Abstract
Purpose
The study objectives are to examine prevalence of current smoking, and to assess the association of both health insurance (HI) and access to care with smoking cessation among cancer survivors.
Methods
We performed an analysis from a cross-sectional study of cancer survivors aged 18–64 years using nationally representative data from the 2009 Behavioral Risk Factor Surveillance System survey. We assessed the prevalence of current smoking among cancer survivors. Also, in a subset excluding never smokers, we assessed cessation status of cancer survivors operationalized as comparing current to former smokers.
Results
The study population (N=18,896) was predominantly 45–64 years of age, female, and white. The prevalence of current smoking was substantially greater among cancer survivors without HI (40.9 %) than for those with HI (19.5 %). Cancer survivors with no HI had 2-fold greater adjusted odds of not quitting cigarette smoking compared to those with HI. Among those with insurance, cancer survivors who did not have regular health care provider or could not see doctor due to cost or had their last routine checkup ≥1 year ago had 60–80 % fold greater adjusted odds of not quitting cigarette smoking compared to cancer survivors who had better access to health care.
Conclusions
Cancer survivors without HI have substantially greater current smoking rates compared with those with HI. Among cancer survivors with HI, those who experienced health care access-related problems had lower cessation rates than their counterparts.
Implications for Cancer Survivors
Smoking cessation needs to be recognized as a crucial component of preventive care for cancer survivors. Continuous patient engagement and cancer-patient-centered strategies are urgently needed to achieve optimal results for quit rates particularly for young cancer survivors who are most susceptible to current smoking.
Keywords: Cancer survivorship, Cigarette smoking, Health insurance, Access to care, Affordable Care Act, Health disparities
Introduction
Over the past 50 years, since the landmark US Surgeon General's report on “Smoking and Health” [2], tremendous progress has been achieved in smoking prevention and cessation, resulting in lower rates of smoking-related cancers, fewer premature deaths, and better lives for millions in the USA and around the world [2–6]. The prevalence of people identifying themselves as current smokers in the USA has more than halved [3, 7], and yet, smoking still remains an important public health problem. According to the Centers for Disease Control and Prevention (CDC), 18.1 % of the US adults were current smokers in 2012 [8]. Cigarette smoking is still the leading cause of preventable morbidity and mortality [9, 10] and is disproportionately prevalent among young, poor, and uninsured US adults [3, 11, 12].
There is growing interest in lifestyle choices and risk behaviors of cancer survivors. Advances in cancer screening and treatment over the past few decades have markedly increased the number of patients surviving cancer. Currently, there are 14 million cancer survivors in the USA, a number that is projected to reach 18 million by 2022 [13]. The Surgeon General's 2014 report was notable in that [14], for the first time, it addressed adverse health consequences of continued smoking in cancer patients and survivors. During treatment and cancer survivorship, cigarette smoking is associated with increased risk of all-cause and cancer-related mortality, second primary cancers, treatment-related complications and toxicities, and cardiovascular and respiratory problems, and poor quality of life [15–23]. Accordingly, the report called for the use of evidence-based smoking cessation strategies and emphasized the need for assessment of tobacco use in the high-risk population of cancer survivors [14].
While smoking rates are comparable among older adults with and without a history of cancer, the prevalence of current smoking stays at alarmingly high levels for young cancer survivors [24, 25]. In adults 18–44 years of age, almost two fifths (38.1 %) of cancer survivors are current smokers compared to 24.0 % in the general population [24]. Overall, continued smoking is associated with almost 3-fold excess risk of mortality, which could be avoided with appropriate cessation practices [26, 27]. However, health gains from smoking cessation are dependent upon age at quitting. For adults who successfully quit before age 44, the excess risk of death from smoking is <20 %, whereas this could be as high as 70 % for adults quitting between ages 44 and 64 years [26]. Unfortunately, many cancer survivors, predominantly the young, do not have any or adequate health care coverage [28–30], and consequently may not receive evidence-based smoking prevention and cessation care tailored to their needs.
Hopefully, with the Patient Protection and Affordable Care Act (ACA), many previously uninsured or underinsured nonelderly cancer survivors will gain access to health insurance and care, and benefit from the ACA's portfolio of mandates for prevention programs including improved access to smoking cessation services both in private and in public insurance coverage systems [31–33]. For targeted and effective smoking cessation interventions to be developed, it will be important to understand the patterns of current smoking by health insurance status and to assess the role of health care access in promoting smoking cessation among cancer survivors especially in those who could potentially benefit the most from smoking cessation, young adults with a history of cancer. To inform policy and practice, the objectives of the study are to report the prevalence of current cigarette smoking and to examine the association of both (1) health insurance and (2) access to care with smoking cessation among young and middle age cancer survivors.
Methods
Data source
We performed a cross-sectional study using data from the 2009 Behavioral Risk Factor Surveillance System (BRFSS) survey. The BRFSS is the largest random-digit telephone health survey in the world and is administered annually to more than 350,000 adults living in the 50 states of the USA, and in the District of Columbia, American Samoa, Palau, Puerto Rico, the US Virgin Islands, and Guam [34]. The BRFSS questionnaire collects data from survey participants on sociodemographic characteristics, health behaviors, preventive health practices, and health care access [35]. The BRFSS data files contain sample weights that can be used so that the analyses are representative of the US population [36]. The study was reviewed and approved by the University of Maryland Institutional Review Board (IRB).
