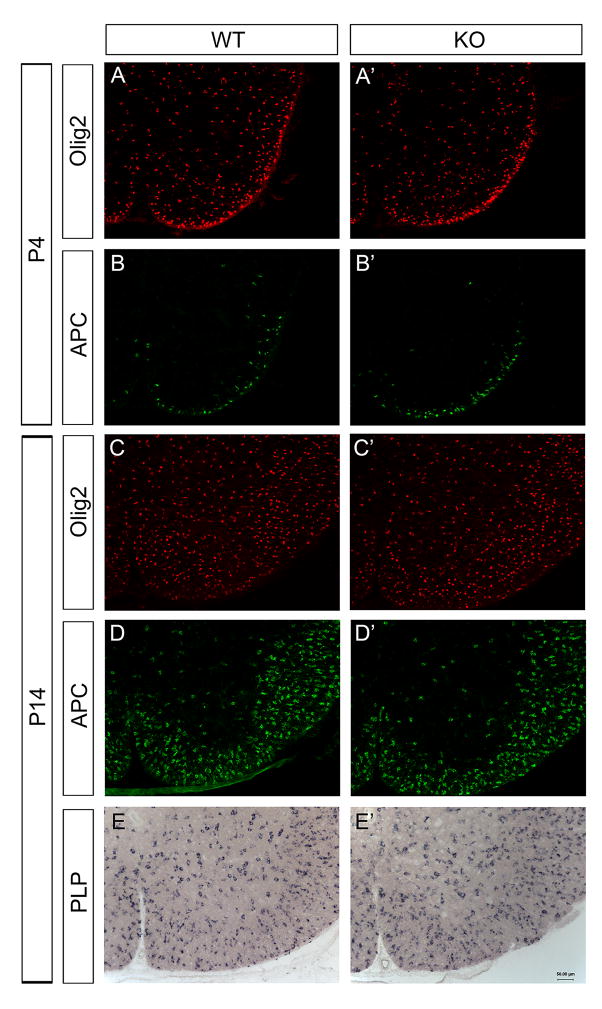
The selective expression of PTPRD in differentiated oligodendrocytes raised the possibility that PTPRD may participate in the oligodendrocyte maturation and myelination process. Therefore, we investigated the expression of several mature markers including APC, MBP and PLP in the PTPRD mutant spinal cords. No apparent difference was detected between wild-type and mutants from P4 to P14 (Fig. 3A–D′, Fig. 4). Comparable MBP expression in wild-type and mutant spinal cords was further verified with Western immunoblotting from P7 up to postnatal 2 months (Fig. 3E). These data suggest that mutation of PTPRD did not significantly affect oligodendrocyte differentiation and myelin gene expression.
Figure 3.
Normal oligodendrocyte differentiation and myelin gene expression in PTPRD mutants. Traverse spinal cord sections from P7 wild-type (A, B, C, D) and PTPRD knock-out (A′, B′, C′, D′) littermates were subjected to immunostaining with anti-Olig2 (A, A′) or anti-APC (B, B′), and in situ hybridization with MBP (C, C′) or PLP (D, D′). Scale bar: 50 μm. E, Western immunoblotting of postnatal day 7, 14 and 2 months spinal cord extracts from wild-type and PTPRD mutants with anti-MBP and anti-β-actin antibodies. Comparable MBP expression was detected in PTPRD knock-out mice as compared to wild-type littermates.
Figure 4.
Normal differentiation and maturation of oligodendrocytes in PTPRD mutant mice. Traverse spinal cords sections from P4 wild-type (A, B) and PTPRD knock-out (A′, B′) littermates were immunostained with anti-Olig2 (A, A′), anti-APC (B, B′). Similarly, spinal cords sections from P14 wild-type (C, D, E) and PTPRD knock-out (C′, D′, E′) were subjected to immunostaining with anti-Olig2 (C, C′) or anti-APC (D, D′), and in situ hybridization with PLP (E, E′). No significant difference is detected between wild-type and knock-out mutants. Scale bar: 50 μm.