Study population
Our study population included cancer survivors aged 18 to 64 years. Cancer survivors were identified as respondents who answered “yes” to the question “Have you ever been told by a doctor, nurse, or other health professional that you had cancer?” Using the American Cancer Society's definition of cancer survivors [37], our study population did not include subjects with nonmelanoma skin cancers. Subjects who responded “Other skin cancer” (nonmelanoma skin cancer) to the question, “With your most recent diagnosis of cancer, what type of cancer was it?”, were excluded from our analyses. Nonmelanoma skin cancers, which are most often basal cell or squamous cell skin cancers, almost never metastasize, are treated differently than melanoma skin cancers, and are not included in state cancer counts.
Smoking status
Smoking status was categorized according to the subject's responses to two questions. Participants who responded “no” to the question, “Have you smoked at least 100 cigarettes in your entire life?”, were classified as never smokers. Those who responded “yes” were asked “Do you now smoke cigarettes every day, some days, or not at all?” Participants who responded “every day or some days” were categorized as current smokers; those who responded “not at all” were categorized as former smokers.
Health insurance and access to care
Our primary independent variable, health insurance status, was classified as insured vs. noninsured according to the response to the question, “Do you have any kind of health care coverage, including health insurance, prepaid plans such as HMOs, or government plans such as Medicare?”.
Three additional questions were used to ascertain the access to routine health care. These questions asked respondents (1) “Do you have at least one person you think of as your personal doctor or health care provider?”, (2) “Was there a time in the last year you needed to see a doctor but could not because of cost?”, and (3) “About how long has it been since you last visited a doctor for a routine checkup?”.
Sociodemographic characteristics and cancer type
Demographic data used in the analyses included age group (18–44, 45–64 years), sex, race/ethnicity (white, African American, Hispanic, other), education status (<high school, high school, >high school), employment status (employed, out of work, other [retired, unable to work, student, homemaker]), household income (<$25 k, $25–50 k, >$50 k), and US census region (Northeast, Midwest, West, South, other [Puerto Rico, the US Virgin Islands, and Guam]). Type of cancer was determined by the survivors' response to a question asking them the type of cancer with which they were most recently diagnosed.
Statistical methods
Cancer survivors with missing information for smoking status, health insurance status, or age were excluded from the study sample. Dummy variables were created for the other variables that had missing information and were included in the regression analyses. Estimates for these dummy variables are not reported as they are not meaningfully interpretable.
Differences in sociodemographic characteristics of the cancer survivors by health insurance status were assessed using weighted chi-squared analyses. We used weighted multivariable Poisson regression with a robust error variance [38], adjusted for sociodemographic characteristics, to determine the prevalence of being a current smoker within age- and health insurance-specific groups.
Among the three smoking groups that we studied (never, former, and current smokers), only two of the groups, former and current smokers, had the opportunity to try to quit their smoking habit. In order to assess smoking cessation status of cancer survivors, we calculated the odds of being a current smoker vs. former smoker in the subset of cancer survivors that excluded never smokers. We used weighted multivariable logistic regression, adjusted for sociodemographic characteristics, to determine the effect of having health insurance on smoking cessation. Furthermore, among subjects with health insurance from this subset, we used weighted logistic regression to explore the association between other health care access variables and smoking cessation. SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used for all analyses in this study.
Results
Sociodemographic characteristics of cancer survivors
Of 432,607 respondents in the 2009 BRFSS database, 24,485 were identified as cancer survivors who were 18–64 years of age. Nonmelanoma skin cancer survivors (N=5515) and participants with missing data for their smoking or health insurance status were excluded yielding a sample of 18,896 cancer survivors.
Among the study population, 11.0 % of the cancer survivors did not have any health care coverage (Table 1). Consistent with the national estimates for cancer survivors [37], the study sample was predominantly 45–64 years of age (72.4 %), female (65.4 %), and white (76.5 %). More than half of the subjects were high school graduates (55.0 %), and a similar proportion were employed (56.3 %). Almost half of the cancer survivors had an annual income of $50,000 or more. Cancers in female or male reproductive organs, breast cancer, and melanoma were the most common cancers reported.
Table 1. Characteristics of cancer survivors aged 18–64 years by health insurance status, 2009, N=18,896.
| Total, N=18,896 | With insurance, N=16,790 | No insurance, N=2106 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Characteristic | N | Weighted %a | N | Weighted %a | N | Weighted %a | Chi-squared P value |
| Smoking status | <0.001 | ||||||
| Current smoker | 4244 | 22.0 | 3418 | 19.5 | 826 | 40.9 | |
| Former smoker | 5927 | 28.9 | 5362 | 29.8 | 565 | 22.7 | |
| Never smoker | 8725 | 49.0 | 8010 | 50.7 | 715 | 36.5 | |
| Age group | <0.001 | ||||||
| 18–44 years | 3101 | 27.6 | 2576 | 25.6 | 525 | 42.4 | |
| 45–64 years | 15,795 | 72.4 | 14,214 | 74.4 | 1581 | 57.6 | |
| Sex | 0.14 | ||||||
| Female | 13,646 | 65.4 | 12,007 | 65.0 | 1639 | 68.4 | |
| Male | 5250 | 36.4 | 4783 | 35.0 | 467 | 31.6 | |
| Race/ethnicity | <0.001 | ||||||
| White | 15,577 | 76.5 | 14,015 | 78.0 | 1562 | 65.3 | |
| African American | 1204 | 8.6 | 999 | 8.2 | 205 | 12.2 | |
| Hispanic | 900 | 8.3 | 744 | 7.6 | 156 | 13.0 | |
| Otherb | 1215 | 6.6 | 1032 | 6.2 | 183 | 9.4 | |
| Educationc | <0.001 | ||||||
| < High school | 1388 | 8.1 | 1032 | 6.7 | 306 | 18.1 | |
| High school | 10,679 | 55.0 | 9272 | 54.0 | 1407 | 62.9 | |
| > High school | 6865 | 36.9 | 6474 | 39.3 | 391 | 18.9 | |
| Employment | <0.001 | ||||||
| Employed | 10,508 | 56.3 | 9523 | 60.0 | 985 | 44.0 | |
| Out of work | 1376 | 9.3 | 934 | 7.2 | 442 | 24.7 | |
| Otherd | 7012 | 34.3 | 6333 | 34.8 | 679 | 31.3 | |
| Household incomee | <0.001 | ||||||
| <$25 k | 4893 | 22.6 | 3697 | 18.5 | 1196 | 53.2 | |
| $25–49 k | 4278 | 20.0 | 3833 | 20.0 | 445 | 20.6 | |
| >$50 k | 7959 | 48.0 | 7716 | 52.5 | 243 | 14.6 | |
| US region | <0.001 | ||||||
| Northeast | 4295 | 20.4 | 4013 | 21.2 | 282 | 14.3 | |
| Midwest | 4241 | 22.2 | 3819 | 22.7 | 422 | 18.2 | |
| West | 4894 | 20.7 | 4355 | 20.7 | 539 | 20.7 | |
| South | 5240 | 35.9 | 4399 | 34.5 | 841 | 46.6 | |
| Otherf | 226 | 0.8 | 204 | 0.8 | 22 | 0.2 | |
| Cancer typeg | <0.001 | ||||||
| Breast | 3930 | 17.5 | 3599 | 18.5 | 331 | 9.8 | |
| Gastrointestinal | 1228 | 6.3 | 1113 | 6.5 | 115 | 4.7 | |
| Head and neck | 1050 | 5.5 | 954 | 5.6 | 96 | 4.5 | |
| Leukemia/lymphoma | 911 | 6.6 | 839 | 6.9 | 72 | 4.1 | |
| Lung | 385 | 2.2 | 357 | 2.4 | 28 | 0.7 | |
| Melanoma | 2475 | 12.9 | 2271 | 13.4 | 204 | 9.7 | |
| Reproductive | 5679 | 30.3 | 4803 | 28.4 | 876 | 44.1 | |
| Otherg | 3238 | 18.8 | 2854 | 18.3 | 384 | 22.3 | |
Weighted percentages were estimated by using sample weights so that the findings are representative of the US population
Other (race/ethnicity) includes participants of non-Hispanic other race only, non-Hispanic multiracial, or participants with unknown/missing information for race/ethnicity
Thirty-six participants had missing information for education
Other (employment) includes participants who were retired, student, homemaker, unable to work, or had missing information for employment
Of participants, 9.4 % (N=1766) had missing information for annual household income
Other (region) includes participants who were residents of Puerto Rico, the US Virgin Islands, or Guam, or had missing information for region of residence
For participants diagnosed with >1 type of cancer, cancer type relates to the most recent diagnosis of cancer
Other (cancer type) includes participants with diagnosis of other cancers or with missing/unknown information for cancer type
Compared to cancer survivors with health insurance, cancer survivors with no health insurance represented a greater proportion of young subjects, 18–44 years old (42.4 vs. 25.6 %), nonwhites (34.7 vs. 22.0 %), and residents of Southern states (46.6 vs. 34.5 %). Having less than high school education (18.1 vs. 6.7 %), being out of work (24.7 vs. 7.2 %), and having annual household income less than $25,000 (53.2 vs. 18.5 %) were more common among cancer survivors with no health insurance than those with insurance.
Prevalence of current smoking
Although the prevalence of current smoking among cancer survivors with health insurance (19.5 %, Table 1) was similar to that seen in the entire US adult population in 2009 (20.6 %) [39], the prevalence in cancer survivors without insurance, 40.9 %, was twice that seen in the US population. The adjusted prevalence of current smoking was higher in cancer survivors without health insurance than those with health insurance in both of the two age-groups we studied (Fig. 1).
Fig. 1.
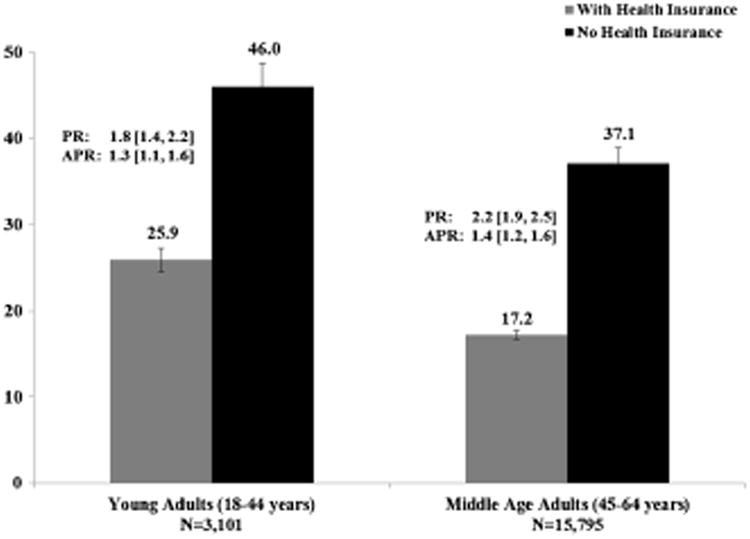
Age group specific prevalence of current smoking among cancer survivors by health insurance status, 2009, N= 18,896. PR prevalence ratio (weighted), APR adjusted prevalence ratio (weighted)— adjusted for age group, insurance status, an interaction term between age group and insurance status, sex, race/ethnicity, education, employment, household income, US region
Health insurance and smoking cessation
Table 2 reports proportion and odds of being a current smoker vs. former smoker in a subset of cancer survivors that excluded never smokers. Overall, 18–44-year olds had greater proportion of current smokers compared with 45–64-year olds. The proportion of current smokers was profoundly greater among cancer survivors without health insurance (78.1 % in 18–44-year olds; 55.4 % in 45–64-year olds) than cancer survivors with health insurance (57.3 % in 18–44-year olds; 34.1 % in 45–64-year olds), regardless of the age group. Of note, a vast majority of former smokers reported to have had ceased smoking more than 5 years ago at the time of survey (data not shown).