PTPRD mutation results in a transient axonal myelination defect
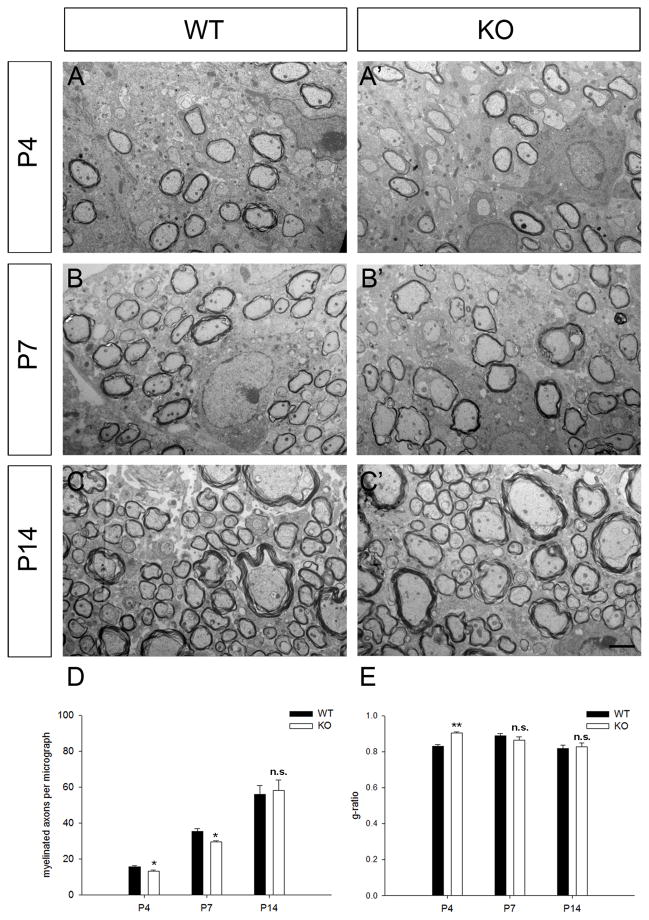
Next we analyzed the myelin ensheathment in PTPRD mutants (Fig. 5). Axons in mouse spinal cord started to be myelinated as early as at P4 (Fig. 5A, A′), and fewer axons were myelinated in PTPRD mutant spinal cords at P4 and P7 (Fig. 5D). Also, g-ratio (the ration of axon diameter/fiber diameter) was increased significantly in PTPRD mutants (0.9±0.006) as compared to wild-type (0.82±0.01) at P4, indicating the reduced thickness of myelin sheaths in the mutants (Fig. 5E). Interestingly, no significant difference was found in the number of myelinated axons and g-ratio between wild-type and PTPRD mutant starting from P14 (Fig. 5D). Thus, there appears to be a mild delay of CNS myelination in the spinal cords of PTPRD mutant mice.
Figure 5.
Axon myelination defects in the spinal cords of PTPRD mutants. A–C′, EM analysis of myelinated fibers in spinal cord sections from wild type and PTPRD mutant mice at P4 (A-A′), P7 (B-B′) and P14 (C-C′). D, Percentage of myelinated axons per micrograph in the wild type and mutant mice at different developmental stages (n=3). E, g-ratio of fibers in the wild-type and mutant mice at various stages. Data were represented as Mean±S.E.M. *, p < 0.05. **, p < 0.01. n.s., not significant. Scale bar: 2 μm.
Normal response of adult PTPRD mutant mice in the electrophysiological experiments and behavior tests
We also performed transcranial magnetic motor-evoked potential (tcMMEP) in adult PTPRD knockout mice as a means to evaluate conduction through long descending motor pathways (Fehlings MG, 1987, Magnuson DS, 1999, Loy DN, 2002, Cao Q and SR, 2005a, Cao Q, 2005b, Beaumont E, 2006). No remarkable difference in latency (Fig. 6A) and amplitude (Fig. 6B) was found between PTPRD knockout and wild-type littermates. Similarly, locomotor function assessed by beam walking behavior score did not change in PTPRD mutant group (Fig. 6C).
Figure 6.
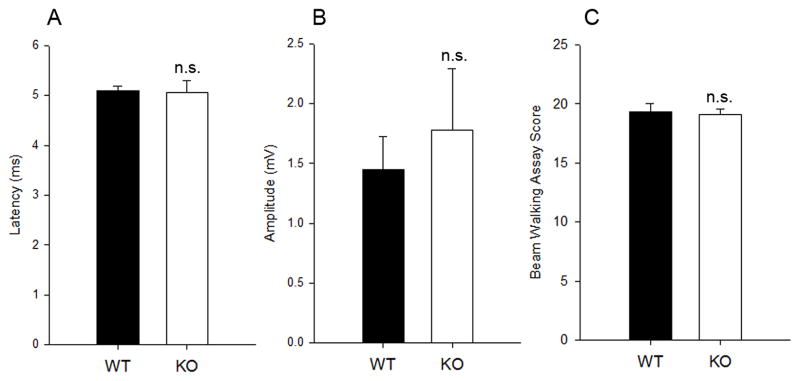
Electrophysiological and behavior test in PTPRD mutants. A, B, Latencies and amplitudes of the tcMMEP recordings from adult wild-type and PTPRD mutants. C, Beam walking assay. Data were represented as Mean±S.D., n=4 animals. n.s., not significant.