Table 2. Odds of being a current smoker (vs. being a former smoker) among cancer survivors aged 18–64 years, 2009, N=10,171.
| Current smokers | Former smokers | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Characteristic | N | Weighted %a | N | Weighted %a | OR | 95 % CI | AOR | 95 % CI |
| Insurance status × age group | ||||||||
| 18–44-year olds | ||||||||
| With Insurance | 723 | 57.3 | 492 | 42.7 | 1 | Reference | 1 | Reference |
| No Insurance | 249 | 78.1 | 86 | 21.9 | 2.7 | 1.7–4.3 | 2.0 | 1.2–3.2 |
| 45–64-year olds | ||||||||
| With Insurance | 2695 | 34.1 | 4870 | 65.9 | 1 | Reference | 1 | Reference |
| No Insurance | 577 | 55.4 | 479 | 44.6 | 2.6 | 2.1–3.2 | 1.6 | 1.2–2.1 |
| Sex | ||||||||
| Female | 3277 | 48.3 | 3903 | 51.7 | 1.8 | 1.5–2.1 | 1.4 | 1.2–1.7 |
| Male | 967 | 34.3 | 2024 | 65.7 | 1 | Reference | 1 | Reference |
| Race/ethnicity | ||||||||
| White | 3408 | 41.9 | 5056 | 58.1 | 1 | Reference | 1 | Reference |
| African American | 252 | 41.3 | 331 | 58.7 | 1.0 | 0.7–1.3 | 0.8 | 0.5–1.1 |
| Hispanic | 168 | 39.5 | 217 | 60.5 | 0.9 | 0.6–1.4 | 0.7 | 0.5–1.1 |
| Otherb | 416 | 63.5 | 323 | 36.5 | 2.4 | 1.8–3.2 | 2.0 | 1.5–2.6 |
| Educationc | ||||||||
| < High school | 572 | 60.6 | 412 | 37.4 | 1.7 | 1.3–2.3 | 1.2 | 0.9–1.6 |
| High school | 2889 | 47.2 | 3487 | 52.8 | 1 | Reference | 1 | Reference |
| > High school | 779 | 27.3 | 2023 | 72.7 | 0.4 | 0.4–0.5 | 0.6 | 0.5–0.7 |
| Employment | ||||||||
| Employed | 1877 | 39.4 | 3144 | 60.6 | 1 | Reference | 1 | Reference |
| Out of work | 461 | 53.6 | 410 | 46.4 | 1.8 | 1.4–2.3 | 1.0 | 0.8–1.3 |
| Otherd | 1906 | 45.3 | 2373 | 54.7 | 1.3 | 1.1–1.5 | 1.0 | 0.8–1.2 |
| Household incomee | ||||||||
| <$25 k | 1835 | 60.4 | 1421 | 39.6 | 3.6 | 3.0–4.4 | 2.3 | 1.8–2.8 |
| $25–49 k | 1011 | 45.5 | 1384 | 54.5 | 2.0 | 1.6–2.4 | 1.5 | 1.2–1.8 |
| >$50 k | 984 | 29.8 | 2584 | 70.2 | 1 | Reference | 1 | Reference |
| US region | ||||||||
| Northeast | 831 | 36.3 | 1536 | 63.7 | 1 | Reference | 1 | Reference |
| Midwest | 979 | 45.5 | 1289 | 54.5 | 1.5 | 1.2–1.8 | 1.3 | 1.0–1.7 |
| West | 962 | 36.7 | 1539 | 63.3 | 1.0 | 0.8–1.3 | 0.8 | 0.7–1.1 |
| South | 1445 | 49.1 | 1514 | 50.9 | 1.7 | 1.4–2.1 | 1.3 | 1.1–1.7 |
| Otherf | 27 | 32.0 | 49 | 68.0 | 0.8 | 0.3–2.1 | 0.8 | 0.3–2.3 |
Never smokers (N=8725) were excluded
OR odds ratio of being a current smoker (vs. being a former smoker), AOR adjusted odds ratio of being a current smoker (vs. being a former smoker)— adjusted for age group, insurance status, an interaction term between age group and insurance status, sex, race/ethnicity, education, employment, household income, US region, CI confidence interval
Weighted percentages were estimated by using sample weights so that the findings are representative of the US population
Other (race/ethnicity) includes participants of non-Hispanic other race only, non-Hispanic multiracial, or participants with unknown/missing information for race/ethnicity
Nine participants had missing information for education
Other (employment) includes participants who were retired, student, homemaker, unable to work, or had missing information for employment
Of participants, 9.4 % (N=952) had missing information for annual household income
Other (region) includes participants who were residents of Puerto Rico, the US Virgin Islands, or Guam, or had missing information for region of residence
Cancer survivors who were female (48.3 %), out of work (53.6 %), and residents of Midwestern (45.5 %) or Southern (49.1 %) states had greater proportion of current smokers compared with their counterparts. Being a current smoker was comparable across racial/ethnic groups. Cancer survivors without a high school diploma or with an annual household income<$25,000 had the highest level of current smoking (>60.0 %).