Regenerated OPCs differentiate normally in a cuprizone-induced demyelination model
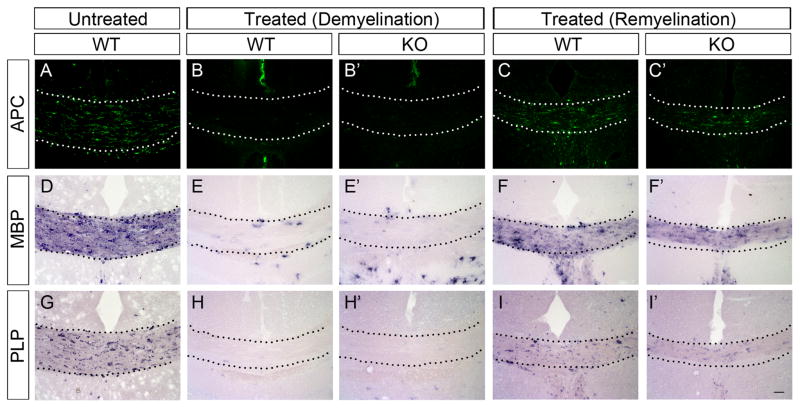
To study the possible function of PTPRD in axonal demyelination and remyelination, we created a chemical-induced demyelination model through cuprizone treatment. We observed an increased expression of PTPRD in regenerated oligodendrocytes during remyelination stage, suggestiong potential roles of PTPRD in differentiation of regenerated OPCs (Fig. 7). After being fed with cuprizone food for 6 weeks, both wild-type and PTPRD mutant mice exhibited a severe demyelination of the corpus callosum (Fig. 8B-B′, E-E′, H-H′). After one week of recovery by removing the cuprizone food, comparable APC, MBP and PLP expression were detected in corpus callosum of mutants (Fig. 8C-C′, F-F′, I-I′). These results indicated that PTPRD is not essential for axonal demyelination and remyelination.
Figure 7.
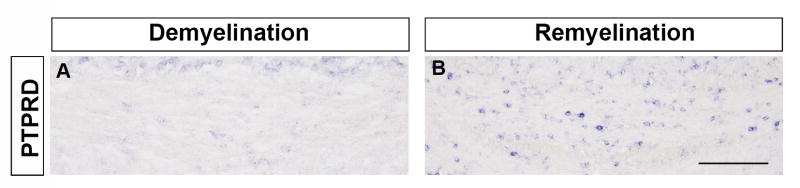
Elevated expression of PTPRD in regenerated oligodendrocytes during remyelination. In situ hybridization with PTPRD probe in the corpus callosum in cuprizone-treated mice during demyelination (A) and remyelination (B). Scale bar: 50 μm.
Figure 8.
Similar OPC differentiation and myelin gene expression in PTPRD mutants during remyelination. Immunostaining of anti-APC (B-B′), in situ hybridization with MBP (E-E′) or PLP probes (H-H′) showed severe demyelination of the corpus callosum (delimited by dotted lines) in wild-type (B, E, H) and PTPRD mutant (B′, E′, H′) mice after 6 weeks cuprizone treatment. Compared with wild-type mice, similar expression of APC (C,C′), MBP (F,F′), and PLP (I,I′) was observed in the corpus callosum area of PTPRD mutant mice after recovery for 1 week. Scale bar: 50 μm.
DISCUSSION
The level of protein tyrosine phosphorylation inside cells is exquisitely balanced by the activities of both protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). PTPs are originally believed to terminate signaling pathways initiated by PTKs. However, now it has been appreciated that through removing inhibitory phosphates, they can also activate kinases and other enzymes (Sallee et al., 2006). Mounting evidence suggests that PTPs are involved in oligodendrocyte maturation and axonal myelination. Many PTPs, including SHP-1, PTPα, PTPβ/ζ, PTPε and PTPγ, are reported to be expressed in oligodendrocyte cells (Levy et al., 1993, Sahin et al., 1995, Ranjan and Hudson, 1996, Massa et al., 2000). Similarly, the present study demonstrated that PTPRD is exclusively expressed in myelinating oligodendrocytes.