After adjusting for the sociodemographic differences, not having health insurance was strongly associated with being a current smoker, in both age groups. Among young adults, cancer survivors with no health insurance had 2-fold greater adjusted odds of being a current smoker (vs. being a former smoker) compared to those with health insurance. Adjusted odds ratio (AOR) of being a current smoker (vs. being a former smoker) was also significantly greater among female cancer survivors (AOR=1.4) and among cancer survivors who were residents of Southern or Midwestern states (AOR=1.3) when compared with their counterparts. Cancer survivors with annual household income less than $25,000 had >2-fold greater adjusted odds of being a current smoker (vs. being a former smoker).
Health care access and smoking cessation among the insured
In a subset of cancer survivors with health insurance who were either current or former smokers, health care access-related problems were commonly reported: 6.7 % did not have a regular health care provider, 15.2 % reported there was a time in the past year they could not see a provider due to cost, and more than 20 % had their last routine checkup ≥1 year ago (data not shown).
Despite having health insurance, about half of the cancer survivors who had a health care access-related problem reported not quitting smoking (Table 3). Cancer survivors who did not have regular a health care provider or could not see doctor due to cost had 1.8-fold greater adjusted odds of being a current smoker (vs. being a former smoker) compared with cancer survivors who did not experience such access-related problems. Among the insured, cancer survivors who had their last routine checkup ≥1 year ago was associated with increased current smoking (AOR=1.6) compared with cancer survivors who potentially had more frequent routine checkups.
Table 3. Association between access to routine care and not quitting smoking among cancer survivors aged 18–64 years with health insurance, 2009, N=8780.
| Current smoker | Former smoker | OR | 95 % confidence interval | AOR | 95 % confidence interval | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N | Weighted %a | N | Weighted %a | |||||
| Regular health care provider | ||||||||
| Yes | 3150 | 38.3 | 5099 | 61.7 | 1 | Reference | 1 | Reference |
| No | 268 | 56.3 | 263 | 43.7 | 2.1 | 1.5–2.9 | 1.8 | 1.2–2.7 |
| Cost-related access problem | ||||||||
| Yes | 717 | 58.5 | 566 | 41.5 | 2.5 | 2.0–3.1 | 1.8 | 1.5–2.3 |
| No | 2701 | 36.1 | 4796 | 63.9 | 1 | Reference | 1 | Reference |
| Last routine checkup | ||||||||
| Within past year | 2597 | 36.8 | 4348 | 63.2 | 1 | Reference | 1 | Reference |
| ≥1 year ago | 821 | 49.9 | 1014 | 50.1 | 1.7 | 1.4–2.1 | 1.6 | 1.3–2.1 |
The analytical sample only included the insured who were either former or current smokers (excluding never smokers)
OR odds ratio of being a current smoker (vs. being a former smoker), AOR adjusted odds ratio of being a current smoker (vs. being a former smoker) by access to care—adjusted for age group, sex, race/ethnicity, education, employment, household income, US region
Weighted percentages were estimated by using sample weights so that the findings are representative of the US population
Discussion
The recent US Surgeon General's report [14] and the American Association for Cancer Research policy statement [21] have drawn attention to the need for assessment of tobacco use and cessation efforts among cancer survivors. Current smoking is a serious public health problem and is disproportionality prevalent among young adults with a history of cancer [24, 29, 30, 40, 41]. However, public health efforts have mostly focused on the general population. In our study, we characterized current smoking rates and examined the role of health insurance and access to routine health care on smoking cessation using a nationally representative sample of community-dwelling cancer survivors aged <65 years. We demonstrate a 2-fold greater prevalence of current smoking among cancer survivors with no health insurance compared to those with health insurance. In the absence of health insurance, almost half of cancer survivors aged 18–44 years were current smokers, pointing out to an urgent need in systematic public health efforts in smoking cessation tailored to young cancer survivors.
To our knowledge, our paper is the first to examine the association between access to health care and smoking cessation in cancer survivors. Comparing current to former smokers in a subset excluding never smokers allows us to better characterize nonelderly cancer survivors with the greatest need for smoking cessation. Being uninsured was associated with more than 2-fold greater odds of not quitting smoking, and among the uninsured, more than three fourths of 18–44-year olds and more than half of 45–64-year olds did not quit smoking. Without access to evidence-based smoking cessation programs, these young cancer survivors are at heightened risk for developing second primary tumors, premature death, and excess morbidity. In addition, given the recent public health concerns over the expanded use, safety and effectiveness of over-the-counter smoking cessation products such as electronic cigarettes [42–45], there is a compelling need for evidence-based and cancer-patient-centered smoking cessation strategies.
Adults younger than 65 years of age constitute half of cancer survivors in the USA [40, 46], many of whom are burdened by loss or lack of health insurance. The 2010 Patient Protection and ACA offers provisions that provide coverage to millions of uninsured or underinsured young Americans and provisions designed to improve access to smoking cessation programs. Private insurance plans are now required to provide smoking cessation services to their enrollees, and state Medicaid programs can no longer exclude medications approved for smoking cessation from their coverage [31–33]. In addition, there are financial incentives for Medicaid programs, in the form of bonus payments in their federal matching funds, to cover tobacco cessation services [31, 33]. Starting in 2016, mandated “Essential Health Benefits” will be evaluated and reconsidered for coverage based on available evidence of medical effectiveness [47]. Our results support continued and expanded coverage of smoking cessation for cancer survivors and for all other insured adults.