Functional analyses revealed that mice with deficiency in SHP-1 display diminished myelination in the spinal cord (Massa et al., 2004). PTPβ/ζ mutant mice exhibit reduced survival of mature oligodendrocytes following inflammatory demyelination (Harroch et al., 2000, Harroch et al., 2002). Furthermore, broad-spectrum blockade of PTP activity in vitro prevents oligodendrocyte differentiation (Ranjan and Hudson, 1996). Given the fundamental function of other PTP members in oligodendrocyte development, it is conceivable that PTPRD exclusively expressed in myelinating oligodendrocytes might involve reversible phosphorylation process and control oligodendrocyte myelination. In addition, PTPRD is known to bind homophilically in vitro, and this homophilic interaction serves to promote neurite outgrowth and adhesion in cultured forebrain neurons (Wang and Bixby, 1999). Since PTPRD is expressed in both axons and myelinating oligodendrocytes and is capable of binding homophilically, it is plausible that PTPRD participates in the axonal-oligodendroglial adhesion and regulates myelin sheath formation by homophilic interaction.
Similar expression of mature oligodendrocyte markers in postnatal spinal cords of wild-type and PTPRD mutants (Fig. 3–4) suggests that PTPRD is not essential for oligodendrocyte differentiation, in keeping with the observation that it is only up-regulated in mature oligodendrocytes. However, in PTPRD mutants, there is a mild defect in the early CNS myelination (Fig. 5), suggesting PTPRD may play an important role in initiating axon-oligodendrocyte recognition and conveying extracelluar signals inside to start myelination process. Intriguingly, this myelination defect is transient (overcome by P14). Consistently, adult PTPRD homozygous mutants are viable and display no gross motional problems. Electrophysiological and behavior studies did not detect significant changes in axonal electrical transmission in adult PTPRD mutant littermates (Fig. 6).
The cuprizone model is widely used to study experimental demyelination and remyelination in rodents. Spontaneous remyelination occurs in the corpus callosum after removal of the toxin (Matsushima and Morell, 2001, Liu et al., 2010). In our study, a complete demyelination was induced in the corpus callosum area after 6-week cuprizone treatment (Fig. 8B–H′). However, we did not find significant differences in differentiation and myelin gene expression of newly generated OPCs between PTPRD mutant and wild-type mice during the first week recovery (Fig. 8C–I′).
Despite the strong up-regulation of PTPRD expression in mature myelinating oligodendrocytes, the relatively mild phenotype in axonal myelination has raised the possibility of functional redundancy from other related molecules. It is known that RPTPs are composed of a large family, and the loss of function of one member could be easily compensated by the presence of other family members. For example, PTPRD and PTPRS have been proven to have complementary function in axon targeting in the developing mammalian nervous system (Uetani et al., 2006). In addition, non-receptor protein tyrosine phosphatase such as SHP2 has also been implicated in the control of oligodendrocyte differentiation and myelination formation (Zhu et al., 2010). Future studies are needed to investigate these possible functional redundancies in axonal myelination with compound mutants. In addition, many other surface adhesion molecules including integrin, Necl1, Necl4 have been implicated in axon-oligodendrocyte interaction (Buttery PC 1999, Park J 2008, Barros CS 2009, Laursen LS 2011, Zhu Y 2013). Thus, axonal myelination in the CNS appears to be controlled by multiple surface factors, and this could explain why disruption of one particular factor leads to mild or no phenotype in CNS myelination.
Conclusion
Here we describe the selective expression of PTPRD in mature oligodendrocytes and its contribution to initial myelination during development. PTPRD mutation does not significantly alter oligodendrocyte differentiation in mice, suggesting possible function redundancy from other axon-oligodendrocyte interaction factors or RPTP family members.
PTPRD is selectively expressed in mature oligodendrocytes.
Mutation of PTPRD delays initial myelination during development.
Mutation of PTPRD does not significantly affect the differentiation of oligodendrocytes.
Acknowledgments
This work is supported by NIH (R01NS37717); The National Basic Research Program of China (973 Program 2013CB531300); National Natural Sciences Foundation of China (Grant No: 31201697; 31372150; 31471955) and Kentucky Spinal Cord and Head Injury Trust.