Although the ACA has increased health care coverage, it presents challenges with respect to acquiring health insurance particularly among those with the greatest need for smoking cessation. For one, uninsured smokers now face higher annual premiums in obtaining a health insurance [48]. While such financial penalties were intended to encourage smoking cessation, they may actually discourage uninsured adults who are current smokers from signing up for a health insurance, which may then subsequently halt their access to care and evidence-based smoking cessation services. Cancer survivors are particularly vulnerable to such policies. Compared to adults with no history of cancer, cancer survivors represent a greater proportion of adults with low education attainment and low annual household income [30, 40]. Cancer survivors are at increased risk for unemployment [29, 46], and therefore at increased risk for losing their employer-sponsored health care coverage. Our study extends these associations by demonstrating that compared to those who had insurance, cancer survivors with no health insurance had lower educational attainment, lower household incomes, and higher unemployment rates. In our study, regardless of insurance status, these subgroups of cancer survivors had greater odds of not quitting smoking compared with their counterparts. Even with access to insurance, high out-of-pocket costs, in the form of deductibles, copay or coinsurance, that are built into the ACA coverage plans' benefit design, may impede continuity of care, decrease adherence to prescribed smoking cessation medications or decrease utilization of other services, such as cognitive behavioral therapy, particularly among adults of low socioeconomic status [33, 49].
Access to health insurance does not always translate into access to health care [50]. In a subset of cancer survivors who had health insurance, we show that those who did not have a personal doctor, or did not have a routine checkup within the last year, or could not see doctor due to cost had 60–80 % greater odds of not quitting smoking compared with cancer survivors who did not experience these health care access problems. As described in the Institute of Medicine (IOM) report [51], many cancer patients are “lost in transition” from the acute cancer treatment phase to long-term survivorship. The ACA has ambitious goals for transforming traditional fee-for-service care into patient-centered medical homes where interdisciplinary team of health care providers are actively engaged in providing comprehensive medical care to patients. As stated in the IOM report, health care delivery models incorporating prevention, surveillance, intervention, and coordination of care need to be examined and designed specifically for cancer survivors [51, 52]. In a survey of American Society of Clinical Oncology members [53], despite asking their patients about smoking status at the first visit, less than half of the physicians reported providing smoking cessation support. Continuous patient engagement and patient-centered strategies targeting cancer survivors are needed to achieve optimal results for quit rates [54–56].
Similar to smoking rates in the general population [8], nonelderly cancer survivors residing in the Southern or Midwestern states had the greatest rates of not quitting smoking compared with cancer survivors from other US regions. As of March 2014, 27 states including the District of Columbia are implementing Medicaid expansion in 2014 [57]. Among the remaining states that are either in an open debate or not moving forward with the implementation, almost all are located in Southern or Midwestern states. Of the 16 Southern states, only 5 are moving forward with the Medicaid expansion [57], resulting in yet another barrier that cancer survivors have to surmount if they are to gain access to care and smoking cessation services. An important finding of our study was the significantly higher ratio of current to former smoker seen in female cancer survivors compared to male cancer survivors; whereas in the entire US adult population [39], more men (23.5 %) are likely to be current smokers than women (17.9 %). Excessive smoking in women, particularly among young cancer survivors, has not been adequately addressed. The ACA provides new comprehensive preventive care coverage for women without cost-sharing requirements, including annual “well-woman” preventive care visits to determine what preventive services are needed [58]. Smoking cessation needs to be recognized as a crucial component of preventive care for cancer survivors and women in general.
Our study has several limitations. First, we did not use the most recent BRFSS data available as cancer survivorship questions were asked of the entire sample only in the 2009 questionnaire. Nevertheless, our data were collected within the past 5 years and provide nationally representative data for smoking rates among cancer survivors prior to the implementation of the ACA in 2010. In 2009, the BRFSS was exclusively a landline telephone survey, and hence, the findings may not be generalizable to cancer survivors who do not have a phone or have a cell phone but not a landline [59, 60]. In our study, we assessed cigarette smoking status and did not consider use of other tobacco products, which also are a growing public health problem. We also did not assess intensity of current smoking. Due to the fact that our data are self-reported, our findings might be subject to social-desirability bias on current smoking. This bias is unlikely to affect comparison of one group to another as we would expect the bias to be similar across comparison groups. Additionally, our cross-sectional data cannot assess temporality of events. Whether cessation occurred prior or after having insurance cannot be determined. Nevertheless, our findings suggest that possession of health insurance is strongly associated with current smoking. Furthermore, we could not ascertain whether our study subjects were either in acute treatment phase or in long-term survivorship care. However, more than 85 % of the study sample reported having their initial cancer diagnosis >2 years ago at the time of survey (data not shown).
In conclusion, cancer survivors without health insurance had substantially greater current smoking rates compared with cancer survivors who had health insurance. Furthermore, among cancer survivors with health insurance, those who did not have access to routine health care had lower smoking cessation rates than those who did not experience health care access-related problems. Given tobacco smoking is still the leading cause of morbidity and mortality in the USA, expanded and improved coverage of evidence-based smoking cessation services is needed in particular for young cancer survivors who are most susceptible to current smoking. Sustained public health efforts are warranted to improve health care delivery to vulnerable and high-risk populations of nonelderly cancer survivors.
Acknowledgments
The study was supported by the Baltimore VA GRECC (Geriatric Research, Education, and Clinical Center), Claude D. Pepper Older Americans Independence Center (NIA P30 AG028747 08), and the Mid Atlantic Nutrition Obesity Research Center (NIDDK 5P30DK072488-09).
Role of the sponsors No role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of interest The authors do not have any financial conflict of interest to disclose regarding the content of this study.
Author contributions Study concept and design: all authors
Acquisition, analysis, or interpretation of data: all authors
Drafting of the manuscript: Burcu
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: Burcu, Sorkin
Obtained funding: Sorkin
Administrative, technical, or material support: all authors
Study supervision: Steinberger, Sorkin
Contributor Information
Mehmet Burcu, Email: mburc001@umaryland.edu.
Eileen K. Steinberger, Email: eileensteinberger@gmail.com.
John D. Sorkin, Email: jsorkin@grecc.umaryland.edu.
References
- 1.Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: Public Health Service; 1964. [Accessed 21 March 2015]. http://profiles.nlm.nih.gov/ps/access/NNBBMQ.pdf. [Google Scholar]
- 2.Cole HM, Fiore MC. The war against tobacco: 50 years and counting. JAMA. 2014;311(2):131–2. doi: 10.1001/jama.2013.280767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holford TR, Meza R, Warner KE, Meernik C, Jeon J, Moolgavkar SH, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964-2012. JAMA. 2014;311(2):164–71. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370(1):60–8. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 5.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder SA, Koh HK. Tobacco control 50 years after the 1964 surgeon general's report. JAMA. 2014;311(2):141–3. doi: 10.1001/jama.2013.285243. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Trends in current cigarette smoking among high school students and adults, United States, 1965–2011. [Accessed 14 Jan 2015]; http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/
- 8.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults—United States, 2005-2012. MMWR Morb Mortal Wkly Rep. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 9.US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 11.Kiefe CI, Williams OD, Greenlund KJ, Ulene V, Gardin JM, Raczynski JM. Health care access and seven-year change in cigarette smoking. The CARDIA Study. Am J Prev Med. 1998;15(2):146–54. doi: 10.1016/s0749-3797(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 12.Kiefe CI, Williams OD, Lewis CE, Allison JJ, Sekar P, Wagenknecht LE. Ten-year changes in smoking among young adults: are racial differences explained by socioeconomic factors in the CARDIA study? Am J Public Health. 2001;91(2):213–8. doi: 10.2105/ajph.91.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Accessed 21 March 2015]. The health consequences of smoking—50 years of progress: a report of the surgeon general. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. [Google Scholar]
- 15.Clair C, Rigotti NA, Porneala B, Fox CS, D'Agostino RB, Pencina MJ, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309(10):1014–21. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Des Rochers C, Dische S, Saunders MI. The problem of cigarette smoking in radiotherapy for cancer in the head and neck. Clin Oncol (R Coll Radiol) 1992;4(4):214–6. doi: 10.1016/s0936-6555(05)81053-2. [DOI] [PubMed] [Google Scholar]
- 17.Ehdaie B, Furberg H, Zabor EC, Hakimi AA, Russo P. Comprehensive assessment of the impact of cigarette smoking on survival of clear cell kidney cancer. J Urol. 2014;191(3):597–602. doi: 10.1016/j.juro.2013.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klosky JL, Tyc VL, Garces-Webb DM, Buscemi J, Klesges RC, Hudson MM. Emerging issues in smoking among adolescent and adult cancer survivors: a comprehensive review. Cancer. 2007;110(11):2408–19. doi: 10.1002/cncr.23061. [DOI] [PubMed] [Google Scholar]
- 19.Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Ann Oncol. 2013;24(10):2699–704. doi: 10.1093/annonc/mdt279. [DOI] [PubMed] [Google Scholar]
- 20.Thompson TL, Pagedar NA, Karnell LH, Funk GF. Factors associated with mortality in 2-year survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2011;137(11):1100–5. doi: 10.1001/archoto.2011.179. [DOI] [PubMed] [Google Scholar]
- 21.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19(8):1941–8. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang S, Prizment A, Haddad T, Robien K, Lazovich D. Smoking and quality of life among female survivors of breast, colorectal and endometrial cancers in a prospective cohort. study J Cancer Surviv. 2011;5(2):115–22. doi: 10.1007/s11764-010-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253–61. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute Cancer survivors and smoking. [Accessed 14 Jan 2015]; http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2011&chid=101&coid=1038&mid=
- 25.Tseng TS, Lin HY, Martin MY, Chen T. Partridge EE Disparities in smoking and cessation status among cancer survivors and non-cancer individuals: a population-based study from National Health and Nutrition Examination Survey. J Cancer Surviv. 2010;4(4):313–21. doi: 10.1007/s11764-010-0127-9. [DOI] [PubMed] [Google Scholar]
- 26.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 27.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health Policy Brief: Young Adults and the Affordable Care Act. [Accessed 14 Jan 2015];Health affairs. 2013 Dec 16; http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_105.pdf.
- 29.Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118(19):4884–91. doi: 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underwood JM, Townsend JS, Tai E, White A, Davis SP, Fairley TL. Persistent cigarette smoking and other tobacco use after a tobacco-related cancer diagnosis. J Cancer Surviv. 2012;6(3):333–44. doi: 10.1007/s11764-012-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Lung Association. Affordable care act and tobacco control: a timeline. [Accessed 14 Jan 2015]; http://www.lung.org/stop-smoking/tobacco-control-advocacy/reports-resources/2012/hsq-aca-timeline-2012.pdf.