Footnotes
AUTHOR CONTRIBUTIONS
QZ: study designs, performed experiments, data analysis and interpretation, wrote the manuscript with MQ; MQ: study designs, data analysis and interpretation; ZT, SZ, HH, XZ: performed PTPRD ISH combined with IHC; HX: EM microscopy; YZ, CS: design and implementation of electrophysiological and behavior experiments; NU: contributed toward PTPRD mutant study design.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barros CSNT, Spencer KS, Nishiyama A, Colognato H, Müller U. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136:2717–2724. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Beaumont EOS, Reed WR, Magnuson DS. Magnetically evoked inter-enlargement response: an assessment of ascending proprio- spinal fibers following spinal cord injury. Exp Neurol. 2006;201:428–440. doi: 10.1016/j.expneurol.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- Buttery PCf-CC. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Molecular and cellular neurosciences. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Cao QXX, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005b;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao QZY, Iannotti C, DeVries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005a;191(Suppl 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Fehlings MGT, Linden RD, Piper IR. Motorevoked potentials recorded from normal and spinal cord-injured rats. Neurosurgery. 1987;20:125–130. doi: 10.1097/00006123-198701000-00027. [DOI] [PubMed] [Google Scholar]
- Gershon TR, Baker MW, Nitabach M, Macagno ER. The leech receptor protein tyrosine phosphatase HmLAR2 is concentrated in growth cones and is involved in process outgrowth. Development. 1998;125:1183–1190. doi: 10.1242/dev.125.7.1183. [DOI] [PubMed] [Google Scholar]
- Haj F, McKinnell I, Stoker A. Retinotectal ligands for the receptor tyrosine phosphatase CRYPalpha. Mol Cell Neurosci. 1999;14:225–240. doi: 10.1006/mcne.1999.0785. [DOI] [PubMed] [Google Scholar]
- Harroch S, Furtado GC, Brueck W, Rosenbluth J, Lafaille J, Chao M, Buxbaum JD, Schlessinger J. A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions. Nat Genet. 2002;32:411–414. doi: 10.1038/ng1004. [DOI] [PubMed] [Google Scholar]
- Harroch S, Palmeri M, Rosenbluth J, Custer A, Okigaki M, Shrager P, Blum M, Buxbaum JD, Schlessinger J. No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase beta. Mol Cell Biol. 2000;20:7706–7715. doi: 10.1128/mcb.20.20.7706-7715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature genetics. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Laursen LSCC, Ffrench-Constant C. Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K. The Journal of cell biology. 2011;192:797–811. doi: 10.1083/jcb.201007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JB, Canoll PD, Silvennoinen O, Barnea G, Morse B, Honegger AM, Huang JT, Cannizzaro LA, Park SH, Druck T, et al. The cloning of a receptor-type protein tyrosine phosphatase expressed in the central nervous system. J Biol Chem. 1993;268:10573–10581. [PubMed] [Google Scholar]
- Liu L, Belkadi A, Darnall L, Hu T, Drescher C, Cotleur AC, Padovani-Claudio D, He T, Choi K, Lane TE, Miller RH, Ransohoff RM. CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: relevance to multiple sclerosis. Nat Neurosci. 2010;13:319–326. doi: 10.1038/nn.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DNMD, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Magnuson DSTT, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Massa PT, Saha S, Wu C, Jarosinski KW. Expression and function of the protein tyrosine phosphatase SHP-1 in oligodendrocytes. Glia. 2000;29:376–385. [PubMed] [Google Scholar]
- Massa PT, Wu C, Fecenko-Tacka K. Dysmyelination and reduced myelin basic protein gene expression by oligodendrocytes of SHP-1-deficient mice. J Neurosci Res. 2004;77:15–25. doi: 10.1002/jnr.20155. [DOI] [PubMed] [Google Scholar]
- Mastracci TL, Lin CS, Sussel L. Generation of mice encoding a conditional allele of Nkx2.2. Transgenic research. 2013;22:965–972. doi: 10.1007/s11248-013-9700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Hayes JE, Dyer KL, Sussman CR. Mechanisms of oligodendrocyte commitment in the vertebrate CNS. Int J Dev Neurosci. 1999;17:753–763. doi: 10.1016/s0736-5748(99)00068-4. [DOI] [PubMed] [Google Scholar]