- 32.Madison K, Schmidt H, Volpp KG. Smoking, obesity, health insurance, and health incentives in the Affordable Care Act. JAMA. 2013;310(2):143–4. doi: 10.1001/jama.2013.7617. [DOI] [PubMed] [Google Scholar]
- 33.The Staff of The Washington Post. Landmark: the inside story of America's new health-care law and what it means for us all. New York: Public Affairs; 2010. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Behavioral risk factor surveillance system. [Accessed 14 Jan 2015]; http://www.cdc.gov/brfss/about/about_brfss.htm.
- 35.Centers for Disease Control and Prevention. Behavioral risk factor surveillance system 2009 questionnaire. [Accessed 14 Jan 2015]; http://www.cdc.gov/brfss/questionnaires/pdf-ques/2009brfss.pdf.
- 36.Centers for Disease Control and Prevention (CDC) Methodologic changes in the Behavioral Risk Factor Surveillance System in 2011 and potential effects on prevalence estimates. MMWR Morb Mortal Wkly Rep. 2012;61(22):410–3. [PubMed] [Google Scholar]
- 37.American Cancer Society. Cancer Treatment & Survivorship: Facts & Figures, 2014-2015. [Accessed 14 Jan 2015]; http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf.
- 38.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135–40. [PubMed] [Google Scholar]
- 40.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40(6):702–11. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Mayer DK, Carlson J. Smoking patterns in cancer survivors. Nicotine Tob Res. 2011;13(1):34–40. doi: 10.1093/ntr/ntq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–6. doi: 10.1001/jama.2013.285347. [DOI] [PubMed] [Google Scholar]
- 43.Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–6. doi: 10.1001/jama.2013.109501. [DOI] [PubMed] [Google Scholar]
- 44.Fairchild AL, Bayer R, Colgrove J. The renormalization of smoking? E-cigarettes and the tobacco “endgame”. N Engl J Med. 2014;370(4):293–5. doi: 10.1056/NEJMp1313940. [DOI] [PubMed] [Google Scholar]
- 45.Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014;174(5):812–3. doi: 10.1001/jamainternmed.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301(7):753–62. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 47.Health policy brief: essential health benefits. [Accessed 14 Jan 2015];Health affairs. 2013 May 2; http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_91.pdf.
- 48.Volpp KG, Galvin R. Reward-based incentives for smoking cessation: how a carrot became a stick. JAMA. 2014;311(9):909–10. doi: 10.1001/jama.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser Family Foundation. Focus on health reform: what the actuarial values in the affordable care act mean. [Accessed 14 Jan 2015];2011 Apr; http://kaiserfamilyfoundation.files.wordpress.com/2013/01/8177.pdf.
- 50.Katz MH. Health insurance is not health care. JAMA Intern Med. 2014;174(6):859–60. doi: 10.1001/jamainternmed.2014.598. [DOI] [PubMed] [Google Scholar]
- 51.Institute of Medicine and National Research Council. From cancer patient to cancer survivor: lost in transition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 52.Viswanathan M, Halpern M, Swinson Evans T, Birken SA, Mayer DK, Basch E. AHRQ Publication No 14-EHC011-EF. Rockville: Agency for Healthcare Research and Quality; 2014. [Accessed 14 Jan 2015]. Models of cancer survivorship care. http://effectivehealthcare.ahrq.gov/ehc/products/523/1881/cancer-survivor-care-transition-report-140318.pdf. [PubMed] [Google Scholar]
- 53.Warren GW, Marshall JR, Cummings KM, Toll BA, Gritz ER, Hutson A, et al. Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9(5):258–62. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blumenthal KJ, Saulsgiver KA, Norton L, Troxel AB, Anarella JP, Gesten FC, et al. Medicaid incentive programs to encourage healthy behavior show mixed results to date and should be studied and improved. Health Aff (Millwood) 2013;32(3):497–507. doi: 10.1377/hlthaff.2012.0431. [DOI] [PubMed] [Google Scholar]
- 55.Cunningham P. Patient engagement during medical visits and smoking cessation counseling. JAMA Intern Med. 2014;174(8):1291–8. doi: 10.1001/jamainternmed.2014.2170. [DOI] [PubMed] [Google Scholar]
- 56.Berg CJ, Carpenter MJ, Jardin B, Ostroff JS. Harm reduction and cessation efforts and interest in cessation resources among survivors of smoking-related cancers. J Cancer Surviv. 2013;7(1):44–54. doi: 10.1007/s11764-012-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaiser Family Foundation. Status of state action on the medicaid expansion decision. [Accessed 14 Jan 2015];2014 http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/
- 58.U.S. Department of Health & Human Services. Affordable care act rules on expanding access to preventive services for women. [Accessed 14 Jan 2015]; http://www.hhs.gov/healthcare/facts/factsheets/2011/08/womensprevention08012011a.html.
- 59.Blumberg SJ, Luke JV, Cynamon ML. Telephone coverage and health survey estimates: evaluating the need for concern about wireless substitution. Am J Public Health. 2006;96(5):926–31. doi: 10.2105/AJPH.2004.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu SS, Balluz L, Battaglia MP, Frankel MR. Improving public health surveillance using a dual-frame survey of landline and cell phone numbers. Am J Epidemiol. 2011;173(6):703–11. doi: 10.1093/aje/kwq442. [DOI] [PubMed] [Google Scholar]