- O’Grady P, Thai TC, Saito H. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J Cell Biol. 1998;141:1675–1684. doi: 10.1083/jcb.141.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JLB, Chen T, Li H, Hu X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J, Peng X, Qiu M. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. The Journal of neuroscience. 2008;28:12815–12819. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Ranjan M, Hudson LD. Regulation of tyrosine phosphorylation and protein tyrosine phosphatases during oligodendrocyte differentiation. Mol Cell Neurosci. 1996;7:404–418. doi: 10.1006/mcne.1996.0029. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle NP, Yu WP, Hall AC. Origins of spinal cord oligodendrocytes: possible developmental and evolutionary relationships with motor neurons. Dev Neurosci. 1997;19:58–68. doi: 10.1159/000111186. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Smith HK, Sun T, Pringle NP, Hall A, Woodruff R. Oligodendrocyte lineage and the motor neuron connection. Glia. 2000;29:136–142. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Sahin M, Dowling JJ, Hockfield S. Seven protein tyrosine phosphatases are differentially expressed in the developing rat brain. J Comp Neurol. 1995;351:617–631. doi: 10.1002/cne.903510410. [DOI] [PubMed] [Google Scholar]
- Sallee JL, Wittchen ES, Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. The Journal of biological chemistry. 2006;281:16189–16192. doi: 10.1074/jbc.R600003200. [DOI] [PubMed] [Google Scholar]
- Schaapveld RQ, Schepens JT, Bachner D, Attema J, Wieringa B, Jap PH, Hendriks WJ. Developmental expression of the cell adhesion molecule-like protein tyrosine phosphatases LAR, RPTPdelta and RPTP sigma in the mouse. Mech Dev. 1998;77:59–62. doi: 10.1016/s0925-4773(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Sommer C, Bele S, Kiessling M. Expression of cerebellar specific glutamate and GABAA receptor subunits in heterotopic cerebellar grafts. Brain Res Dev Brain Res. 1997;102:225–230. doi: 10.1016/s0165-3806(97)00103-x. [DOI] [PubMed] [Google Scholar]
- Southwood C, He C, Garbern J, Kamholz J, Arroyo E, Gow A. CNS myelin paranodes require Nkx6–2 homeoprotein transcriptional activity for normal structure. J Neurosci. 2004;24:11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AW. Isoforms of a novel cell adhesion molecule-like protein tyrosine phosphatase are implicated in neural development. Mech Dev. 1994;46:201–217. doi: 10.1016/0925-4773(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Thompson KM, Uetani N, Manitt C, Elchebly M, Tremblay ML, Kennedy TE. Receptor protein tyrosine phosphatase sigma inhibits axonal regeneration and the rate of axon extension. Mol Cell Neurosci. 2003;23:681–692. doi: 10.1016/s1044-7431(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Tian YE, Wu LH, Mueller WT, Chung FZ. A Screening Strategy Based on Differential Binding of Ligand to Receptor and to Binding Proteins: Screening for Compounds Interacting with Corticotrophin-Releasing Factor-Binding Protein. J Biomol Screen. 1999;4:319–326. doi: 10.1177/108705719900400607. [DOI] [PubMed] [Google Scholar]
- Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, Yakura H, Asano M, Iwakura Y. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bixby JL. Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecular for CNS neurons. Mol Cell Neurosci. 1999;14:370–384. doi: 10.1006/mcne.1999.0789. [DOI] [PubMed] [Google Scholar]
- Wegner M. Transcriptional control in myelinating glia: the basic recipe. Glia. 2000;29:118–123. [PubMed] [Google Scholar]
- Zhang JS, Honkaniemi J, Yang T, Yeo TT, Longo FM. LAR Tyrosine Phosphatase Receptor: A Developmental Isoform Is Present in Neurites and Growth Cones and Its Expression Is Regional- and Cell-Specific. Mol Cell Neurosci. 1998;10:271–286. doi: 10.1006/mcne.1998.0663. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhao X, Zheng K, Li H, Huang H, Zhang Z, Mastracci T, Wegner M, Chen Y, Sussel L, Qiu M. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development. 2014;141:548–555. doi: 10.1242/dev.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YLH, Li K, Zhao X, An T, Hu X, Park J, Huang H, Bin Y, Qiang B, Yuan J, Peng X, Qiu M. Necl-4/SynCAM-4 is expressed in myelinating oligodendrocytes but not required for axonal myelination. PloS one. 2013;8:e64264. doi: 10.1371/journal.pone.0064264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Park J, Hu X, Zheng K, Li H, Cao Q, Feng GS, Qiu M. Control of oligodendrocyte generation and proliferation by Shp2 protein tyrosine phosphatase. Glia. 2010;58:1407–1414. doi: 10.1002/glia.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]